-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
N-terminal pro-brain natriuretic peptide levels associated with severe hand, foot and mouth disease
Background:
Severe hand, foot, and mouth disease (HFMD) is sometimes associated with serious complications such as acute heart failure that can cause substantial child mortality. N-terminal pro-brain natriuretic peptide (NT-proBNP) is a sensitive and specific biomarker of congestive heart failure. The aim of this study was to use plasma NT-proBNP levels to establish the severity of childhood HFMD.Methods:
A retrospective study was performed in 128 Chinese patients with severe HFMD and 88 patients with mild HFMD treated between January 2014 and October 2015. Univariate and multiple logistic regression analyses were used to analyze the risk factors for severe HFMD. NT-proBNP levels were analyzed in 128 severe HFMD patients, and the predictive value of NT-proBNP was assessed by receiver operating characteristic analyses.Results:
Multivariate analysis controlling for several potential confounders showed that enterovirus 71 infection [odds ratio (OR) 19.944, 95 % confidence interval (CI) 6.492–61.271], peripheral WBC count (OR 3.428, 95 % CI 1.186–9.914), fasting glucose (OR 19.428, 95 % CI 2.236–168.784), procalcitonin (OR 9.084, 95 % CI 3.462–23.837, and NT-proBNP (>125 pg/mL) (OR 16.649, 95 % CI 4.731–58.585) were each associated with the severity of HFMD. The 45 dead severe patients had higher pre-procedural levels of NT-proBNP than the 83 cured severe patients (12776 ± 13115 versus 1435 ± 4201 pg/mL, P < 0.001). An NT-proBNP cutoff value of 982 pg/mL predicted mortality with 87 % sensitivity and 86 % specificity.Conclusion:
Plasma NT-pro-BNP level appears to be a useful biological marker for predicting the severity and mortality of HFMD.Keywords:
Hand, foot, and mouth disease, N-terminal pro-brain natriuretic peptide, Disease severity, Mortality
Authors: Hui-Ling Deng 1,2; Yu-Feng Zhang 2; Ya-Ping Li 1; Yu Zhang 2; Yan Xie 2; Jun Wang 2; Xiao-Yan Wang 2; Shuang-Suo Dang 1*
Authors place of work: Department of Infectious Diseases, Second Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an 710004, China. 1; Department of Infectious Diseases, Xi’an Children’s Hospital, Xi’an 710003, China. 2
Published in the journal: BMC Infectious diseases 2016, 16:585
Category: Research article
doi: https://doi.org/10.1186/s12879-016-1929-9© 2016 The Author(s).
Open access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The electronic version of this article is the complete one and can be found online at: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1929-9Summary
Background:
Severe hand, foot, and mouth disease (HFMD) is sometimes associated with serious complications such as acute heart failure that can cause substantial child mortality. N-terminal pro-brain natriuretic peptide (NT-proBNP) is a sensitive and specific biomarker of congestive heart failure. The aim of this study was to use plasma NT-proBNP levels to establish the severity of childhood HFMD.Methods:
A retrospective study was performed in 128 Chinese patients with severe HFMD and 88 patients with mild HFMD treated between January 2014 and October 2015. Univariate and multiple logistic regression analyses were used to analyze the risk factors for severe HFMD. NT-proBNP levels were analyzed in 128 severe HFMD patients, and the predictive value of NT-proBNP was assessed by receiver operating characteristic analyses.Results:
Multivariate analysis controlling for several potential confounders showed that enterovirus 71 infection [odds ratio (OR) 19.944, 95 % confidence interval (CI) 6.492–61.271], peripheral WBC count (OR 3.428, 95 % CI 1.186–9.914), fasting glucose (OR 19.428, 95 % CI 2.236–168.784), procalcitonin (OR 9.084, 95 % CI 3.462–23.837, and NT-proBNP (>125 pg/mL) (OR 16.649, 95 % CI 4.731–58.585) were each associated with the severity of HFMD. The 45 dead severe patients had higher pre-procedural levels of NT-proBNP than the 83 cured severe patients (12776 ± 13115 versus 1435 ± 4201 pg/mL, P < 0.001). An NT-proBNP cutoff value of 982 pg/mL predicted mortality with 87 % sensitivity and 86 % specificity.Conclusion:
Plasma NT-pro-BNP level appears to be a useful biological marker for predicting the severity and mortality of HFMD.Keywords:
Hand, foot, and mouth disease, N-terminal pro-brain natriuretic peptide, Disease severity, MortalityWhat is Known
• Severe HFMD cases and over 90 % of fatal cases were caused by EV71. Acute heart failure is one of the most common causes of death in severe HFMD. N-terminal pro-brain natriuretic peptide (NT-proBNP) are useful biomarkers for the assessment of congestive heart failure.
What is New
• EV71 infection, fasting glucose, procalcitonin (PCT) and NT-proBNP levels were each associated with the severity of HFMD. Plasma NT-pro-BNP level appears to be a useful biological marker for predicting the severity and mortality of HFMD.
Background
Severe hand, foot and mouth disease (HFMD) associated with enterovirus (EV) 71 in children can result in high morbidity and mortality [1, 2, 3]. Most children with HFMD have mild symptoms including fever and cutaneous lesions on their hands, feet and buttocks, along with oral lesions [4, 5]. However, in rare cases, patients may also develop severe and life-threatening complications such as encephalitis, acute pulmonary edema, and cardiopulmonary failure [2, 6]. Consequently, acute heart and pulmonary failure is proposed as an important cause of rapid deterioration of HFMD, leading to mortality [3, 7].
Since 2008, nationwide epidemics of EV71 have occurred [8, 9, 10] in China, affecting 4.5 million children, and >3500 have died from the disease due to neurogenic cardiopulmonary failure and brainstem encephalitis, according to the Chinese Center for Disease Control and Prevention (http://www.chinacdc.cn/). These outbreaks of HFMD and high mortality represent a significant public health problem in China. However, there is still no available vaccine against EV71 and other enteroviruses that cause HFMD [11, 12,13]. Current management in the clinic is only to relieve symptoms. Actually, it is important and arduous to predict whether patients will develop severe or life-threatening illness at their first clinical visit. Severe HFMD progresses quickly and has high mortality. Feasible prognosis prediction of severe HFMD would aid clinical decisions and management.
The 108-amino-acid (aa) pro-hormone brain-type natriuretic peptide (BNP) was first isolated from porcine brains in 1988 [14, 15]. BNP is a cardiac neuro-hormone secreted from ventricular myocytes in response to increased intraventricular pressure [15, 16, 17]. Once BNP has been segregated, it divides into a biologically active 32-aa BNP and inactive 76-aa NT-proBNP [18, 19]. Excessive volume or pressure loading of the heart can lead to increased BNP synthesis and secretion. In clinical applications, BNP levels are related to cardiac function and are thought to be a sensitive and specific biomarker of congestive heart failure [16, 19, 20]. Some studies have established that BNP is a risk marker in patients with HIV infection [21], severe sepsis [18], acute Kawasaki disease [22], and acute Puumala hantavirus infection [23]. A recent HFMD investigation has demonstrated that high levels of NT-proBNP are associated with the complication of cardiopulmonary collapse [24].
In this study, we explored the hypothesis that higher levels of NT-proBNP might be associated with severe HFMD.
Methods
Patients
This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of the Medical College of Xi’an Jiaotong University and Xi’an Children’s Hospital, Xi’an, China. A total of 2320 HFMD patients were collected from all cases admitted to the two hospitals between January 2014 and October 2015. We retrospectively enrolled 216 patients whose clinical data were elaborate, comprising 128 Chinese patients with severe HFMD and 88 patients with mild HFMD in this case–control study. Among the 128 patients with severe HFMD, 45 died. Children with severe HFMD were treated as cases, while those with mild disease served as controls and were used as the reference group when calculating odds ratios (ORs).
The definition of HFMD was based on the criteria in the Hand, Foot and Mouth Disease Clinical Guide (2010 edition) [25], which contains the following criteria: (1) symptoms and signs occur during epidemics in preschool children; and (2) patients show typical exanthema on the hands, feet, mouth and/or buttocks, with or without fever. Patients with mild HFMD had rashes on their hands, feet, mouths, and buttocks, with or without fever. Patients with severe disease may have one of the followings: (1) neurological manifestations; (2) respiratory manifestations; and (3) circulatory manifestations. The diagnosis of fatal HFMD was based on one of the following criteria: (1) frequent convulsions, coma and cerebral hernia; (2) dyspnea, cyanosis, bloody frothy sputum, and pulmonary rales; and (3) shock and circulatory failure. Other concomitant viruses positive for IgM were ruled out in this study.
Pan-enterovirus RT-PCR assay for identification of EV71 infection
EV71 infection in patients with clinically suspected HFMD was confirmed by positive EV71 identification using pan-enterovirus real-time RT-PCR. Viral RNA extraction from throat swabs was performed using an RNA extraction kit (Qiagen, Germany). Real-time RT-PCR for enterovirus was performed with the primers 5′-TCCTCCGGCCCCTGAATG -3′ and 5′-AATTGTCACCATAAGCAGCCA-3′. This triplex RT-PCR reaction was performed in a 25 μL total reaction mixture containing 17 μL buffer, 1.5 μL primer and probe mix, 1.5 μL enzyme mix, and 5 μL extracted RNA template. On a CFX96 Real-Time PCR Detection System (Bio-Rad Inc., USA) 30 min reverse transcription at 50 °C and 10 min denaturation at 95 °C, followed by 5 circles of pro-amplification at 94 °C for 10 s, 50 °C for 30 s, 72 °C for 30 s, and 40 cycles at 94 °C for 10 s, and 58 °C for 30 s were performed to collect fluorescence signal.
Plasma NT-proBNP measurement
A rapid, comer cially available NT-proBNP immunoassay (Cobas, Germany) was used for the NT-proBNP measurement. Blood samples were collected by venipuncture into standard EDTA tubes and centrifuged within 30 min of collection. Plasma NT-proBNP was measured using a commercially available horse-radish peroxidase, and colorimetric end-point assay was carried out for the quantitative determination of feline NT-proBNP.
Data collection
The authors (Deng HL and Zhang YF) used standardized forms to extract data independently from medical records. Detailed demographic data including sex and age were collected from medical records. Clinical manifestations including highest fever temperature, vomiting, limb weakness, hypersomnia, convulsion, high blood pressure, consciousness disorder, dysfunction of respiratory rhythm, and circulatory disturbance were collected and 128 severe cases were identified. The laboratory data collected included enterovirus infection, white blood cell (WBC) count, fasting glucose, plasma NT-proBNP levels, myocardial enzyme spectrum [creatine kinase (CK), CK-MB, lactate dehydrogenase (LDH)] and procalcitonin (PCT).
Statistical analysis
All statistical analyses were performed using SPSS version 13.0 (IBM, Chicago, IL, USA). Data for continuous variables were summarized as mean ± SD or median (range), and inter-group differences were assessed for significance using the Wilcoxon rank-sum test or Student’s t test. Data for categorical variables were summarized as numbers and percentages, and the χ2 test was used to assess differences between patients with mild or severe HFMD. Univariate and multivariate logistic regression analyses were used to identify risk factors associated with severe HFMD using ORs. All variables with a univariate P < 0.20 along with those deemed to be clinically significant were considered for inclusion in multivariate models. Receiver operating characteristic (ROC) method was used to define the optimal cut-off values of baseline NT-proBNP. The threshold of significance for all statistical tests was defined to be P < 0.05.
Results
Patient characteristics and clinical manifestations
There were 216 patients diagnosed with HFMD including 128 severe cases, 45 of whom died (20.8 %). Table 1 shows the clinical manifestations of the 216 children with HFMD who were eligible for enrollment. There were significant differences between mild and severe cases in terms of fever, peak temperature, duration of fever, symptoms of central nervous and cardiopulmonary systems, WBC count (>15 × 109/L), fasting glucose (>8.3 mmol/L), current EV71 infection, CK (>229 U/L), CK-MB (>25 U/L) and LDH (>450 U/L), PCT (>0.1 ng/mL) and plasma NT-proBNP (log10 pg/mL) (2.18 ± 0.35 vs 3.07 ± 0.75, P < 0.001) levels. Pan-enterovirus real-time RT-PCR was performed to confirm the cases caused by EV71 infection. Additional file 1: Table S1 shows the clinical characteristics of HFMD caused by EV71. The proportion of severe cases among children with HFMD caused by the EV71 infection increased significantly than non-EV71 infection (P < 0.05). The plasma NT-proBNP (log10 pg/mL) levels in the EV71 infection cases were significantly higher than non-EV71 infection (3.04 ± 0.88 vs 2.49 ± 0.58, P < 0.001).
Tab. 1. Risk factors for severe HFMD 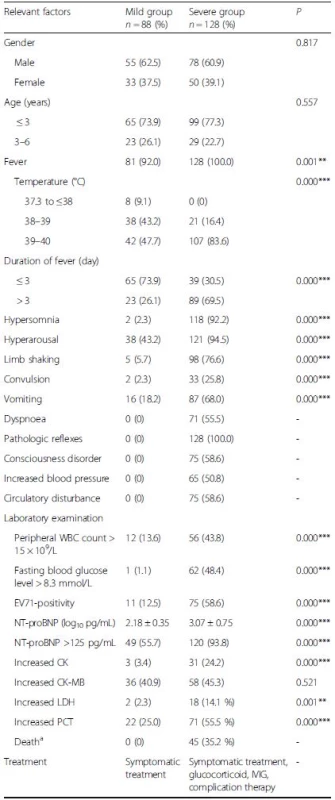
HFMD hand, foot, and mouth disease, EV71 enterovirus 71, WBC White blood cell, NT-proBNP N-terminal of the prohormone brain natriuretic peptide, CK Creatine kinase isoenzyme, CK-MB Creatine kinase isoenzymeMB, LDH Lactate dehydrogenase, PCT procalcitonin, IVIG Intravenous immunoglobulins aCauses of death were acute pulmonary edema, brainstem encephalitis and circulatory failure *P <0.05, **P <0.01,***P <0.001 Risk factors for severe disease
Risk factors for severe HFMD are summarized in Table 2. In the univariate analysis, current EV71 infection [(OR 9.906, 95 % confidence interval (CI) 4.807–20.413], WBC count (OR 4.926, 95 % CI 2.442–9.938), fasting glucose (OR 81.727, 95 % CI 11.045–604.760), PCT (OR 3.737, 95 % CI 2.061–6.777) and NT-proBNP levels (>125 pg/mL) (OR 11.939, 95 % CI 5.205–27.383) were risk factors for severe HFMD. In the multivariate model, EV71 infection (OR 19.944, 95 % CI 6.492–61.271), WBC count (OR 3.428, 95 % CI 1.186–9.914), fasting glucose (OR 19.428, 95 % CI 2.236–168.784), PCT (OR 9.084, 95 % CI 3.462–23.837) and NT-proBNP (>125 pg/mL) (OR 16.649, 95 % CI 4.731–58.585) were each associated with the severity of HFMD.
Tab. 2. ORs for severe HFMD 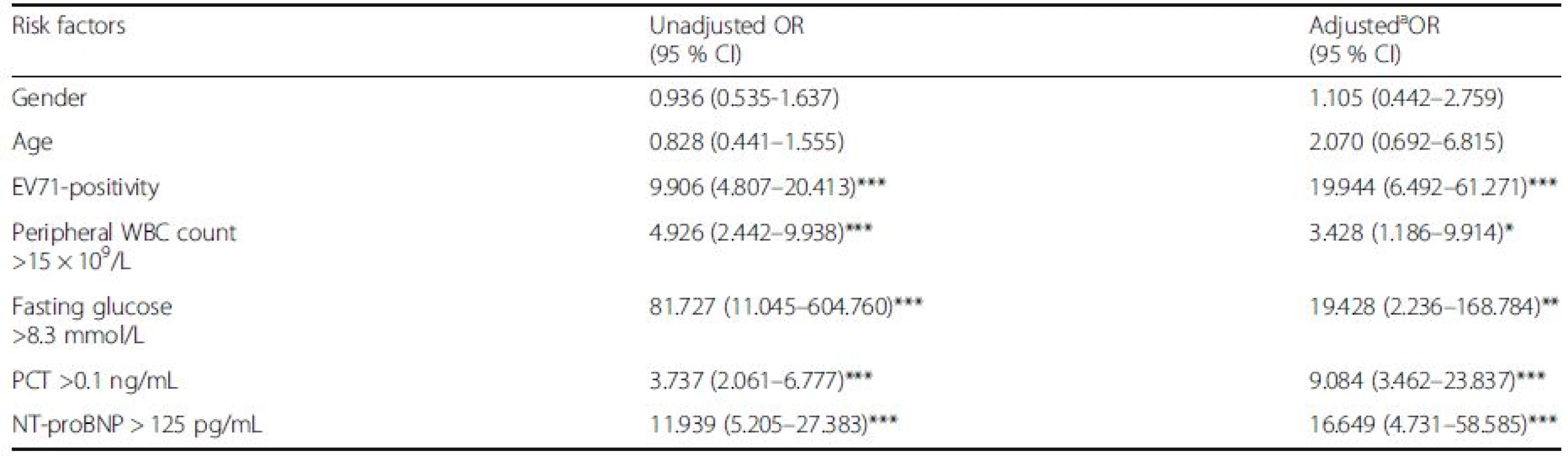
HFMD hand, foot, and mouth disease, EV71 enterovirus 71, PCT procalcitonin, NT-proBNP N-terminal of the prohormone brain natriuretic peptide CI Confidence interval, OR odds ratio. OR was calculated using the mild children as a reference group aIn multivariate logistic regression model (n = 216), we controlled for age, gender, EV71-seropositivity, WBC, fasting glucose, CK, LDH, PCT and NT-proBNP. After adjusting for potential confounding factors, there was significant difference in EV71 infection, peripheral WBC count, fasting blood glucose, PCT and plasma NT-proBNP levels *P <0.05, **P <0.01, ***P <0.001 NT-proBNP levels in severe HFMD
Children with severe HFMD were divided into two groups based on cure or death. Table 3 shows that hypersomnia, convulsion, peripheral WBC count, fasting blood glucose level, NT-proBNP levels, CK and LDH were associated with mortality in children with severe HFMD (P < 0.05). The ROC curve and interactive dot diagram for calculating the optimal cut-off value of NT-proBNP in predicting mortality is shown in Fig. 1. At an NT-proBNP cut-off value of >982.45 pg/mL, the sensitivity and specificity were 86.7 and 85.5 %, respectively.
Tab. 3. Risk factors associated with death of children with severe HFMD 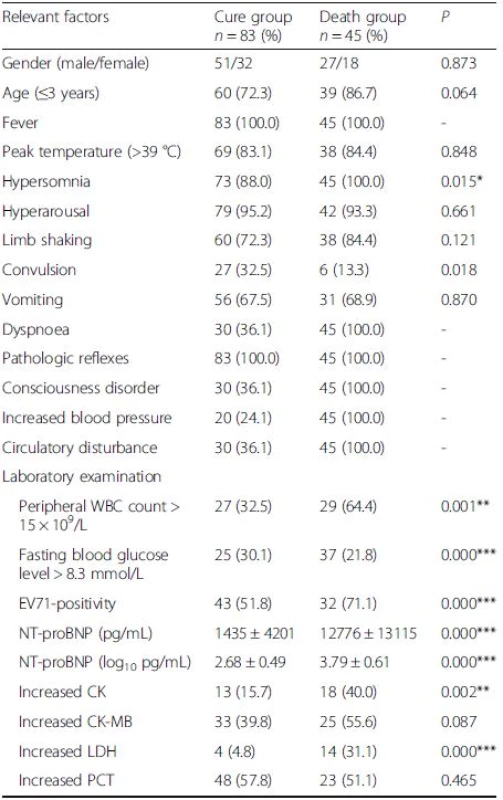
HFMD hand, foot, and mouth disease, EV71 enterovirus 71, WBC White blood cell, NT-proBNP N-terminal of the prohormone brain natriuretic peptide, CK Creatine kinase isoenzyme, CK-MB Creatine kinase isoenzyme-MB, LDH Lactate dehydrogenase, PCT procalcitonin, IVIG Intravenous immunoglobulins *P <0.05, **P <0.01,***P <0.001 Fig. 1. ROC curve and interactive dot diagram for calculating optimal cut-off value of NT-proBNP in predicting mortality 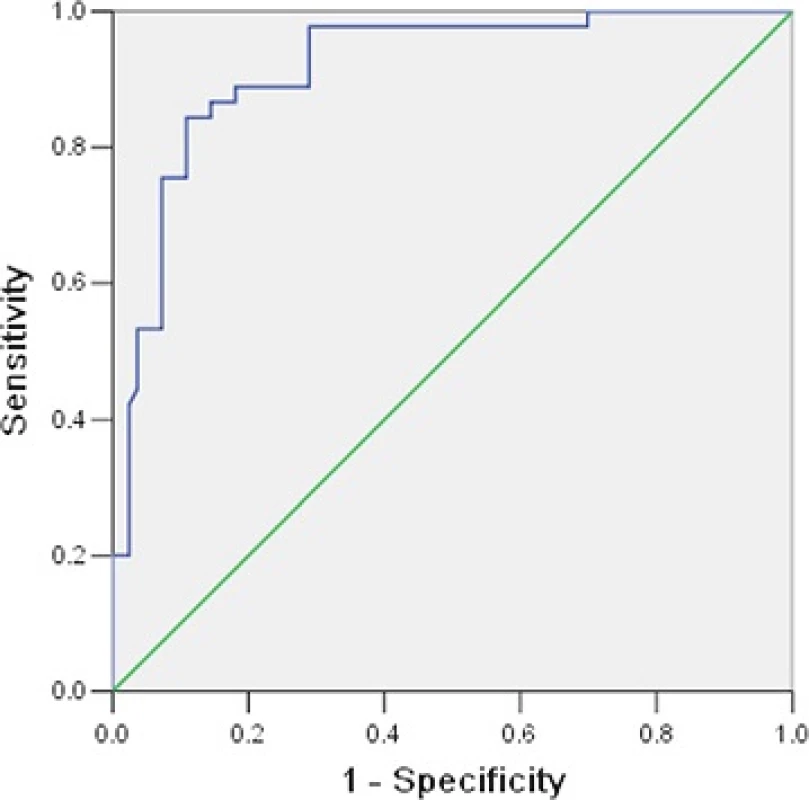
Discussion
We found that current EV71 infection, peripheral WBC count, fasting glucose, PCT and NT-proBNP levels (>125 pg/mL) were each associated with severity of HFMD. Consequently, ROC curve analysis revealed that an NT-proBNP value of ≥982.45 pg/mL had a sensitivity of 86.7 % and specificity of 85.5 % in predicting death in patients with severe HFMD. Therefore, early identification of these risk factors and timely and effective intervention are important in controlling mortality of severe HFMD.
Serum BNP is mainly synthesized and secreted by ventricular myocytes, and increased intraventricular pressure stress could modulate synthesis of BNP [15, 16, 17]. In addition to hemodynamic stress, inflammation of the myocardial tissue may also induce the production of BNP [22, 26]. As a clinically valuable biomarker, NT-proBNP can fulfill most of these criteria in patients with heart failure and ventricular overload [27]. In the cardiology literature, NT-proBNP had emerged as an independent and crucial warning factor of clinical outcome in patients with heart failure [19, 20]. We speculated that fatal HFMD involves brainstem and autonomic nerve dysfunction, leading to a “sympathetic storm” and significantly increased catecholamine concentration in the blood [28]. This may lead to increased blood flow to the heart and ventricular preload, resulting in increased NT-proBNP secretion and release. Previous studies have reported that NT-proBNP levels are significantly increased in severe HFMD, with cardiopulmonary collapse [24] or EV71 infection [16], which is consistent with our findings that children with severe HFMD, and those who have died, had an increase in the levels of NT-proBNP.
EV71 is a neurotropic virus that can cause severe complications involving the central neurogenic pulmonary edema, aseptic meningitis, brainstem encephalitis, and cardiopulmonary failure [6]. Many clinical studies have shown that the symptoms of HFMD caused by EV71 are more severe than those caused by other enteroviruses [29, 30]. In this study, we found that EV71 infection was associated with severe HFMD, which is consistent with previous studies. Peripheral WBC count, increased fasting glucose and PCT levels were other risk factors identified in our study, which is also consistent with other studies. PCT is a helpful biomarker for early diagnosis of inflammatory reactions, and high plasma PCT concentrations were associated with severe fatal HFMD. Increased fasting glucose was a significant risk factor in fatal cases, which is consistent with previous studies. The specific mechanism of increased fasting glucose is still unclear.
This study had some limitations. The first was that the cases of HFMD in this study were inpatients. We excluded outpatients because of incomplete medical records, and this may have introduced selection bias. The second was the small sample size in our study, and this may have led to negative results for some analyzed factors. Further studies with larger numbers of cases are required to confirm these results.
Conclusions
EV71 infection, peripheral WBC count, fasting glucose, PCT and NT-proBNP levels (>125 pg/mL) were each associated with the severity of HFMD. Plasma NT-pro-BNP level appears to be a useful biological marker for predicting the severity and mortality of HFMD. Further studies are needed to confirm our findings.
Abbreviations
BNP: Brain-type natriuretic peptide; CI: Confidence interval; CK: Creatine kinase; CK-MB: Creatine kinase isoenzyme-MB; EV71: Enterovirus 71; HFMD: Hand foot, and mouth disease; LDH: Lactate dehydrogenase; NTproBNP: N-terminal of the prohormone brain natriuretic peptide; OR: Odds ratio; PCT: Procalcitonin; WBC: White blood cell
Acknowledgments
We thank the doctors of the Department of Infectious Diseases of Xi’an Children’s Hospital and Second Affiliated Hospital of Medical College of Xi’an Jiaotong University for their help in data collection.
Funding
This work was not supported by the national funding.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its Additional File).
Authors’ contributions
Deng HL conceptualized and designed the study, carried out the initial analyses, collected the clinical data, drafted the manuscript; Zhang YF collected the clinical data and statistics; and all authors have approved the final manuscript to be published.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of the Medical College of Xi’an Jiaotong University and Xi’an Children’s Hospital, Xi’an, China.
Additional files
Additional file 1: Table S1. The characteristics of HFMD causing by EV71. (DOC 57 kb)
Received: 25 March 2016
Accepted: 13 October 2016
Published: 19 October 2016* Correspondence:
Shuang-Suo Dang
Department of Infectious Diseases,
Second Affiliated Hospital of Medical College of Xi’an Jiaotong University,
Xi’an 710004, Chinadang212@126.com
Zdroje
1. Choi CS, Choi YJ, Choi UY, Han JW, Jeong DC, Kim HH, et al. Clinical manifestations of CNS infections caused by enterovirus type 71. Korean J Pediatr. 2011;54 : 11–6. doi:10.3345/kjp.2011.54.1.11.
2. Lee MS, Lin TY, Chiang PS, Li WC, Luo ST, Tsao KC, et al. An investigation of epidemic enterovirus 71 infection in Taiwan, 2008: clinical, virologic, and serologic features. Pediatr Infect Dis J. 2010;29 : 1030–4. doi:10.1097/INF.0b013e3181e52945.
3. Shekhar K, Lye MS, Norlijah O, Ong F, Looi LM, Khuzaiah R, et al. Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia–clinical and pathological characteristics. Med J Malaysia. 2005;60 : 297–304.
4. Kushner D, Caldwell BD. Hand-foot-and-mouth disease. J Am Podiatr Med Assoc. 1996;86 : 257–9.
5. Xu W, Liu CF, Yan L, Li JJ, Wang LJ, Qi Y, et al. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol J. 2012;9 : 8. doi:10.1186/1743-422X-9-8.
6. Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999; 341 : 936–42.
7. Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis. 2000;31 : 678–83.
8. Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, et al. The persistent circulation of enterovirus 71 in People’s Republic of China: causing emerging nationwide epidemics since 2008. PLoS One. 2011;6:e25662. doi: 10.1371/journal.pone.0025662.
9. Zhang Y, Tan X, Cui A, Mao N, Xu S, Zhu Z, et al. Complete genome analysis of the C4 subgenotype strains of enterovirus 71: predominant recombination C4 viruses persistently circulating in China for 14 years. PLoS One. 2013;8:e56341. doi:10.1371/journal.pone.0056341.
10. Zhu J, Luo Z, Wang J, Xu Z, Chen H, Fan D, et al. Phylogenetic analysis of Enterovirus 71 circulating in Beijing, China from 2007 to 2009. PLoS One. 2013;8:e56318. doi:10.1371/journal.pone.0056318.
11. Kung YA, Hung CT, Liu YC, Shih SR. Update on the development of enterovirus 71 vaccines. Expert Opin Biol Ther. 2014;14 : 1455–64. doi:10.1517/14712598.2014.935330.
12. Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an Enterovirus 71 (EV71) vaccine. Vaccine. 2010;28 : 7731–6. doi:10.1016/j.vaccine.2010.09.065.
13. Lee MS, Tseng FC, Wang JR, Chi CY, Chong P, Su IJ. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis. 2012;6:e1737. doi:10.1371/journal.pntd.0001737.
14. Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1998;332 : 78–81.
15. Davidson NC, Struthers AD. Brain natriuretic peptide. J Hypertens. 1994;12 : 329–36.
16. Jan SL, Lin SJ, Fu YC, Lin MC, Chan SC, Hwang B. Plasma B-type natriuretic peptide study in children with severe enterovirus 71 infection: a pilot study. Int J Infect Dis. 2013;17:e1166–71. doi:10.1016/j.ijid.2013.06.012.
17. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87 : 1402–12.
18. McLean AS, Huang SJ. Brain not processing: is finding a role for BNP in sepsis like fitting a square peg into a round hole? Crit Care. 2014;18 : 161. doi: 10.1186/cc13960.
19. Jan SL, Fu YC, Hwang B, Lin SJ. B-type natriuretic peptide in children with atrial or ventricular septal defect: a cardiac catheterization study. Biomarkers. 2012;17 : 166–71. doi:10.3109/1354750X.2011.649494.
20. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347 : 161–7.
21. Olalla J, Crespo E, De la Torre J, Sempere M, Del Arco A, Prada JL, et al. Factors related to NT-proBNP levels in HIV patients aged over 40 years. AIDS Res Ther. 2015;12 : 17. doi:10.1186/s12981-015-0058-7.
22. Lin KH, Chang SS, Yu CW, Lin SC, Liu SC, Chao HY, et al. Usefulness of natriuretic peptide for the diagnosis of Kawasaki disease: a systematic review and meta-analysis. BMJ Open. 2015;5:e006703. doi:10.1136/bmjopen-2014-006703.
23. Rajaniemi SM, Hautala N, Sironen T, Vainio O, Vapalahti O, Vaheri A, et al. Plasma B-type natriuretic peptide (BNP) in acute Puumala hantavirus infection. Ann Med. 2014;46 : 38–43. doi:10.3109/07853890.2013.862960.
24. Song C, Yibing C, Guo Y, Jin Z, Cui Y, Gu X. Risk factors of severe hand, foot and mouth disease complicated with cardiopulmonary collapse. Infect Dis (Lond). 2015;47 : 453–7. doi:10.3109/23744235.2015.1015051.
25. Ministry of Health of the People’s Republic of China. Hand, Foot and Mouth Disease Clinic Guide (2010 edition). Available: http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohyzs/s3586/201004/46884.htm (in Chinese). Accessed 19 Jan 2012.
26. Takemura G, Fujiwara H, Takatsu Y, Fujiwara T, Nakao K. Venticular expression of atrial and brain natriuretic peptides in patients with myocarditis. Int J Cardiol. 1995;52 : 213–22.
27. Ribeiro AL, Reis AM, Teixeira MM, Rocha MO. Brain natriuretic peptide in Chagas’ disease: further insights. Lancet. 2003;362 : 333.
28. Maron MB, Holcomb PH, Dawson CA, Rickaby DA, Clough AV, Linehan JH. Edema developmerit and recovery in neurogenie pulmonary edema. J Appl Physiol. 1994;77 : 1155–63.
29. McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26 : 91–107.
30. Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–81. doi:10.1016/j.ijid.2010.07.006.
Štítky
Infekční lékařství
Článek vyšel v časopiseBMC Infectious diseases
Nejčtenější tento týden
2016 Číslo 585- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
Nejčtenější v tomto čísle
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



