-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mammaglobin Immunostaining in the Differential Diagnosis Between Cutaneous Apocrine Carcinoma and Cutaneous Metastasis from Breast Carcinoma
Imunohistologický průkaz mamaglobinu v diferenciální diagnostice mezi apokrinním karcinomem kůže a kožní metastázou karcinomu prsu
Diferenciální diagnóza mezi apokrinním karcinomem kůže (AKK) a kožní metastázou karcinomu prsu je často obtížná. Spíše než imunohistologie může pomoci klinická informace.
Použili jsme protilátku mamaglobin k vyšetření 10 případů kožní metastázy duktálního karcinomu prsu a 2 případů AKK. V jednom z případů AKK byly pozitivní jen ojedinělé buňky, druhý případ byl negativní. Z 10 případů metastázy karcinomu prsu byly v 5 pozitivní buňky četné (až 60 % nádorových buněk), ve 4 byly pozitivní ojedinělé buňky a 1 případ byl negativní.
Jsme si vědomi, že by bylo v budoucnu vhodné vyšetřit více případů AKK než bude možno vyslovit kategorický závěr; naše výsledky však ukazují, že imunohistologický průkaz exprese mamaglobinu v četných buňkách nádoru může svědčit pro jeho metastatický původ.Klíčová slova:
mamaglobin – karcinom apokrinní žlázky – metastatický karcinom – duktální karcinom – prs
Authors: A. Fernandez-Flores
Authors place of work: Service of Cellular Pathology, Clinica Ponferrada, Ponferrada, Spain
Published in the journal: Čes.-slov. Patol., 45, 2009, No. 4, p. 108-112
Category: Původní práce
Summary
The differential diagnosis between cutaneous apocrine carcinoma (CAC) and cutaneous metastases from breast carcinoma is commonly difficult. Many times, clinical information is crucial in the final diagnosis, because help that can be obtained from immunohistochemistry is usually limited concerning this subject.
We used the antibody mammaglobin in order to study 10 cases of cutaneous metastasis of ductal breast carcinoma, and 2 cases of CAC. One of the CAC cases showed only scattered positive cells, while the other did not show any positivity. Four cases of metastatic breast carcinoma also showed scattered positive cells. In other five metastatic cases, positive cells were abundant, representing up to 60% of the tumoral cells. One case of metastatic breast carcinoma did not show any expression of mammaglobin at all. Although, more cases of CAC should probably be studied in the future before any categorical conclusion can be obtained, our results seem to indicate that a pattern of immunostaining with expression of mammaglobin in many cells would favor a metastatic origin of the tumor.Key words:
mammaglobin – apocrine gland carcinoma – metastatic carcinoma – ductal carcinoma – breastCutaneous apocrine carcinoma (CAC) is an elusive malignancy among the adnexal tumors. On the contrary to other adnexal tumors, the differential diagnosis with a cutaneous metastasis from a breast carcinoma is extremely difficult, up to the point that many reports emphasize how crucial the clinical information is. The immunohistochemistry has not been of much help in order to discriminate between both conditions. Many of these thoughts are presented in a recent article by Adámková et al. (3).
Mammaglobin is a relatively new antibody that intensively stains ductal breast carcinomas. Although the staining pattern with mammaglobin has been investigated in certain benign apocrine tumors, its expression has not been checked in CAC.
In this report, we investigated the expression of mammaglobin by two CACs, as well as by 10 cutaneous metastases from ductal breast carcinoma, in order to check if mammaglobin might help in the differential diagnosis between them.
Material and methods
The cases were recovered from our archives, revising the hematoxylin-eosin slides.
We performed an immunohistochemical study in all the cases, with the monoclonal mouse anti-human mammaglobin antibody of DakoCytomation (Clone 304-1A5; code N1637), and with the Dako REAL EnVision detection system.
Results
The details about the selected cases, including location of the tumors and gender and age of the patients are shown in table 1.
Tab. 1. Details about the cases investigated for expression of mammaglobin 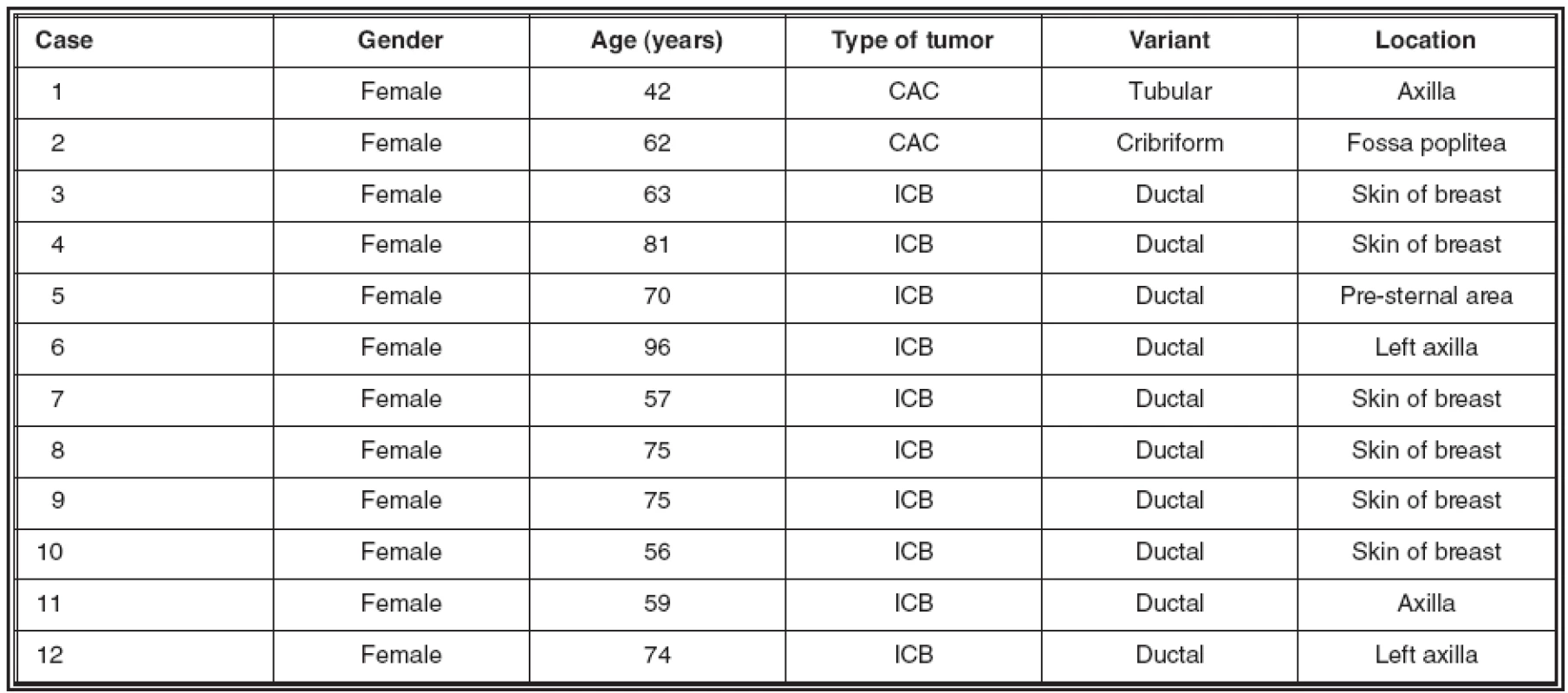
CAC: cutaneous apocrine carcinoma; ICB: infiltrating carcinoma of breast One of the CACs showed a common tubular morphology (Fig.1; bottom), while the second had a cribriform pattern (Fig. 1; top). This latter case has been reported on its own before (13).
Fig. 1. The two cases of CAC showed a cribriform (top) and tubuliform (bottom) pattern, respectively. 
In the immunohistochemical study, one of the cases of CAC (case number 2) showed only scattered positive cells (Fig.2.A), while the other did not show any positivity. Four cases of metastatic breast carcinoma showed a similar pattern of immunostain like the one observed in case number 2, with scattered cells expressing the antigen (Fig 2.B). In other five metastatic cases, positive cells were abundant and represented up to 60% of the tumoral cells (Fig.3). One case of meatastatic breast carcinoma did not show any expression of mammaglobin.
Fig. 2. A) Scattered positive cells for mammaglobin in one of the CAC cases (number 2) B) A similar pattern was observed in four cases of metastatic carcinoma from the breast. 
Fig. 3. Abundant positive cells expressing mammaglobin were observed in five of the metastatic cases. 
Discussion
Our results lead us to conclude that a pattern of immunostaining with many positive cells for mammaglobin would favor a metastasis from breast duct carcinoma. With “many”, we mean a pattern of expression in which more than only scattered cells are stained. Although the definition might sound ambiguous, a posititivity of more than 10% of the tumoral cells sounds as a reasonable condition. On the contrary, a pattern of immunostaining with “few scattered positive cells only” would not favor any of the two possibilities, and the same would happen if there was no expression of the marker. With the limited number of CAC cases that we studied, one should be cautious before such conclusions can be categorized and more studies with the antibody would be necessary in the future.
Another limitation of our study is that all cases studied from breast were ductal carcinomas instead of the specific apocrine carcinoma of breast. Althought breast is considered by many as a modified apocrine gland (1, 2), it could be claimed that perhaps the expression of apocrine carcinomas of the breast would have been different. Nevertheless, some studies on the subject have demonstrated that “breast tumoral cells with both apocrine and non-apocrine features express mammaglobin with roughly equal frequency and intensity” (39).
The information obtained gives us some help from the field of immunohistochemistry in a subject which is always difficult: the main differential diagnosis when facing a cutaneous apocrine carcinoma (CAC) is a metastasis from a breast carcinoma or also a carcinoma that arises in an axillary breast prolongation (17) or in ectopic mammary tissue (7, 20, 26, 35, 37, 39).
Some morphologic clues have been mentioned in literature in order to distinguish between both entities (32, 35), and one of the most helpful ones is the evidence of an in situ sweat gland component, which points out towards a CAC (9, 39).Since that finding is far from being the rule, the differential diagnosis between a primary tumor and a metastasis can sometimes be impossible without the appropriate clinical information (7, 20, 26, 35, 37, 39).
The immunophenotype of the tumor is only of a relative help in distinguishing its origin. In the past, some authors pointed out that an intense immunolabelling for CEA, especially in the absence of expression of GCDFP-15 by tumoral cells, would favour a primary cutaneous CAC over a metastasis from a breast carcinoma (20, 39). In fact, many of the CACs reported have shown a weak and focal expression of GCDFP-15 (26), or have failed to show any expression at all of the marker (7, 23, 24, 40). This is in spite of the fact that GCDFP-15 is considered as a very specific marker for apocrine differentiation (23, 39). Nevertheless, in a series, GCDFP-15 failed to mark four ductal breast carcinomas, while it was expressed by the only CAC studied (4),therefore demonstrating the relative use of the marker in this specific differential. Others demonstrated GCDFP-15 in less than half of their cases of breast carcinoma skin metastases (36).
It was sometime suggested that an immunophenotype androgen receptor (AR)+, estrogen receptor (ER)-, progesterone receptor (PR)-, would favour an apocrine origin (11, 25), since it is expressed not only by normal apocrine glands (11), but also by apocrine carcinomas (11, 22, 34) and by extrammary Paget disease, which is alleged by some to origin from apocrine glands (11). This latter point is nevertheless highly controversial, since the discovery of Toker cells also in the vulva (42). These cells are claimed as the precursor of extrammamary Paget disease by some (5, 15). An immunophenotype ER - PR - AR+ has not been the rule in all CACs studied in literature. Some for instance have demonstrated expression of ER in cribriform CAC (13). Some others have demonstrated expression of PR by apocrine adenomas, as well as by papillary CACs (23). Recently, Robson et al. studied a large series of CACs and demonstrated that 62 % were ER+, 60 % were PR+ and 36 % were AR - (30).
Cytokeratin (CK) 7 has been demonstrated as a good marker for Toker cells (14, 21, 41, 42) as well as for Paget disease, either mammary (26), or extrammary. It has not been found as useful in the diagnosis between a primary adnexal tumor and a metastasis, unless used as a part of an antibody panel (29). It is interesting how the pattern of immunostaining is important: focal CK7 expression was suggestive of a primary adnexal tumor, while diffuse immunostaining was mainly seen in a metastasis (29). This is similar to our results with mammaglobin, with a focal pattern favouring a primary tumor. This rule regarding CK7, nevertheless, seems to faint when distinguishing between CAC and a cutaneous metastasis of a breast carcinoma. CK7 has been demonstrated strongly and diffusely expressed by primary CAC (13).
Recently, p63 has been found to be of much use in the differential diagnosis of primary adnexal tumors versus metastatic adenocarcinomas to the skin (18, 19, 28).
However, CAC has been proved to be an exception, since not only its metastases but also the primary tumor does not express any p63 (19).
Other markers which are sometimes mentioned in the literature, in the diagnosis of primary cutaneous adnexal tumors, are only of relative help when facing a possible CAC. Cytokeratin (CK) 5/6, for instance, is usually expressed strongly and diffusely by primary cutaneous adnexal neoplasms (28).On the contrary, only a small percentage of cutaneous metastases express CK 5/6 and they usually do it in a weak way (28). Even so, these findings are not specific, and by no means CK 5/6 can be the only marker in which a diagnosis should be supported.
Mammaglobin is a 93 aminoacids protein which originally was identified in breast carcinoma cell lines (12). Mammaglobin is secreted as a glycosylated peptide (10). The expression of mammaglobin has been described in other tissues apart from breast, like lung tumors, tumors from the female genital tract (16, 29, 33), salivary gland tumors (29), and malignant mesothelioma (8). Mammaglobin is also expressed by eccrine and apocrine sweat glands (12), but the expression is quite different from the one observed in breast tissue. While eccrine glands show strong cytoplasmic staining of the coiled cells, in the immunohistochemical study for mammaglobin, the apocrine glands showed only staining of scattered cells (31).
Logically, this pattern might be expected for adnexal tumors of apocrine origin. For instance, cylindroma has been negative in most cases in which mammaglobin has been investigated, and when positive, only a small group of cells expressed the marker (31). Apocrine hidrocystoma showed a pattern of staining similar to the normal apocrine gland, i.e. just some scattered cells were positive (31); and the same pattern was the one observed in hidradenoma papilliferum (31). This is quite different from the pattern of staining that is observed in the adenocarcinomas developing from the breast, in which an intense and diffuse expression for mammaglobin is quite the rule (38). CAC, on the contrary, has not been investigated till now for mammaglobin expression, to the best of our knowledge, but the differences in expression by the breast tissue and apocrine tumors make us think that it could be one of the first reliable markers in the differential diagnosis when facing a possible CAC.
Our results seem to indicate that some additional help in the differential diagnosis between these entities could be obtained from the use of this marker when facing difficult cases. This opinion is in a way contrary to what has previously been claimed in literature. Bhargava et al., for instance, asserted that “mammaglobin does not seem to be a useful stain to distinguish breast from sweat gland carcinomas” (6). Nevertheless, they do not specified the type of sweat gland carcinoma studied in their report. That information is important, not only because the CAC is the most difficult to distinguish from a metastasis, but also because mammaglobin is strongly expressed by the normal eccrine gland (31).
Corresponding author:
Angel Fernandez-Flores, MD, PhD
S. Patología Celular
Avenida Galicia 1
24400 Ponferrada
Spain
Telephone: (00 34) 987 42 37 32
Fax: (00 34) 987 42 91 02
e-mail: gpyauflowerlion@terra.es
Zdroje
1. Ackerman AB, Kessler G, Gyorfi T, Tsou HC, Gottlieb GJ.: Contrary view: the breast is not an organ per se, but a distinctive region of skin and subcutaneous tissue. Am. J. Dermatopathol., 29 : 211-218, 2007.
2. Ackerman AB.: The breast is not an organ. Am J Dermatopathol., 30 : 304, 2008.
3. Adämková K, Kajo K, Kajová Y, Fetisovová Ž, Mellová Y.: Mammary adenocarcinoma in male and its differential diagnosis. Cesk. Dermatol., 82 : 347-351, 2007.
4. Ansai S, Koseki S, Hozumi Y, Kondo S.: An immunohistochemical study of lysozyme, CD-15 (Leu M1), and gross cystic disease fluid protein-15 in various skin tumors. Assessment of the specificity and sensitivity of markers of apocrine differentiation. Am. J. Dermatopathol., 17 : 249-255, 1995.
5. Belousova IE, Kazakov DV, Michal M, Suster S.: Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary paget disease. Am. J. Dermatopathol., 28 : 84-86, 2006.
6. Bhargava R, Beriwal S, Dabbs DJ.: Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am. J. Clin. Pathol., 127 : 103-113, 2007.
7. Cameseille-Teijeiro J, Cameseille-Teijeiro JF.: Homologous carcinomas of the breast and skin. Am. J. Clin. Pathol., 111 : 709-712, 1999.
8. Carletti AM, Roncella S, Canessa PA, et al.: Expression of human mammaglobin in pleural effusions of patients with malignant mesothelioma. Thorax, 61 : 271, 2006.
9. Chamberlain RS, Huber K, White JC, et al.: Apocrine gland carcinoma of the axilla. Review of the literature and recommendations for treatment. Am. J. Clin. Oncol., 22 : 131-135, 1999.
10. Colpitts TL, Billing-Medel P, Friedman P, et al.: Mammaglobin is found in breast tissue as a complex with BU101. Biochemistry, 40 : 11048-11059, 2001.
11. Diaz de Leon E, Carcangiu ML, Prieto VG, McCue PA, Burchette JL, To G, Norris BA, Kovatich AJ, Sanchez RL, Krigman HR, Gatalica Z.: Extramammary Paget disease is characterized by the consistent lack of estrogen and progesterone receptors but frequently expresses androgen receptor. Am. J. Clin. Pathol., 113 : 572-575, 2000.
12. Fanger GR, Houghton RL, Retter MW, et al.: Detection of mammaglobin in the sera of patients with breast cancer. Tumour. Biol., 23 : 212-221, 2000.
13. Fernandez-Flores A, Pol A, Juanes F, Crespo LG.: Immunohistochemical phenotype of cutaneous cribriform carcinoma with a panel of 15 antibodies. Med. Mol. Morphol., 40 : 212-217, 2007.
14. Fernandez-Flores A.: Toker cell related to the folliculo-sebaceous-apocrine unit: a study of horizontal sections of the nipple. Rom. J. Morphol. Embryol., 49 : 339-343.
15. Fernandez-Flores A.: Toker-cell pathology as a unifying concept. Histopathology, 52 : 889-891, 2008.
16. Grünewald K, Haun M, Fiegl M, et al.: Mammaglobin expression in gynaecologic malignancies and malignant effusions detected by nested reverse transcriptase-polymerase chain reaction. Lab. Invest., 82 : 1147-1153, 2002.
17. Gutermuth J, Audring H, Voit C, et al.: Primary carcinoma of ectopic axillary breast tissue. J. Eur. Acad. Dermatol. Venereol., 20 : 217-221, 2006.
18. Ivan D, Diwan AH, Prieto VG.: Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod. Pathol. 18 : 137-142, 2005.
19. Ivan D, Nash JW, Prieto VG, et al.: Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to the skin. J. Cutan. Pathol., 34 : 474-480, 2007.
20. Katagiri Y, Ansai S.: Two cases of cutaneous apocrine ductal carcinoma of the axilla. Dermatology,199 : 332-337, 1999.
21. Lundquist K, Kohler S, Rouse RV.: Intraepidermal cytokeratin 7 expression is not restricted to Paget cells but is also seen in Toker cells and Merkel cells. Am. J. Surg. Pathol., 23 : 212-219, 1999.
22. MacNeill KN, Riddell RH, Ghazarian D.: Perianal apocrine adenocarcinoma arising in a benign apocrine adenoma; first case report and review of the literature. J. Clin. Pathol., 58 : 217-219, 2005.
23. Miyamoto T, Hagari Y, Inoue S, et al.: Axillary apocrine carcinoma with benign apocrine tumours: a case report involving a pathological and immunohistochemical study and review of the literature. J. Clin. Pathol. 58 : 757-761, 2005.
24. Nishikawa Y, Tokushashi Y, Saito Y, et al.: A case of apocrine adenocarcinoma associated with hamartomatous apocrine gland hyperplasia of both axillae. Am. J. Surg. Pathol., 18 : 832-836, 1994.
25. Obaidat NA, Alsaad KO, Ghazarian D.: Skin adnexal neoplasms—part 2: an approach to tumours of cutaneous sweat glands. J. Clin. Pathol. 60 : 145-159, 2007.
26. Ohnishi T, Watanabe S.: The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget’s disease. Br. J. Dermatol. 142 : 243-247, 2000.
27. Paties C, Taccagni L, Papotti M, et al.: Apocrine carcinoma of the skin. A clinicopathologic, immunohistochemical, and ultrastructural study. Cancer, 71 : 375-381, 1993.
28. Plumb SJ, Argenyi ZB, Stone MS, et al.: Cytokeratin 5/6 immunostaining in cutaneous adnexal neoplasms and metastatic adenocarcinoma. Am. J. Dermatopathol., 26 : 447-451, 2004.
29. Qureshi HS, Ormsby AH, Lee MW, et al.: The diagnostic utility of p63, CK5/6, CK7, and CK20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J. Cutan. Pathol., 31 : 145-152, 2004.
30. Robson A, Lazar AJ, Ben Nagi J, Hanby A, Grayson W, Feinmesser M, Granter SR, Seed P, Warneke CL, McKee PH, Calonje E.: Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am. J. Surg. Pathol., 32 : 682-690, 2008.
31. Sasaki E, Tsunoda N, Hatanaka Y, et al.: Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod. Pathol., 20 : 208-214, 2007.
32. Sjödin A, Guo D, Hofer P-A, et al.: Mammaglobin in normal human sweat glands and human sweat gland tumors. J. Invest. Dermatol., 121 : 428-429, 2003.
33. Stout AP, Cooley SGE.: Carcinoma of the sweat glands. Cancer, 4 : 521-536, 1951.
34. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR.: Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am. J. Surg. Pathol., 15 : 835-841, 1991.
35. Tassi RA, Bignotti E, Rossi E, et al.: Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol. Oncol., 105 : 578-585, 2007.
36. Wallace ML, Longacre TA, Smoller BR.: Estrogen and progesterone receptors and anti-gross cystic disease fluid protein 15 (BRST-2) fail to distinguish metastatic breast carcinoma from eccrine neoplasms. Mod. Pathol. 8 : 897-901, 1995.
37. Warkel RL, Hekwig EB.: Apocrine gland adenoma and adenocarcinoma of the axilla. Arch. Dermatol., 114 : 198-203, 1978.
38. Warkel RL.: Selected apocrine neoplasms. J. Cutan. Pathol., 11 : 437-449, 1984.
39. Watson MA, Dintzis S, Darrow CM, et al.: Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res., 59 : 3028-3031, 1999.
40. Wick MR, Ockner DM, Mills SE, et al.: Homologous carcinomas of the breast, skin, and salivary glands. A histologic and immunohistochemical comparison of ductal mammary carcinoma, ductal sweat gland carcinoma, and salivary duct carcinoma. Am. J. Clin. Pathol., 109 : 75-84, 1998.
41. Willman JH, Golitz LE, Fitzpatrick JE.: Clear cells of Toker in accessory nipples. J. Cutan. Pathol. 30 : 256-260, 2003.
42. Willman JH, Golitz LE, Fitzpatrick JE.: Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am. J. Dermatopathol. 27 : 185-188, 2005.
43. Yoshida A, Kodama Y, Hatanaka S, et al.: Apocrine adenocarcinoma of the bilateral axillae. Acta Pathol. Jpn., 41 : 927-932, 1991.
Štítky
Patologie Soudní lékařství Toxikologie
Článek Jaká je vaše diagnóza?Článek Plán akcí IPZV – jaro 2010Článek Prof. Hlava v PardubicíchČlánek JAKÁ JE VAŠE DIAGNÓZA?Článek JAK SE VÁM LÍBÍ?Článek Pláč českého jazyka
Článek vyšel v časopiseČesko-slovenská patologie

2009 Číslo 4-
Všechny články tohoto čísla
- Jaká je vaše diagnóza?
- Detection of Regulatory Protein p16/INK4A in the Dysplastic Cervical Squamous Cell Epithelium is a Diagnostic Tool for Carcinoma Prevention
- Plán akcí IPZV – jaro 2010
- Mammaglobin Immunostaining in the Differential Diagnosis Between Cutaneous Apocrine Carcinoma and Cutaneous Metastasis from Breast Carcinoma
- Prof. Hlava v Pardubicích
- Unusual Clinical Presentation of Hepatic Yolk Sac Tumour in Periappendical Region. A Case Report and Review of the Literature
- Congenital Granular Cell Epulis: a Case Report
- JAKÁ JE VAŠE DIAGNÓZA?
- JAK SE VÁM LÍBÍ?
- ROČNÍK 45 (Česko-slovenská patologie)
- Pláč českého jazyka
- New Aspects of Tumor Pathobiology
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Detection of Regulatory Protein p16/INK4A in the Dysplastic Cervical Squamous Cell Epithelium is a Diagnostic Tool for Carcinoma Prevention
- Congenital Granular Cell Epulis: a Case Report
- New Aspects of Tumor Pathobiology
- JAKÁ JE VAŠE DIAGNÓZA?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



