-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Eosinophilic esophagitis – 10 years of experience in five Czech pediatric endoscopy centers
Eozinofilní ezofagitida – 10 let zkušeností pěti českých pediatrických endoskopických center
Úvod: Eozinofilní ezofagitida (EoE) je chronické progresivní zánětlivé onemocnění jícnu charakterizované lokální eozinofilní infiltrací doprovázenou příznaky dysfunkce jícnu. Cílem této studie bylo charakterizovat rysy regionálně dia gnostikovaných případů EoE a popsat lokální strategie léčby.
Metody: V observační studii byla retrospektivně analyzována data dětských pacientů s histologicky prokázanou EoE v ně kte rém z pěti dětských endoskopických center. Tato analýza se zaměřila na obecné charakteristiky pacientů (věk, pohlaví, příznaky) a jejich možnou souvislost s trváním symptomatického onemocnění do stanovení dia gnózy. U pacientů byly dále analyzovány demografické parametry, klinické příznaky, laboratorní, endoskopické a histopatologické nálezy a použití různých modalit léčby.
Výsledky: Od ledna 2010 do září 2020 bylo zaznamenáno 33 nových případů EoE. Silná vazba EoE na mužské pohlaví byla v souladu s výsledky dříve publikovaných studií (81,8 %). Ačkoliv medián věku pacientů v době prvních příznaků byl 7 let, medián věku v době stanovení dia gnózy byl téměř 13 let. Nejčastěji referovaným symptomem byly refluxní příznaky obecně (39,4 %), následované zvracením (36,4 %) a dysfagií (33,3 %). Stanovení senzibilizace proti různým potravinovým alergenům bylo provedeno u 23 (69,7 %) pacientů s EoE. Z těchto pacientů byla u 17 (51,5 % vyšetřovaných) prokázána senzibilizace na ně kte rý z potravinových alergenů. Téměř polovina pacientů (48,5 %) byla vyšetřována stanovením specifických IgE a pouze u 2 pacientů (6,1 %) bylo provedeno vyšetření kožními prick testy. Alergická komorbidita byla zjištěna u 75,8 % pacientů, nejčastěji to bylo bronchiální astma a alergická rinokonjunktivitida (45,5 %; resp. 42,2 %). U více než dvou třetin pacientů prokázala dia gnostická endoskopie abnormální makroskopické nálezy (69,7 %), nejčastěji podélné rýhy a bílé exsudáty. Nejčastěji zvoleným způsobem léčby byly inhibitory protonové pumpy (93,9 %), následované eliminační dietou (75,8 %) a podáváním kortikosteroidů (63,6 %). Naprostá většina pacientů léčených kortikosteroidy byla léčena topickými formami (90,9 %).
Závěr: Jedná se o první retrospektivní studii u pediatrických pacientů s EoE v České republice. U pacientů s EoE byly prokázány velmi podobné charakteristiky jako v dříve publikovaných zahraničních pracích. Dlouhodobě sbíraná prospektivní data v národním registru EoE pacientů České republiky by významně napomohla v dalším získávání znalostí o tomto onemocnění.
Klíčová slova:
eozinofilní ezofagitida – deti – pediatrie – endoskopie
Authors: Pecl J. 1,2; Karásková E. 3; Kunovsky L. 2,4,5; Jimramovský F. 1,2; Schneiderová H. 1,2; Pinkasová T. 1,2; Veverková M. 1,2; Jouza M. 1,2; Hloušková E. 1,2; Bajerová K. 1,2; Látalová V. 3; Véghová-Velgáňová M. 3; Geryk M. 3; Šuláková A. 6; Ťoukálková L. 7; Jakšič D. 7; Zimen M. 8; Ježová M. 2,9; Urík M. 2,10; Wiesnerová M. 11; Jabandžiev P. 1,2,12
Authors place of work: Department of Pediatrics, University Hospital Brno, Brno, Czech Republic 1; Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Department of Pediatrics, Faculty of Medicine and Dentistry, Palacký University, University Hospital, Olomouc, Czech Republic 3; Department of Gastroenterology and Internal Medicine, University Hospital Brno, Brno, Czech Republic 4; Department of Surgery, University Hospital Brno, Brno, Czech Republic 5; Department of Pediatrics, Faculty of Medicine, University of Ostrava, University Hospital Ostrava, Ostrava, Czech Republic 6; Department of Pediatrics, Tomas Bata Regional Hospital, Zlin, Czech Republic 7; Department of Pediatrics, Jihlava Hospital, Jihlava, Czech Republic 8; Department of Pathology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 9; Department of Pediatric Otorhinolaryngology, University Hospital Brno, Faculty of Medicine, Masaryk University Brno, Brno, Czech Republic 10; Institute of Biostatistics and Analyses Ltd., Brno, Czech Republic 11; Central European Institute of Technology, Masaryk University, Brno, Czech Republic 12
Published in the journal: Gastroent Hepatol 2020; 74(6): 469-480
Category: Original article
doi: https://doi.org/10.48095/ccgh2020469Summary
Background: Eosinophilic esophagitis (EoE) is a chronic, progressive inflammatory disease of the esophagus characterized by local eosinophilic infiltration accompanied by symptoms of esophageal dysfunction. The aim of this study was to characterize features of EoE dia gnosed regionally and to describe local strategies for its treatment.
Methods: The observational survey retrospectively analyzed a data set of child patients with histologically proven EoE from five pediatric endoscopy centers. This analysis focused on describing their general situation (age, gender, symptoms) and also aimed to investigate any possible linkage between age and symptoms or length of dia gnosis period. Demographic features; clinical symptoms; laboratory, endoscopic, and histopathological findings; and chosen treatment of patients were recorded and analyzed.
Results: From January 2010 to September 2020, 33 new cases of EoE were reported. Strong association of EoE with male sex is consistent with the results from formerly published studies (81.8%). The median age of symptom onset was 7 years, while the median age for dia gnosis was almost 13 years. The most common symptoms were reflux symptoms in general (39.4%), followed by vomiting (36.4%), and dysphagia (33.3%). Examination for sensitization to food allergens was performed on 23 (69.7%) patients with dia gnosed EoE. Out of these, 17 (51.5% of all cases) were found to be sensitive to some allergens. Most of this subgroup (and 48.5% of all cases) were examined by specific IgE testing, and just 2 (6.1% of all cases) patients were tested only by skin prick tests. Another allergic comorbidity was present in 75.8% of patients, and the most common of these were bronchial asthma and allergic rhinoconjunctivitis (45.5% and 42.2%, respectively). More than two-thirds of patients (69.7%) had abnormal macroscopic findings during dia gnostic endoscopy, and the most common were longitudinal furrows and white exudates. The most common initial modality of treatment was to use proton-pump inhibitors (PPI; 93.9%), followed by food elimination (75.8%) and then by corticosteroid administration (63.6%). The vast majority of patients treated with corticosteroids received a topical preparation (90.9%).
Conclusion: This is the first retrospective study on pediatric patients with EoE in the Czech Republic. We found similar features of EoE as reported in formerly published works elsewhere. Collecting long-term prospective observational data in a national EoE register of patients in the Czech Republic would significantly improve our knowledge of this disease.
Keywords:
eosinophilic esophagitis – children – Pediatrics – endoscopy
Introduction
Eosinophilic esophagitis (EoE) is a chronic progressive inflammatory disease characterized by local eosinophilic infiltration of the esophageal mucosa and symptoms resulting from its dysfunction. EoE and gastroesophageal reflux disease (GERD) are today the most commonly identified esophageal causes of difficulty in swallowing among children and adolescents [1]. EoE is currently a relatively common finding in esophagoscopy (up to 7%) and can no longer be considered rare (overall prevalence 0.5–1 case per 1,000 persons) [2]. Despite the number of publications ana lyzing various attributes of EoE, the cause of the exponential increase in its prevalence within Western Europe over the past three decades remains unclear. This is the first retrospective analysis of pediatric EoE in the Czech Republic. The aims of this study were to analyze the demographic, clinical, laboratory, and endoscopic features of pediatric EoE from a large geographic region, to better understand EoE across a wide range of variable manifestations, and to provide feedback to improve the dia gnostic-therapeutic process.
Material and methods
The data for patients with the EoE dia gnosis were collected from three university hospitals in Moravia and two regional pediatric departments that are capable of endoscopic examinations and further to look after patients with EoE. All participating pediatricians, pediatric gastroenterologists, and allergologists were sent an e-mail asking them to report all dia gnoses of histologically proven pediatric EoE (≥ 15 eosinophils per high-power field [HPF] in a sample of esophageal mucosa with the highest number of eosinophils) made in the previous 10 years. The identities of all cases remained anonymous. Data collected via a standardized questionnaire included patient demogra phics, symptoms, macroscopic and histopathological findings at dia gnosis and control endoscopy, therapeutic mana gement, allergic comorbidity, and exa mination of sensitization. The collected data are presented as means or medians, with categorical variables summarized as percentages. The data were analyzed using the SAS and SPSS programs. The threshold for statistical significance of tests used in the analysis was 5%.
Results
Demographics
We have collected reports from 33 cases of pediatric EoE, 27 of which were for male patients (81.8%) and 6 (18.2%) for female patients. The male-to-female ratio is thus approximately 4 : 1.
Clinical symptoms
Mean age for the occurrence of EoE symptoms was 8.05 years (SD 5, median 7.0) and the mean age for dia gnosis was almost 11.4 years (SD 4.60, median 12.75). Various presenting symptoms leading up to the dia gnosis irrespective of and according to age are described in Tab. 1.
Tab. 1. Presenting symptoms leading up to diagnosis of EoE in general and in age-related groups.
Tab. 1. Hlavní příznaky vedoucí k diagnóze EoE celkově a v různých věkových skupinách.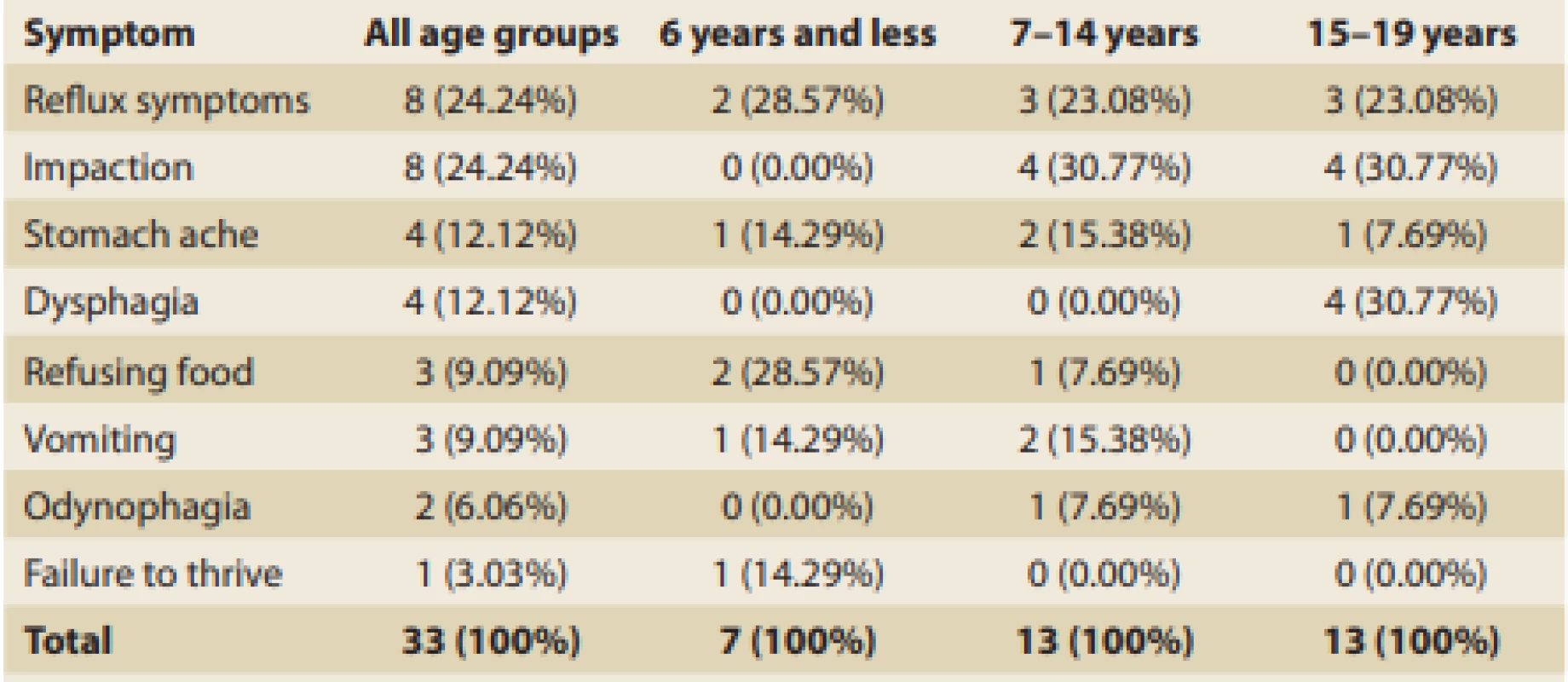
The age of manifestation for each individual presenting symptom of EoE and symptomatic time to dia gnosis were established, and these data are summarized in Tab. 2. A median of fewer than 3 years was needed to dia gnose EoE in patients with food impaction, vomiting, odynophagia, dysphagia, and abdo minal pain. In cases of reflux symptoms, failure to thrive, and food refusal, however, it took a longer time to be referred and dia gnosed. The wide range of symptomatic periods makes it difficult to interpret this data and subsequent collection of data would be appropriate.
Tab. 2. Age of first EoE signs manifestation and symptomatic time distribution according to the main symptom (in years).
Tab. 2. Věk při manifestaci prvních příznaků EoE a délka jejich trvání před stanovením diagnózy (roky).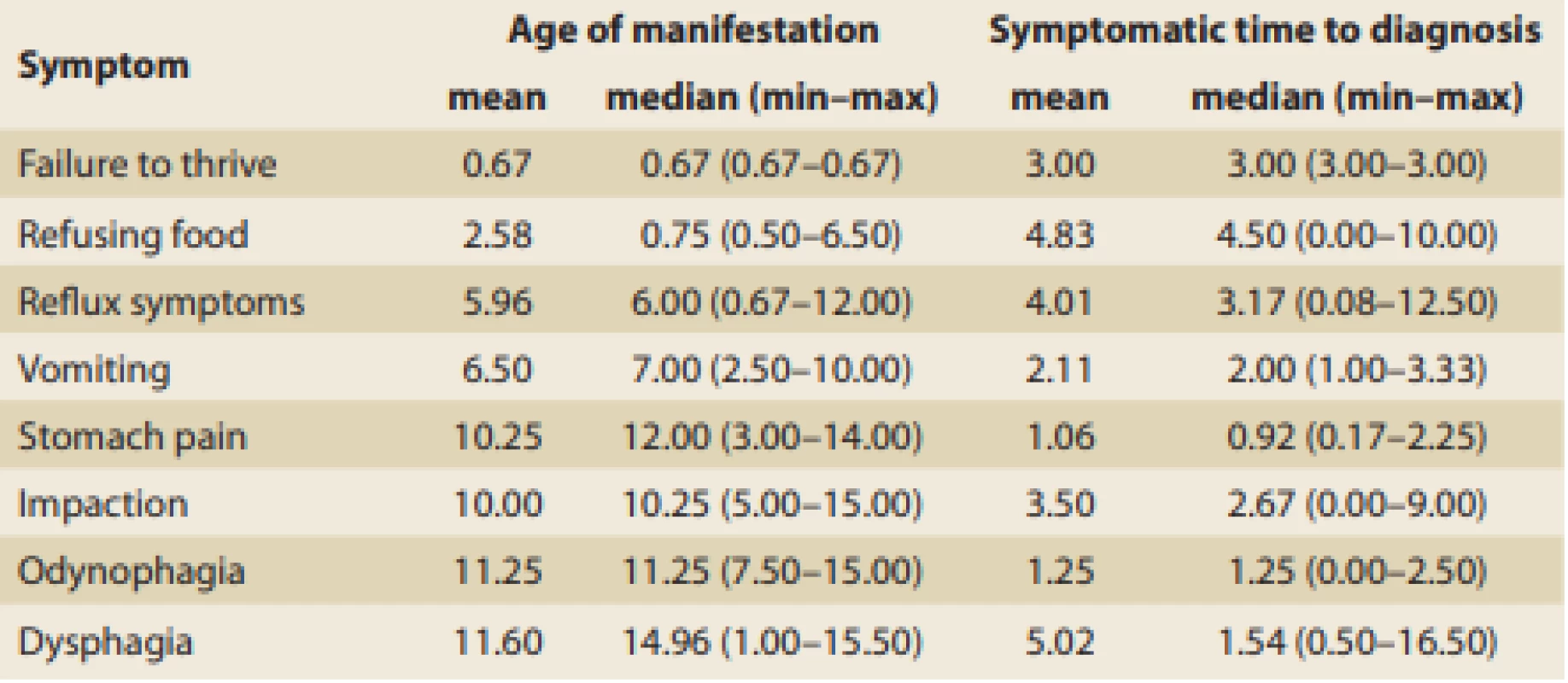
Irrespective of patient age, reflux symptoms were the most common and affected more than 39% of all patients with EoE. This was closely followed by vomiting (36%) and dysphagia (33%), then by food impaction and stomach ache. The occurrence of clinical symptoms significantly varied depending on children’s ages at the time of dia gnosis (Tab. 3).
Tab. 3. Occurrence of clinical symptoms according to the children’s ages.
Tab. 3. Výskyt klinických příznaku podle věku pacientů.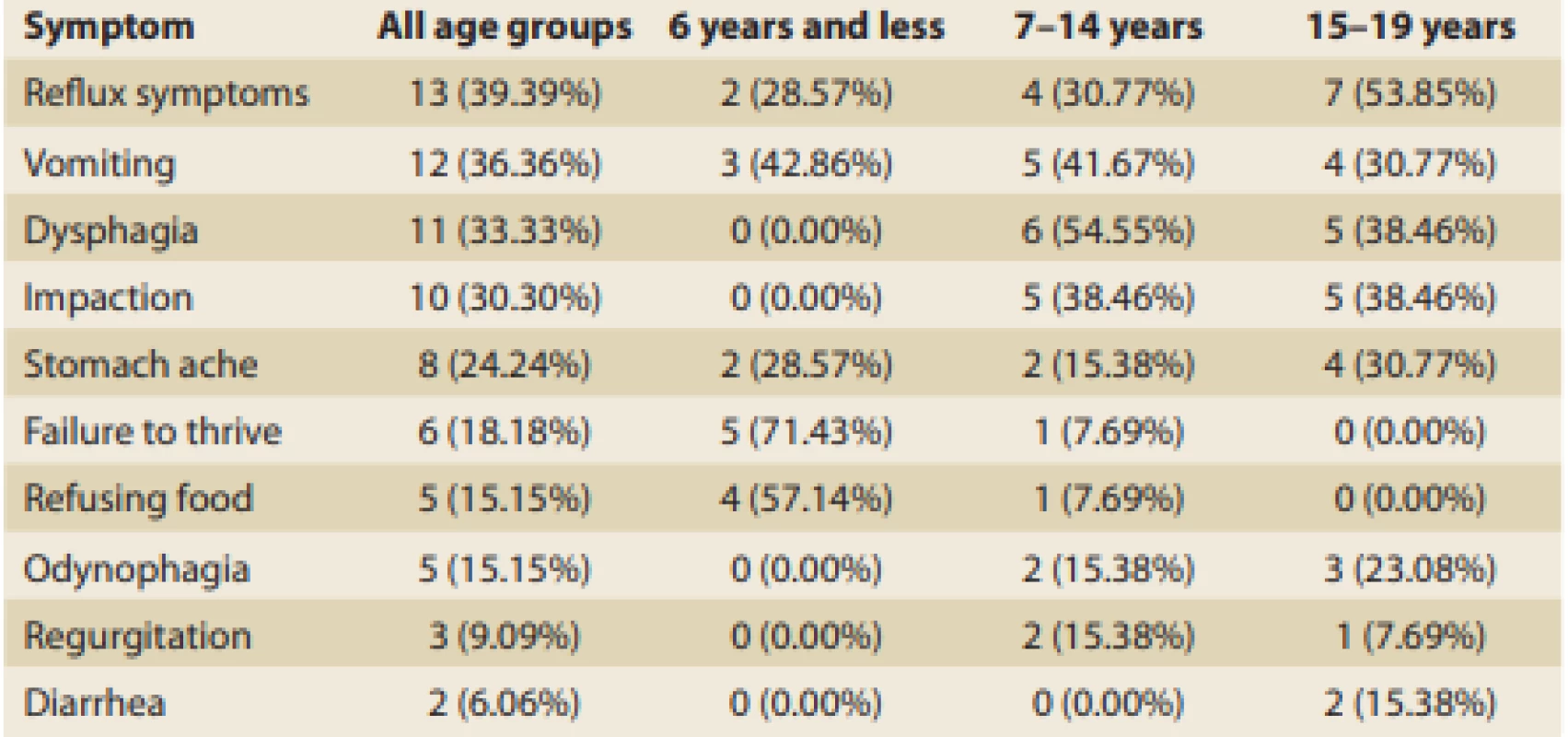
The youngest patients (≤ 6 years) most frequently showed a failure to thrive (71%), followed by food refusal (57%), and vomiting (43%). Patients from 7 to 14 years of age most frequently complained about dysphagia (55%), followed by vomiting and food impaction (both around 40%), then by reflux symptoms (just less than one-third). Symptoms of patients above 15 years of age were pretty similar to those of the previous age-related group. Reflux symptoms in general, including cough and pyrosis, were typical (almost 54%) and were followed by dysphagia and impaction (both 38%). Adolescents relatively often also referred to vomiting (31%), stomach ache (30%), and odynophagia (23%).
The overall numbers of symptoms manifested by patients with EoE generally and within age-related groups are shown in Tab. 4.
Tab. 4. Numbers of symptoms generally and by age group.
Tab. 4. Celkový počet příznaků a jejich počet v různých věkových skupinách.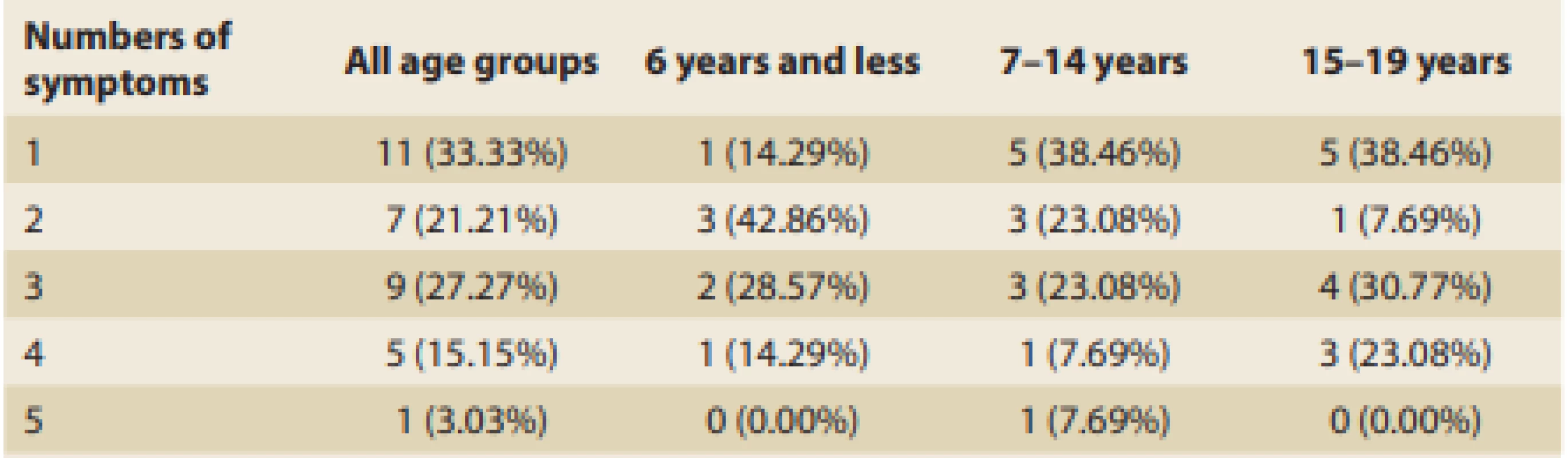
We also looked at the symptomatic time length in relation to the age of the patients at the time of the first symptoms. The data are depicted graphically in Fig. 1.
Fig. 1. Length of symptomatic time before diagnosis achievement in relation to the age of first symptom onset (years).
Obr. 1. Délka trvání příznaků před stanovením diagnózy ve vztahu k věku při jejich manifestaci (roky).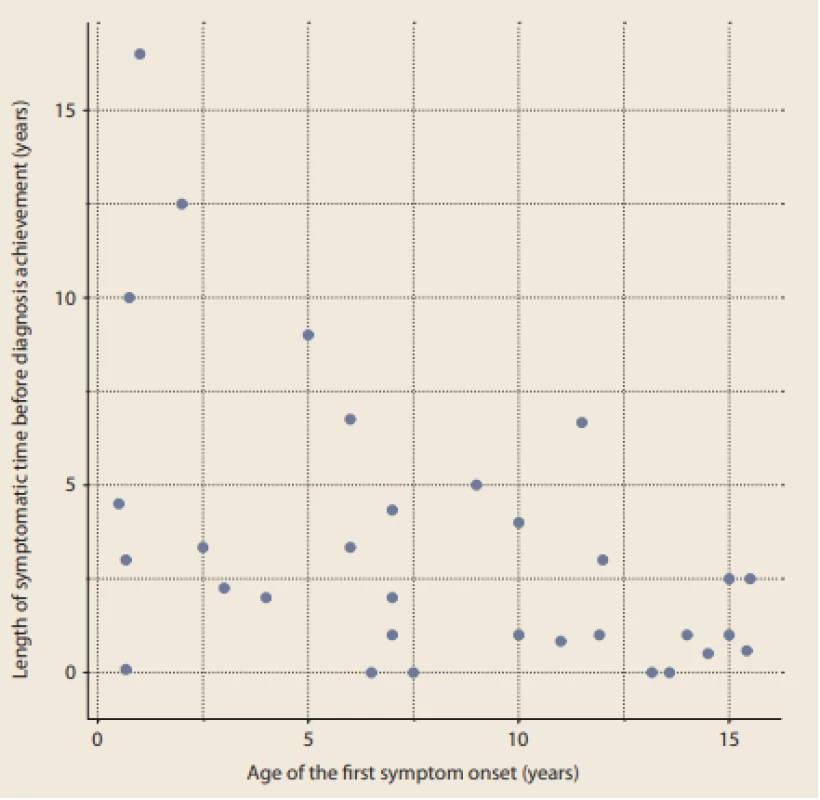
Patients were divided into three groups according to their age at occurrence of the first symptoms, and the results are summarized in Tab. 5.
Tab. 5. The length of symptomatic period before diagnosis achievement according to the age o patients (years).
Tab. 5. Délka symptomatického období před stanovením diagnózy podle věku pacientů (roky).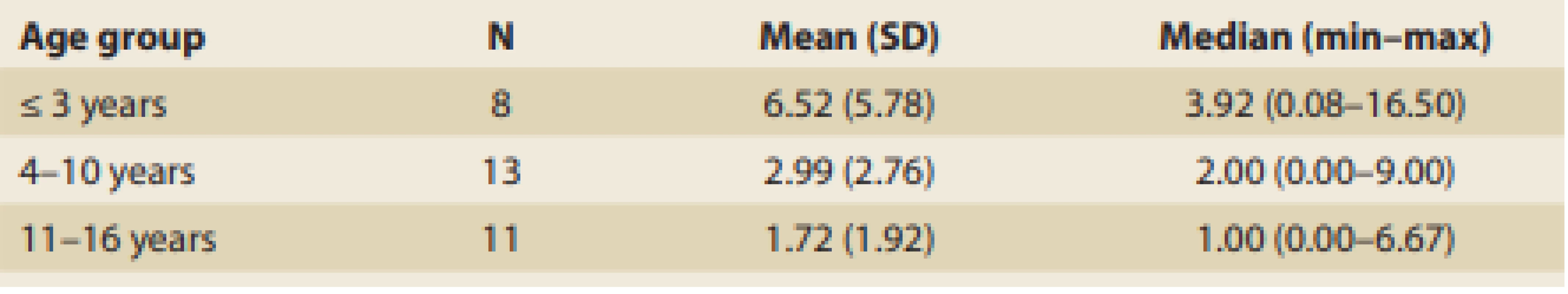
The longest symptomatic period prior to establishing the dia gnosis was reported in a boy nearly 18 years old who had intermittent dysphagia, vomiting, and reflux symptoms already from his second year of life. The longest-wai ting girl was at first dia gnosed as ha ving an eating disorder. From infancy she showed food refusal and failure to thrive, followed later by esophageal reflux symptoms, regurgitation, and nausea. At age 5 years, she refused to swallow solid parts of food and suffered an aspiration episode in attempting to swallow a berry. At 7 years of age, she underwent esophagogastroduodenoscopy, unfortunately without performance of esophageal bio psy. She was referred to a neuro logist for suspicion of autistic spectrum disorder. Finally, at 10 years of age, due to persisting mild problems like dysphagia and regurgitation, she underwent a control endoscopy which, despite normal macroscopic features of esophageal mucosa, showed histopathological features typical of EoE.
Endoscopic and histopathological findings
Almost one-third of the 33 patients with EoE had normal macroscopic findings during the first endoscopy (30%). Other wise longitudinal furrows (sometimes referred to as “crepe paper-like appearance”), white exudates, and mucosal erythema were the most common findings (30%, 8%, and 7%, respectively). Much less frequently, features of chronic inflammation like trachealization or concentric rings (21%) and esophageal strictures (6%) were apparent upon first bio psy. In comparison with the features more frequently seen, however, these had a stronger tendency to persist even after the administration of treatment. Fig. 2 summarizes the presence of various endoscopic findings apparent during dia gnostic and control endoscopy in the 33 patients with EoE.
Fig. 2. Occurrence of various endoscopic fi ndings at fi rst and later endoscopy.
Obr. 2. Výskyt různých endoskopických nálezů při diagnostické a kontrolní endoskopii.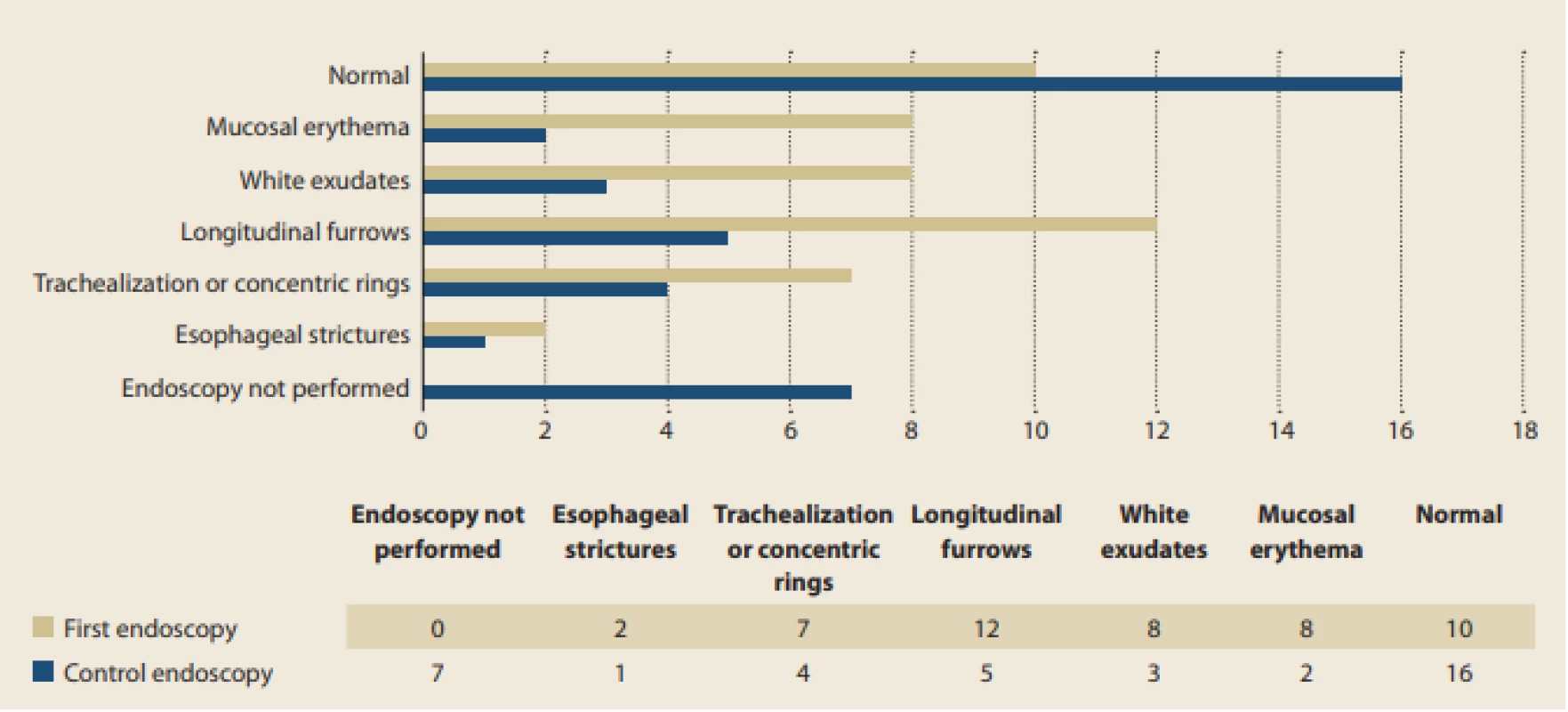
The typical age of patients and symptomatic time to achieving dia gnosis according to the particular endoscopic findings are summarized in Tab. 6. A hypothetical association between the age of patients or symptomatic time to dia gnosis with certain endoscopic fin dings was examined using Mann-Whitney U-test, but no significant relationship was proven. On the other hand, due to the low number of specific endoscopic findings, the hypothetical association of specific endoscopic findings with the length of a symptomatic period is not conclusively debunked, and this possibility is indicated in the present data set.
Tab. 6. Age at diagnosis and symptomatic time before diagnosis in relation to endoscopic fi ndings (in years).
Tab. 6. Věk při stanovení diagnózy a trvání příznaků před stanovením diagnózy u různých endoskopických nálezů (roky).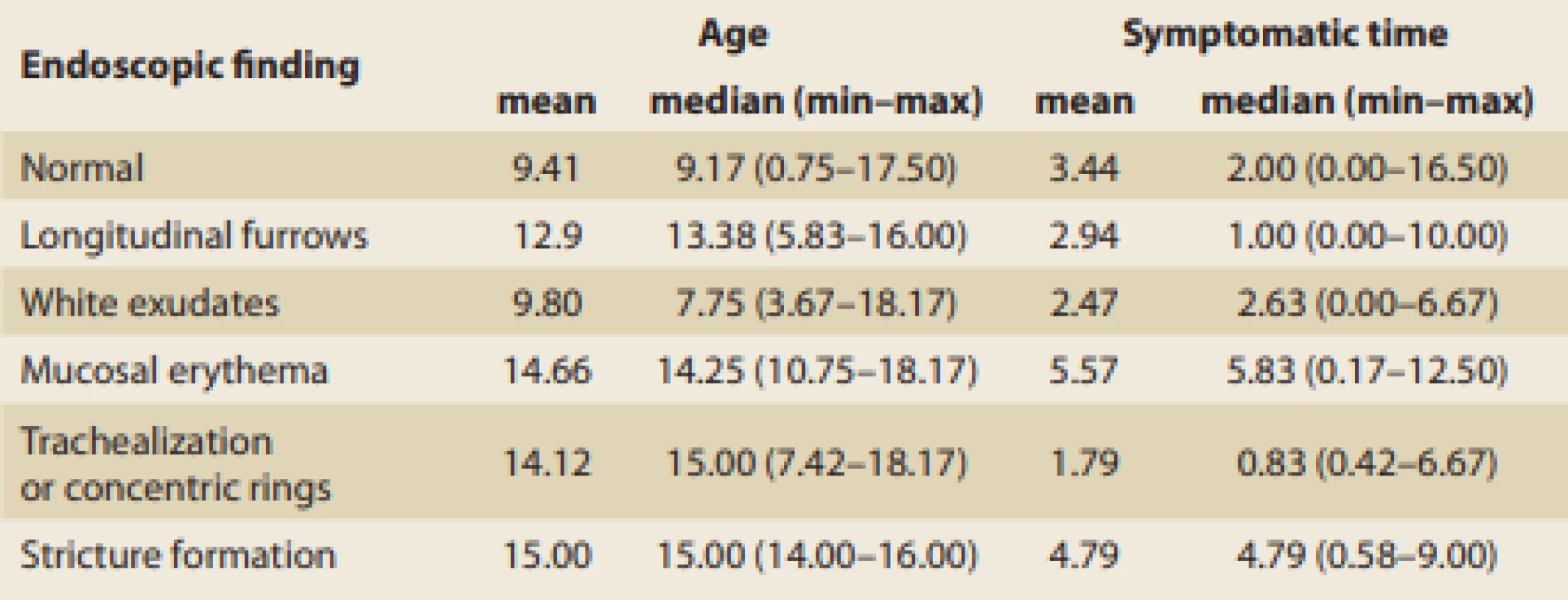
The median intraepithelial eosinophil count (IEE) per high-power field (HPF) in the dia gnostic bio psy samples obtained from the esophagus in EoE patients was 51.16/HPF, while in the control samples it was 35.48/HPF. Decrease in local eosino philic inflammation during treatment was significant, and this was also confirmed by Wilcoxon signed-rank test (p-value = 0.0002). Nevertheless, this median value is still more than twice the cutoff.
Allergological evaluation
The median relative eosinophil count in peripheral blood samples of patients with EoE was 5.6% (1.70–30.70) and the median immunoglobulin E (IgE) level was 300 kU/l (24–1,960). Relative eosinophil counts and especially IgE levels were significantly higher in younger patients and there was a significant decreasing trend with higher age. The correlation of both variables with age at dia gnosis was significant (IgE: Spearman’s Rho = 0.41, p-value = 0.034; Eosinophils: Spearman’s Rho = −0.37, p-value = 0.037). Sensitization to food allergens was examined in 23 (69.70%) patients overall. Of these, 17 (51.52% of all cases) were found to be sensitive to some of the food allergens. Most of this subgroup of patients – 16 (48.48% of all cases) – were examined by laboratory-specific IgE assessment and only 2 (6.06% of all cases) by skin prick-to-prick testing with native foods. Five (15.15%) of the patients were tested by both methods. Three out of 7 patients (42.86%) from the group of the youn gest children (≤ 6 years of age) were sensitized to some food. In 2 (28.57%) cases sensitivity was to cow’s milk, in 2 cases to eggs, and in 2 cases to peanuts. One patient for each (14.29%) had sensiti vity to soy, gliadin, and wheat. In the patient group aged 7–14 years, 7 (53.85%) were sensitized. The most common sensitization was to proteins of cow’s milk, with 4 cases (30.77%), followed by hazelnuts with 3 cases (23.08%). Wheat, walnuts, peanuts, and eggs sensitized 2 (15.38%) patients each, and apples, potatoes, gliadin, and soy 1 (7.69%) patient each. The oldest group of adolescent patients aged 15 years or older had the highest sensitization rate to food allergens: 8 (61.54%) out of 13 overall. These patients were the most often polysensitized to food allergens. The most common food allergens which sensitized this older group were hazelnuts in 7 (53.85%) patients, closely followed by wheat, peanuts, and apples in 6 (46.15%) patients each. Other allergens sensitized adolescents in much lower numbers. Three (23.08%) adolescent patients were allergic to peppers (in 1 case explicitly spe cified as green peppers), 2 (15.38%) patients reported allergy to either egg, soy, oats, cow’s milk, walnuts, almonds, or rice. Allergy to corn, lupine, lentils, peas, beans, peaches, carrots, rye, barley, and gluten, in general, were reported only in 1 (7.69%) older patient each. An evaluation of patient histories revealed bronchial asthma in 45.5% (n = 15), allergic rhinoconjunctivitis in 42.4% (n = 14), food allergy in 27.3% (n = 9), and atopic dermatitis in 24.2% (n = 8). No additional allergic comorbidity was found in 24.4% of patients with EoE (n = 8).
Management
In the 33 patients with EoE analyzed, the most commonly used therapeutic option consisted of proton-pump inhibitors (PPI), which were used in 31 (93.9%) patients. PPI were followed by some type of elimination diet in 25 (75.8%) patients and then by corticosteroids (CS) admi nistration in 21 (63.6%) patients (Tab. 7). Patients usually received a combination of treatments and the most common was all three of the aforementioned modalities, which was seen in 14 (42.4%) patients. PPI was the only therapeutic option used without any of the others only in 3 (9.1%) patients. The rates of administering the various treatments are summarized in Tab. 7.
Tab. 7. Rates of administering various treatment combinations.
Tab. 7. Relativní výskyt různých kombinací léčby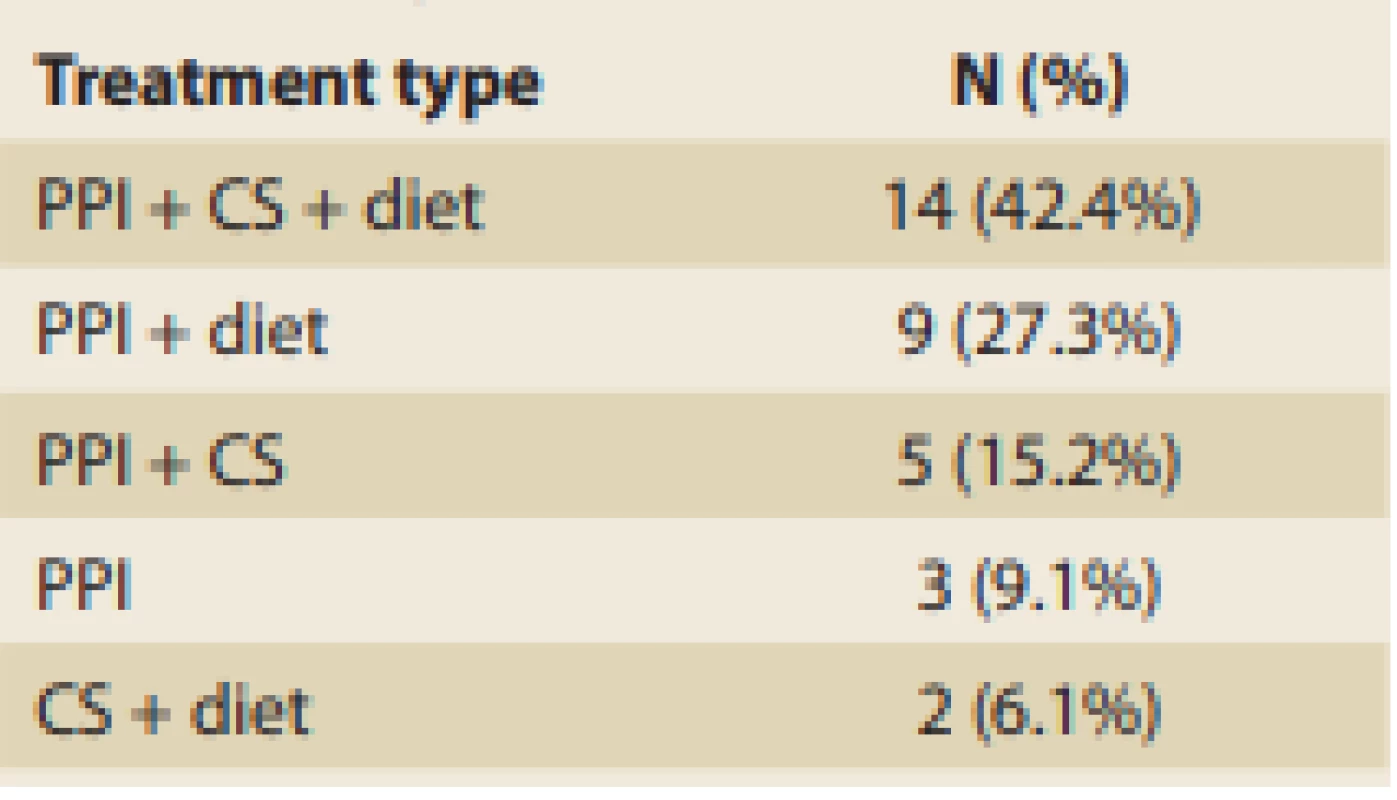
PPI were administered in 93.9% of EoE cases in this data set, and we may state that the vast majority of patients used PPI in an appropriate dosage (ave - rage dose 0.9 mg/kg/day, median 1 mg/kg/day, min. 0.2, max 1.5).
In the group of patients who were treated with topical CS more than half of patients (57.1%) got budesonide suspension with an appropriate median dose of 2 mg/day. A minority of patients was treated with swallowed fluticasone MDI with a median dose 200 µg/day (min. 100.0, max. 375.0). Only 3 patients were treated with systemic corticosteroids.
Food elimination was a very common treatment among our patients, with 25 (75.8%) eliminating at least one. The most commonly eliminated food consisted of nuts (by 17 patients).
Although the strict six-food diet (SFD) was observed by only 1 (4.00%) patient, another 4 (16.0%) patients adhered to the diet with the exception of one food from SFD (fish, wheat, or soy). The numbers of patients within our data set eliminating various foods are summarized in Fig. 3.
Fig. 3. Number of patients eliminating specific foods.
Obr. 3. Počet pacientů eliminujících určité potraviny.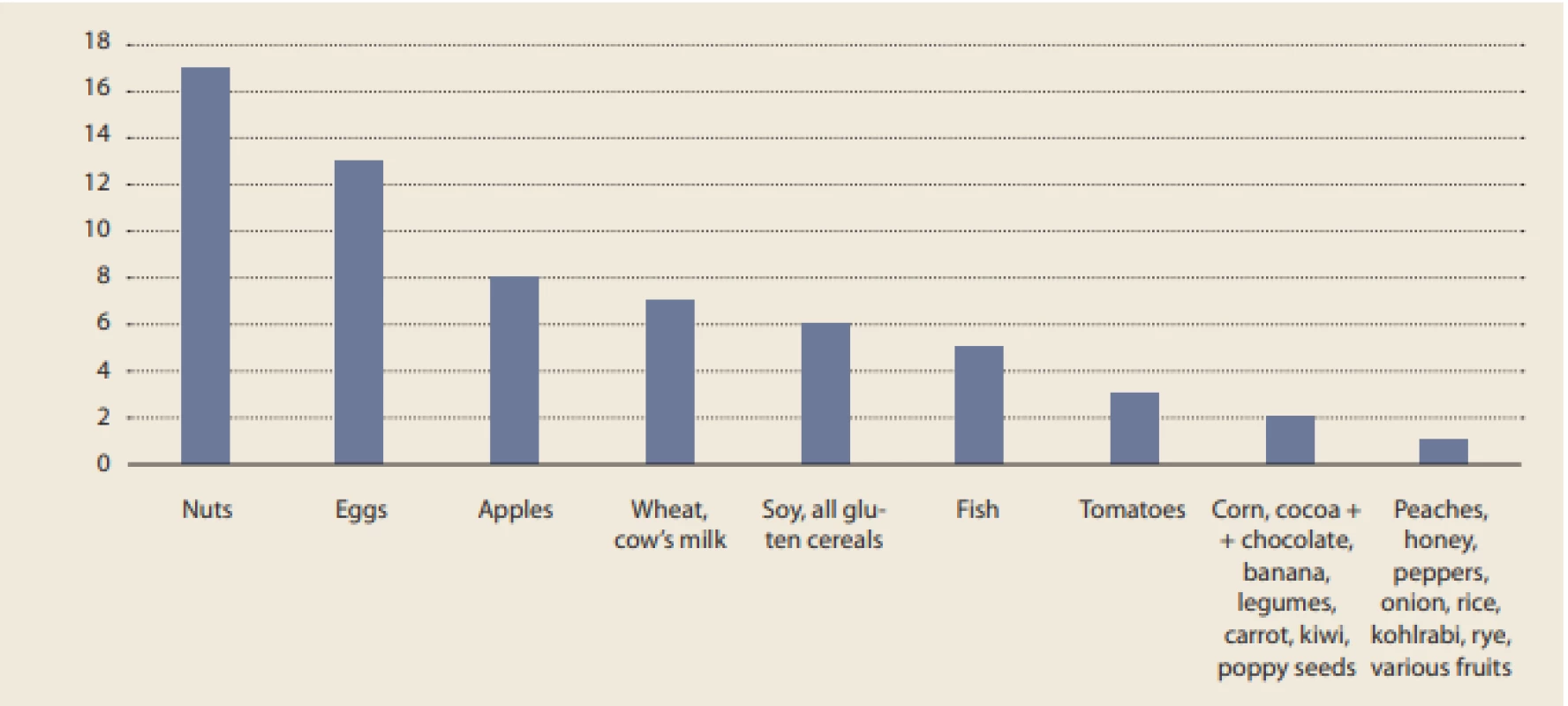
No esophageal dilation was needed, and only 2 of all patients manifes ted complications from treatment. A 13-year-old boy manifested mycotic esophagitis after budesonide suspension administration and an 8-year-old boy complained about itching throat and intermittent dysphagia after fluticasone treatment, which was impossible to differentiate from the manifestation of disease activity.
The rates of clinical, endoscopic, and histopathological remission achievement by the time of the control endoscopy were investigated, and those results are summarized in Tab. 8.
Tab. 8. Remission achievement.
Tab. 8. Dosažení remise.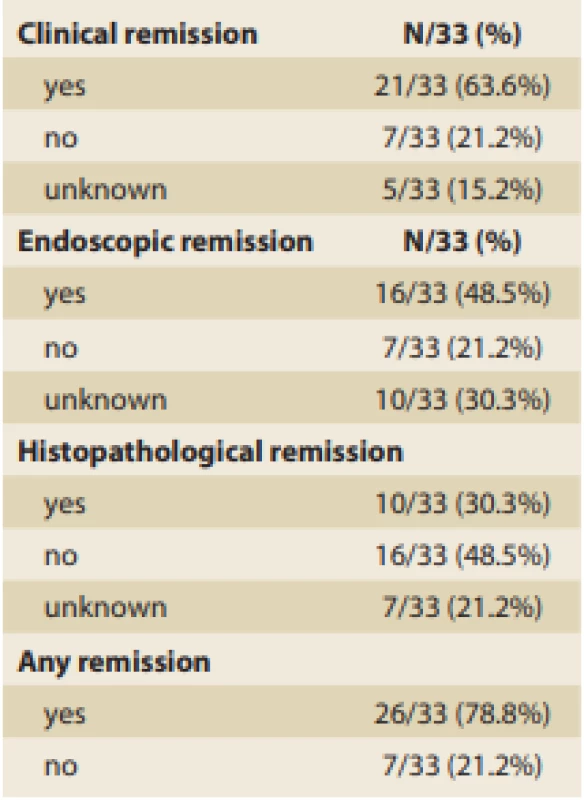
Discussion
In the presented study we found the basic demographic data to be consistent with that from similar published case series. We demonstrated the vast majority of patients to be male (81%) and the median age at onset to be 7 years. This only strengthens the reliability and reproducibility of our subsequent findings for the data set of patients representing the typical demography of an EoE population as reported in large cross-sectional surveys elsewhere [3].
The complex EoE immunopathogene sis constituting the background for histopathological changes resulting in various endoscopic features and clinical symptoms is summarized in Fig. 4.
Fig. 4. Pathophysiology of EoE. Environment, and especially during early childhood, has the dominant impact on the multifactorial development of EoE. The relevant factors are listed in the scheme above. Hereditary factors are generally considered to have a minor effect [4,5]. Nevertheless, genetic predispositions for EoE include mutations in the calpain-14ͺ gene and down-regulation of some additional genes whose products are involved in epithelial barrier function (desmoglein-1, filaggrin, involucrin, and others) against allergens from an external environment, and that barrier’s disruption is one route for sensitization [6] Whole exome sequencing has identifi ed numerous variants of the TSLP (thymic stromal lymphopoietin) gene, which is currently considered to be a major regulator of the TH-2 cellular response in terms of allergenic polarization after contact with allergens [7]. Moreover, the genetic predisposition is associated also with genes for eotaxin-3 in esophageal epithelial cells, which is probably a major atopyindependent genetic factor for EoE development [8]. After an allergen’s penetration of the esophageal mucosa, antigen-presenting cells present the processed antigens to T helpers type 2. Activation of the so-called TH-2 response is accompanied by release of numerous cytokines, of which interleukin-13 (IL), IL-4, and IL-5 are considered to be most important in development of the acute infl ammatory phase of EoE, which results in increased eotaxin 3 expression followed by local eosinophilic hyperfiltration. Esophageal tissue damage leads to additional disruptions of the superficial epithelium followed by lingering release of proinfl ammatory chemokines by activated eosinophils maintaining chronic infl ammatory infiltration and fibrous tissue production that results in irreversible esophageal strictures formation [9]. CCL 26 – chemokine (C-C motif) ligand ͼ, ECP – eosinophil cationic protein, EPX – eosinophil peroxidase, FGFͿ – fi broblast growth factor Ϳ, IL – interleukin, MBP – major basic protein, TGF-α and β – transforming growth factor α and β, TH-2 – T helper type 2, TSLP – thymic stromal lymphopoietin. Figure 4ͺ was prepared in accordance with Davis et al [Ϳ] and created in collaboration with the Service Center for E-Learning at Masaryk University, Faculty of Informatics (part of user support at IS MU, Faculty of Informatics, Masaryk University, Brno).
Obr. 4. Patofyziologie EoE. Environmentální vlivy, zejména pak v raném dětství, mají dominantní dopad na multifaktoriální vývoj EoE. Relevantní faktory jsou uvedeny v předkládaném schématu. Obecně se má za to, že dědičné faktory mají pouze malý vliv [4,5]. Mezi genetické predispozice pro EoE však patří mutace genu calpain-14 a down-regulace některých dalších genů, jejichž produkty se podílejí na funkci epiteliální bariéry (desmoglein-1, filaggrin, involucrin a další) proti alergenům z vnějšího prostředí a narušení této bariéry je jednou z cest senzibilizace [6.] Metodou celoexomového sekvenování byla identifi kována řada variant genu TSLP (thymic stromal lymphopoietin), který je v současné době považován za hlavní regulátor buněčné odpovědi TH-2 z hlediska alergenní polarizace po kontaktu s alergeny [7]. Genetická predispozice je navíc spojena také s geny pro eotaxin-3 v epiteliálních buňkách jícnu, což je pravděpodobně hlavní genetický faktor nezávislý na atopii pro vývoj EoE [8]. Po průniku alergenu do sliznice jícnu antigen prezentující buňky předkládají zpracované antigeny pomocným T lymfocytům typu 2. Aktivace takzvané TH-2 odpovědi je doprovázena uvolňováním mnoha cytokinů, z nichž jsou interleukin-13 (IL), IL-4 a IL-5 považovány za nejdůležitější ve vývoji akutní zánětlivé fáze EoE, která vede ke zvýšené expresi eotaxinu-3 následované lokální eozinofilní infiltrací. Poškození tkáně jícnu vede k dalšímu narušení povrchového epitelu, po kterém následuje přetrvávající uvolňování prozánětlivých chemokinů aktivovanými eozinofily, kdy dochází k chronické zánětlivé infiltraci a fibrotizaci tkáně, což vede k tvorbě ireverzibilních striktur jícnu [9]. CCL 26 – chemokinový ligand 26 (C-C motiv), ECP – eozinofilní kationický protein, EPX – eozinofilní peroxidáza, FGF9 – fibroblastový růstový faktor 9, IL – interleukin, MBP – hlavní bazický protein, TGF-α a β – transformující růstový faktor α a β, TH-2 – pomocný T lymfocyt typu 2, TSLP – thymický stromální lymfopoetin. Obr. 4 byl převzat a upraven dle Davis et al [9] a vytvořen ve spolupráci se Střediskem služeb pro e-learning Masarykovy univerzity (součást uživatelské podpory na IS MU, Fakulta informatiky Masarykovy univerzity, Brno).![Pathophysiology of EoE.
Environment, and especially during early childhood, has the dominant impact on the multifactorial development of EoE.
The relevant factors are listed in the scheme above. Hereditary factors are generally considered to have a minor effect [4,5].
Nevertheless, genetic predispositions for EoE include mutations in the calpain-14ͺ gene and down-regulation of some additional
genes whose products are involved in epithelial barrier function (desmoglein-1, filaggrin, involucrin, and others) against allergens
from an external environment, and that barrier’s disruption is one route for sensitization [6] Whole exome sequencing has
identifi ed numerous variants of the TSLP (thymic stromal lymphopoietin) gene, which is currently considered to be a major
regulator of the TH-2 cellular response in terms of allergenic polarization after contact with allergens [7]. Moreover, the genetic
predisposition is associated also with genes for eotaxin-3 in esophageal epithelial cells, which is probably a major atopyindependent genetic factor for EoE development [8]. After an allergen’s penetration of the esophageal mucosa, antigen-presenting cells present the processed antigens to T helpers type 2. Activation of the so-called TH-2 response is accompanied by
release of numerous cytokines, of which interleukin-13 (IL), IL-4, and IL-5 are considered to be most important in development
of the acute infl ammatory phase of EoE, which results in increased eotaxin 3 expression followed by local eosinophilic
hyperfiltration. Esophageal tissue damage leads to additional disruptions of the superficial epithelium followed by lingering
release of proinfl ammatory chemokines by activated eosinophils maintaining chronic infl ammatory infiltration and fibrous
tissue production that results in irreversible esophageal strictures formation [9].
CCL 26 – chemokine (C-C motif) ligand ͼ, ECP – eosinophil cationic protein, EPX – eosinophil peroxidase, FGFͿ – fi broblast
growth factor Ϳ, IL – interleukin, MBP – major basic protein, TGF-α and β – transforming growth factor α and β, TH-2 – T helper
type 2, TSLP – thymic stromal lymphopoietin.
Figure 4ͺ was prepared in accordance with Davis et al [Ϳ] and created in collaboration with the Service Center for E-Learning
at Masaryk University, Faculty of Informatics (part of user support at IS MU, Faculty of Informatics, Masaryk University, Brno).<br>
Obr. 4. Patofyziologie EoE.
Environmentální vlivy, zejména pak v raném dětství, mají dominantní dopad na multifaktoriální vývoj EoE. Relevantní faktory jsou
uvedeny v předkládaném schématu. Obecně se má za to, že dědičné faktory mají pouze malý vliv [4,5]. Mezi genetické predispozice
pro EoE však patří mutace genu calpain-14 a down-regulace některých dalších genů, jejichž produkty se podílejí na funkci epiteliální
bariéry (desmoglein-1, filaggrin, involucrin a další) proti alergenům z vnějšího prostředí a narušení této bariéry je jednou z cest
senzibilizace [6.] Metodou celoexomového sekvenování byla identifi kována řada variant genu TSLP (thymic stromal lymphopoietin),
který je v současné době považován za hlavní regulátor buněčné odpovědi TH-2 z hlediska alergenní polarizace po kontaktu
s alergeny [7]. Genetická predispozice je navíc spojena také s geny pro eotaxin-3 v epiteliálních buňkách jícnu, což je pravděpodobně
hlavní genetický faktor nezávislý na atopii pro vývoj EoE [8]. Po průniku alergenu do sliznice jícnu antigen prezentující buňky
předkládají zpracované antigeny pomocným T lymfocytům typu 2. Aktivace takzvané TH-2 odpovědi je doprovázena uvolňováním
mnoha cytokinů, z nichž jsou interleukin-13 (IL), IL-4 a IL-5 považovány za nejdůležitější ve vývoji akutní zánětlivé fáze EoE, která vede
ke zvýšené expresi eotaxinu-3 následované lokální eozinofilní infiltrací. Poškození tkáně jícnu vede k dalšímu narušení povrchového
epitelu, po kterém následuje přetrvávající uvolňování prozánětlivých chemokinů aktivovanými eozinofily, kdy dochází k chronické
zánětlivé infiltraci a fibrotizaci tkáně, což vede k tvorbě ireverzibilních striktur jícnu [9].
CCL 26 – chemokinový ligand 26 (C-C motiv), ECP – eozinofilní kationický protein, EPX – eozinofilní peroxidáza, FGF9 – fibroblastový
růstový faktor 9, IL – interleukin, MBP – hlavní bazický protein, TGF-α a β – transformující růstový faktor α a β, TH-2 – pomocný
T lymfocyt typu 2, TSLP – thymický stromální lymfopoetin.
Obr. 4 byl převzat a upraven dle Davis et al [9] a vytvořen ve spolupráci se Střediskem služeb pro e-learning Masarykovy univerzity
(součást uživatelské podpory na IS MU, Fakulta informatiky Masarykovy univerzity, Brno).](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/3c23ef6a78e5a3c38a92a927f003ce33.png)
Basically, immunopathological mecha - nisms involved in the pathogenesis of EoE are IgE-mediated and non-IgE-mediated reactions, the latter of which are more delayed at the onset. Immediate allergic reactivity is in connection with an immunoglobulin isotype switch to allergen-specific IgE formation, which is set as a result of a predominant allergenic type 2 T-helpers signalization (at the expense of tolerogenic signaling of type 1 T-helpers). This results in loss of immune tolerance and leads to a process of sensitization to food - and aeroallergens that occurs at various sites (e. g., respiratory tract, skin) [10].
EoE is often associated with atopy and food allergy, so nonspecific allergy mar kers were analyzed in this study (total serum IgE and relative eosinophilia) and these were just slightly higher in patients with EoE than are the reference intervals for pediatric patients. This is in concor dance with recently published data showing an absence of clinical effect of anti-IgE antibody (omalizumab) in patients with EoE and demonstrating that the non-IgE mechanism is the dominant immunopathological mechanism of EoE development [11–13]. Almost three--quarters of patients in the present study had been examined and more than half of those had proven sensitization to food allergens. These findings confirm a strong association between food allergy and EoE reported previously [14]. In the present study, we found that laboratory assessment of specific IgE levels is the predominant method for detecting sensitization to food allergens. The severity of symptoms cannot be predicted by either the specific IgE level or the degree of skin testing positivity, but it was proven previously that the probabi lity of symptom onset is directly related. Considering this fact, the authors consider it important to mention that skin prick-to-prick testing with native food triggers (i.e., cow’s milk, eggs, peanuts, and fish) has been shown in studies to have very high negative predictive value and thus it provides reliable, noninvasive, and relatively low-cost exclusion of IgE-mediated mechanisms in the background of EoE pathogenesis [15]. There were significant differences among proven food allergens in the different age groups. Cow’s milk, eggs, and peanuts were dominant food allergens of children up to 7 years of age. Above this age, tree nuts, wheat, apples, and peanuts rise in significance. These results are quite similar in comparison to those from other, previously published Western studies [16]. These observations are in agreement with the known phenomenon of oral tolerance and disappearance of allergic reactivity in relationship to some food allergens that is common at an early age and with rising relevance of other food allergens that often are homologous with some of the aeroallergens (i.e., Bet v1) [17]. More than one--quarter (27.3%) of the 33 analyzed EoE patients suffered from oral allergy syndrome (sometimes reported as a food-pollen syndrome), which is caused by cross-reactivity of an IgE-mediated immune reaction to specific aeroallergens having proteins similar to those in some fruits, vegetables, or tree-nuts. Large re - trospective surveys have proven a strong association of EoE with commonly seen atopic diseases (most frequently with allergic rhinoconjunctivitis) [3,18]. Furthermore, three-quarters of all patients in the present study showed some allergic comorbidity, with bronchial asthma and/or allergic rhinoconjunctivitis dia gnosed in almost half of them. It is noteworthy that statistical analysis of the remission achievement rate in relation to other allergic comorbidities in our 33 patients showed no impact of these comorbidities on the results of the treatment.
Allergic inflammation results in eso phageal dysfunction that is accompanied by clinical symptoms related to the age of the patients. Younger children usually manifest only nonspecific symptoms that have a considerable degree of overlap with symptoms of gastroesophageal reflux. Vomiting, food refusal, and failure to thrive are the most common symptoms of EoE in preschool children. Controversially, abdominal pain in this age group is particularly tricky to interpret. Parents of young children are usually referring about so-called “colics”, and preschool children may also complain of epigastric pain despite having only an esophageal disease with chest pain (odynophagia), dysphagia, or pyrosis (heartburn) [19]. When EoE is chronic, without adequate therapy, and usually progressing, the disease shows many and variable combinations, with symptoms changing through time during childhood. Earlier publications mention these clinical features of EoE as a pitfall of EoE dia gnostics, and especially in younger children
The youngest patients in this survey were two boys who both had manifested difficulty feeding, food refusal in combination with various symptoms of gastroesophageal reflux, and failure to thrive at 6 and 9 months of age. It took more than 5 years – and even almost 13 years in the second case – to achieve an appropriate endoscopic dia gnosis of EoE. Our results point to a trend of longer symptomatic time in children having their first symptoms at a younger age, as expected. The correlation between age of first symptoms and length of dia gnosis is statistically significant (Spearman’s rho = −0.42, p-value = 0.014), and it is obvious that EoE in young children is usually underdia gnosed.
Moreover, one-third of all of the patients experienced only one of the EoE symptoms, which then led to the decision to perform endoscopic evaluation and histopathological dia gnosis. This was unusual in the youngest group of patients, and among those children this occurred only once (in the case of a patient refusing food). The appearance of just one symptom before proceeding to bio psy was observed more often among older patients, and most frequently it was abdominal pain or food impaction that led directly to the dia gnosis without demonstrating any additional symptoms. This phenomenon also supports the hypothesis that EoE could be underdia gnosed in young children, as they usually must manifest multiple symptoms to get an appropriate dia gnosis. Furthermore, symptoms, such as food impaction, that seem able to lead to dia gnosis on their own occur not so frequently in young children, further complicating their reaching a dia gnosis of EoE. Nevertheless, the statistical test did not confirm any relationship at the 5% significance level between age at dia gnosis and number of symptoms. Older children with dysmotility of the eso phagus usually complain about concrete difficulties like dysphagia, odynophagia, or chest pain, as well as various gastro esophageal reflux symptoms. The relative manifestation rates of various symptoms among patients in the present study quite closely correspond to previously published data [20,21]. If these symptoms of esophageal dysmotility in older children are not dia gnosed precisely and on time and then adequately treated they may lead to irreversible remodeling of the esophageal wall and irreversible stricture formation with recurrent food impaction [19,22,23].
The mean duration of symptoms in patients involved in this study was 3.36 (SD 3.87) years and the median 2.25 years with a very wide range of symptomatic time to dia gnosis of 0–16.5 years.
Interestingly, the cases of girls with EoE in our study showed an even longer symptomatic period (mean 4.9 years with SD 3.8 and median 4.2 years with range 0–10). Unfortunately, in comparison with data published elsewhere, such long symptomatic intervals before appropriate dia gnosis in children are not unusual [22,24].
Endoscopic findings in the present pediatric EoE data set included both inflammatory (erythematous mucosa, linear furrows, and patchy white exudates) and fibrostenotic changes (crepe paper-like appearance of the mucosa, concentric ring-like esophageal changes or strictures). The most common endoscopic findings in the present pediatric patient data set were longitudinal furrows and white exudates, which is in concordance with previously published pediatric data from Western countries [22].
If EoE is not treated adequately and in a timely manner, the chronic inflammation in the esophageal wall develops complications associated with the remodeling process. The concentric rings and esophageal strictures can be seen among adult patients with EoE dominant macroscopic findings [2]. Also in our data set these macroscopic signs were seen with the highest median patient’s age among all findings. The results of retrospective studies have shown that the most important risk factor for esophageal remodeling and stricture formation is late establishment of the dia gnosis [25].
Detailed guidelines for the management of EoE in pediatric patients are regu - larly published by the Joint Task Force on Allergy-Immunology Practice Para meters, and the respected professional authorities the European Academy of Allergy and Clinical Immunology (EAACI) and European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) publish a joint communication covering current knowledge on EoE respecting the rules of evidence-based medicine [1,26]. Management of EoE in children aims for the resolution of clinical symptoms as well as endoscopic and histological remission. Based on individual evaluation as to the severity of clinical manifestations, the results of allergological examinations, and macroscopic findings, a decision among the various treatment modalities is made. Treatment options include PPI, elimination diets, and topical or systemic CS administration, as well as their combinations [27]. Systemic CS are reserved for severe cases where rapid relief of symptoms is required and other treatment has not proven successful. This conservative attitude regarding their use was appa rent also in our data set [28]. Magistraliter prescription of budesonide suspension and swallowing of fluticasone aerosol forms are off-label administrations of these drugs. Jorveza® is a new preparation of topical CS budesonide in an orodispersible tablet that is registered in the Czech Republic to treat adults with eosinophilic esophagitis. In a double-blind, placebo-controlled phase III clinical trial (EOS-1), Jorveza® showed both high safety and efficacy for induction and long-term treatment of EoE in adults. To date, there are no available data concerning Jorveza® administration in the pediatric population, but clinical trials are currently being conducted [29].
An elimination diet is considered as a first-line therapeutic modality for children and adults with EoE and, together with food challenges, these are irreplaceable in the dia gnostic approach to food allergies. EoE is a chronic disease by its nature, and gradually progressive esophageal wall remodeling is a long - -term process that allows too many patients to adapt to and thus underestimate the symptoms. From these clinical features of EoE there follows a major limitation upon the use of elimination diets, because to objectively measure the complex therapeutic effect of a dia gnostic diet necessitates repeated endoscopy assessment and esophageal bio psies after food challenges. The invasivity of these procedures constitutes the main limitation upon admi nistering individual therapeutic diets in childhood. Unfortunately, strong data suggest that the highest efficiency involves elemental diets (90.8%) in comparison with SFD (72.1%), the four-food diet (53.4%), and allergy skin test result--directed food elimination (45.5%) [30]. All these approaches are recommended for the treatment of EoE under the current guidelines [31]. Nevertheless, in order to achieve the highest possible adherence and maintain the best quality of life, it is appropriate to strive for as much targeted elimination in the diet as is possible, and this must be chosen individually for each patient. Time - and cost-effective procedures may point to recommending a diet eliminating a few of the most allergenic foods (i.e., the four-food diet). Then, in order to increase the effectiveness of the dia gnostic diet, the standard approach in the health care facilities of the present authors consists in conducting skin prick-to-prick testing with a huge set of the most allergenic foods, including certain oral allergy syndrome triggers and individually referred food suspects. Their positivity is then quantified by detection of IgE to specific components of allergens in order to increase reproducibility and clinical relevance of these results [7,32].
In comparison with a recent meta-analysis, our study showed a slightly lower rate of remission achievement. This may be due to a relatively high proportion of incomplete evaluations, occurring, among other reasons, because some patients did not return for ambulatory controls, a significant number of patients did not undergo control endoscopy and/or bio psy, and the availability of nutritional consulting varied by different health care facility. Moreover, our survey had quite strictly set terms for remission achievement (absence of all symptoms, disappearance of all macroscopic findings, and decrease of IEE strictly below the dia gnostic cutoff). Statistical tests were performed to investigate the relationships between certain treatments and rate of remission (clini cal, endoscopic, and histopathological) achievement. Categorical variables and remission achievement were tested using Fisher’s exact test and continuous variables using Mann-Whitney U-test. None of the tested variables (PPI use and its dosage, CS use and its dosage, food elimination, range of food elimi nation, combination of various treatments) proved to be statistically signi ficant. Whereas median IEE count in the control bio psies was still above the dia gnostic cutoff, the authors consider it appropriate to mention that local mucosal eosinophilia is not fully pathognomonic and so it may accompany various gastrointestinal diseases (i.e., inflammatory bowel disease) for which differential dia gnosis must be borne in mind.
The results of the present analysis are to a great extent consistent with the results of pediatric EoE experience previously published elsewhere, and we claim that the analyzed set of patients truly constitute a representative demographic sample of the regional EoE population.
Conclusion
This study’s comprehensive overview of the present regional management of pediatric EoE may increase pediatricians’ awareness about the characteristics of EoE and may lead to an increase in the frequency of endoscopic verifications and bio psies, especially in younger children often presenting with nonspecific symptoms for a long period. Early detection of EoE and initiation of effective treatment during the inflammatory stage are very important. The therapeutic elimination diet is a safe and reliable approach to the long-term effective management of pediatric patients with EoE. The results of this study are limited by its retrospective design, the relatively small number of patients, and the lack of standardization of the treatment protocols as ensues from the multicenter collection of the analyzed data. The authors would appreciate if this survey would contribute to the founding of a national register of patients with EoE in the Czech Republic.
Acknowledgement
Figure 4 was created in cooperation with Service Center for e-learning at MU (part of user sup port at IS MU, Faculty of Informatics, Masaryk University, Brno).
Submitted/Doručeno: 2. 10. 2020
Accepted/Přijato: 24. 11. 2020
Petr Jabandžiev, MD, PhD.
Department of Pediatrics
University Hospital Brno
Černopolní 9
613 00 Brno
Czech Republic
Zdroje
1. Lucendo AJ, Molina-Infante J, Arias Á et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for dia gnosis and management in children and adults. United European Gastroenterol J 2017; 5 (3): 335–358. doi: 10.1177/2050640616689 525.
2. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018; 154 (2): 319–332.e3. doi: 10.1053/j.gastro.2017.06.067.
3. Capucilli P, Cianferoni A, Grundmeier RW et al. Comparison of comorbid dia gnoses in children with and without eosinophilic esophagitis in a large population. Ann Allergy Asthma Immunol 2018; 121 (6): 711–716. doi: 10.1016/j.anai.2018.08.022.
4. Jensen ET, Hoffman K, Shaheen NJ et al. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol 2014; 109 (5): 668–675. doi: 10.1038/ajg.2014.47.
5. Jensen ET, Kappelman MD, Kim HP et al. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2013; 57 (1): 67–71. doi: 10.1097/MPG.0b013e318290d15a.
6. Kubo A, Nagao K, Amagai M. Epidermal bar rier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest 2012; 122 (2): 440–447. doi: 10.1172/JCI57416.
7. Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol 2010; 22 (6): 795–799. doi: 10.1016/j.coi.2010.10.020.
8. Aceves SS, Newbury RO, Chen D et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2010; 65 (1): 109–116. doi: 10.1111/j.1398-9995.2009.02142.x.
9. Davis BP, Rothenberg ME. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol 2016; 11 : 365–393. doi: 10.1146/annurev-pathol-012615-044241.
10. Akei HS, Brandt EB, Mishra A et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol 2006; 118 (1): 62–69. doi: 10.1016/j.jaci.2006.04.046.
11. Rocha R, Vitor AB, Trindade E et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr 2011; 170 (11): 1471–1474. doi: 10.1007/s00431-011 - 1540-4.
12. Clayton F, Fang JC, Gleich GJ et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014; 147 (3): 602–609. doi: 10.1053/j.gastro.2014.05.036.
13. Gonsalves N, Yang GY, Doerfler B et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142 (7): 1451–1459.e1. doi: 10.1053/j.gastro.2012.03.001.
14. Hong S, Vogel NM. Food allergy and eosinophilic esophagitis: learning what to avoid. Cleve Clin J Med 2010; 77 (1): 51–59. doi: 10.3949/ccjm.77a.09018.
15. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001; 107 (5): 891–896. doi: 10.1067/mai.2001.114708.
16. Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE-mediated food allergy. J Allergy Clin Immunol Pract 2017; 5 (2): 369–375. doi: 10.1016/j.jaip.2016.11.020.
17. van Rhijn BD, van Ree R, Versteeg SA et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy 2013; 68 (11): 1475–1481. doi: 10.1111/all.12257.
18. Dauer EH, Freese DK, El‐Youssef M et al. Clinical characteristics of eosinophilic esophagitis in children. Ann Otol Rhinol Laryngol 2005; 114 (11): 827–833. doi: 10.1177/000348940511401103.
19. Liacouras CA, Spergel J, Gober LM. Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am 2014; 43 (2): 219–229. doi: 10.1016/j.gtc.2014.02.012.
20. Spergel JM, Brown-Whitehorn TF, Beausoleil JL et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009; 48 (1): 30–36. doi: 10.1097/MPG.0b013e3181788282.
21. Ristic N, Jankovic R, Dragutinovic N et. al. Dia gnosis of eosinophilic esophagitis in children: a serbian single-center experience from 2010 to 2017. Med Princ Pract 2019; 28 (5): 449–456. doi: 10.1159/000499657.
22. Eroglu Y, Lu H, Terry A et al. Pediatric eosinophilic esophagitis: single-center experience in northwestern USA. Pediatr Int 2009; 51 (5): 612–616. doi: 10.1111/j.1442-200X.2008.02796.
23. Gonsalves N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: is this the same disease? Dig Dis 2014; 32 (12): 89–92. doi: 10.1159/000357078.
24. Roberts AJ, Day AS, Sinclair J et al. Paediatric eosinophilic oesophagitis in New Zealand: a 3-year prospective study. J Paediatr Child Health 2020. In press. doi: 10.1111/jpc.15183.
25. Lipka S, Kumar A, Richter JE. Impact of dia gnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 2016; 50 (2): 134–140. doi: 10.1097/MCG.0000000000000297.
26. Hirano I, Chan ES, Rank MA et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Ann Allergy Asthma Immunol 2020; 124 (5): 416–423. doi: 10.1016/j.anai.2020.03.020.
27. Mikoviny Kajzrlíková I, Vítek P. Eozinofilní ezofagitida – současný pohled na dia gnostiku a léčbu. Gastroent Hepatol 2020; 74 (3): 228–232. doi: 10.14735/amgh2020228.
28. Papadopoulou A, Dias JA. Eosinophilic esophagitis: an emerging disease in childhood – review of dia gnostic and management strategies. Front Pediatr 2014; 2 : 129. doi: 10.3389/fped.2014.00129.
29. Mikoviny Kajzrlíkova I. Jorveza® – očekávaný preparát k léčbě eozinofilní ezofagitidy. Gastroent Hepatol 2020; 74 (4): 357–359. doi: 10.14735/amgh2020357.
30. Arias A, González-Cervera J, Tenias JM et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014; 146 (7): 1639–1648. doi: 10.1053/j.gastro.2014.02.006.
31. Hirano I, Furuta GT. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis, Gastroenterology 2020; 158 (4): 840–851. doi: 10.1053/ j.gastro.2019.09.052.
32. Molina-Infante J, Arias A, Barrio J et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol 2014; 134 (5): 1093–1099.e1. doi: 10.1016/j.jaci.2014.07.023.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek GNET tenkého střevaČlánek Recenzia knihy
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2020 Číslo 6- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Dětská gastroenterologie a hepatologie
- Eozinofilní ezofagitida – 10 let zkušeností pěti českých pediatrických endoskopických center
- Trichohepatoenterický syndrom u pacienta s mutacemi genu TTC37 – kazuistika
- Syndrom arteria mesenterica superior v souvislosti s Crohnovou chorobou – kazuistika
- Eozinofilní enteritida – kazuistika vzácné manifestace onemocnění a stručný přehled novinek
- Společné stanovisko odborných společností k farmakologické léčbě obezity
- Adjustabilní intragastrické balóny v bariatrii – větší zastoupení respondentů ve srovnání s užitím neadjustabilních intragastrických balónů
- Zobrazovací metody u neúrazových náhlých příhod břišních
- Vliv synbiotika ColonFit na symptomy pacientů se syndromem dráždivého tračníku, funkční zácpou a funkčním průjmem
- GNET tenkého střeva
- Inhibitory protonové pumpy – známe je dobře? Jsou skutečně tak bezpečné? – část 2
- Biosimilární adalimumab FKB-327 v léčbě idiopatických střevních zánětů
- Subkutánně podávaný vedolizumab pro ulcerózní kolitidu a Crohnovu chorobu v klinickém programu VISIBLE
- Výběr z mezinárodních časopisů
- Jaký je význam testování na covid-19 před endoskopickým vyšetřením?
- Recenzia knihy
- MUDr. Radoslav Pruška zemřel 10. listopadu 2020
- Dosáhne gastroentero-hepatologický výzkum v Čechách na excelenci?
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zobrazovací metody u neúrazových náhlých příhod břišních
- Syndrom arteria mesenterica superior v souvislosti s Crohnovou chorobou – kazuistika
- Eozinofilní ezofagitida – 10 let zkušeností pěti českých pediatrických endoskopických center
- Eozinofilní enteritida – kazuistika vzácné manifestace onemocnění a stručný přehled novinek
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



