-
Medical journals
- Career
Ischemic peripheral arterial disease and recurrent iliofemoral venous thrombosis in a 24-year-old man with antiphospholipid syndrome
Authors: D. Ručka; K. Bojanovská; S. Heller; P. Procházka; L. Skalická; P. Vařejka; M. Chochola; A. Linhart; D. Karetová
Authors‘ workplace: Second Department of Internal Medicine – Cardiology and Angiology, General University Hospital in Prague, First Faculty of Medicine, Charles University in Prague, head prof. MUDr. Aleš Linhart, DrSc.
Published in: Vnitř Lék 2013; 59(2): 127-131
Category: Case Report
Overview
The following is a case report of a young man with antiphospholipid syndrome, present with a recurrent iliofemoral venous thrombosis and premature peripheral arterial disease. This case report highlights the high risk of recurrent thrombosis upon discontinuation of anticoagulation therapy, particularly in the presence of persistent spontaneously increased aPTT and a high antiphospholipid antibody titer. The case report also reviews the potential of endovascular treatment of iliac vein thrombosis and points out the good 24-month patency rates of stents implanted into the pelvic vein region.
Key words:
antiphospholipid syndrome – iliofemoral deep vein thrombosis – recurrent thrombosis – accelerated atherosclerosis – peripheral arterial diseaseIntroduction
The term antiphospholipid syndrome refers to a thrombophilic disease characterized by the presence of antiphospholipid antibodies in the serum, clinically presented as venous and arterial thrombosis or miscarriage in women [1,2]. Thromboembolism may involve vessels of internal organs, and signs of organ dysfunction ultimately modify the clinical picture [3]. Unlike other thrombophilic conditions, antiphospholipid antibody positivity is associated with an increased risk for atherosclerosis [4]. The most common manifestation of the disease is lower limb deep vein thrombosis and stroke. Primary thromboembolism involving other territories is rare [5,6].
Our report examines the case of a young man with high antiphospholipid antibody titers whose disease manifests itself as peripheral arterial disease (PAD) in the presence of a significant stenosis of the common femoral artery (CFA) and recurrent iliofemoral thrombosis requiring local thrombolytic therapy with stent implantation into both common iliac veins.
Case Report
A 30-years-old obese patient (BMI 30) who is also a smoker, was first hospitalized in our department in 2006 when he was 24-years-old. His medical history included treatment of hepatopathy (with biopsy-based diagnosis of steatosis), hypertriglyceridemia and several attacks of gouty arthritis. His family history did not include cardiovascular or thromboembolic disease.
The reason for the patient’s first hospitalization was a trauma-related non-healing wound on the toe of the left lower limb. Left inguinal bruit, weak pulse in the left common femoral artery (CFA), and a decreased ankle-brachial index (0.8) indicated the presence of peripheral arterial disease (PAD). The patient was indicated for digital subtraction angiography (DSA), which confirmed significant stenosis of the left CFA above the bifurcation (fig. 1a). The patient subsequently had surgical endarterectomy combined with venoplasty, with the medial branch of the great saphenous vein used as a graft; however, the main trunk of the great saphenous vein remained intact. Intraoperatively, the surgeon assessed the finding as a typical atherosclerotic lesion of the artery. Histology revealed a fibroelastic plaque with residual thrombosis. The revascularization procedure and intensive local treatment resulted in complete healing of the wound within the subsequent 4 weeks. Drug therapy after surgery included aspirin, with fibrate and allopurinol administered because of hypertriglyceridemia and hyperuricemia.
Fig. 1 Atherosclerotic lesion of left common femoral artery (CFA). a – CFA before surgical treatment, b – CFA reocclusion after four years. 
The reason for re-hospitalization three year later was a painful edema of the entire right lower limb. Ultrasound diagnosed extensive iliofemoral venous thrombosis. Prior to local thrombolytic therapy, the patient had an abdominal CT, which confirmed iliac vein thrombosis while ruling out vein oppression. In addition, the examination showed hepatosplenomegaly with a liver density suggestive of steatosis. Dilatation of the portal vein to 17 mm and splenic vein to 12 mm led to the suspicion of portal hypertension. Computed tomography and echocardiography excluded steno-occlusive hepatic vein involvement. Gastroscopy did not identify esophageal varices. Once the presence of conditions raising the risk for bleeding had been excluded, local thrombolytic therapy was initiated.
As a rule, we start the procedure with a popliteal vein puncture under ultrasound control, whereby a 6F sheath with a hemostatic valve is inserted into the vein using Seldinger‘s technique to perform initial phlebography (fig. 2a). Next, a guidewire is advanced, under skiasopic control, across the thrombotic occlusion up to the vena cava inferior. Subsequently, a 5F straight catheter with side ports is advanced over the guidewire to perform cavography. The catheter is then retracted to the middle third of the thrombotic occlusion to start local thrombolysis [7] with alteplase (Actilyse, Boehringer Ingelheim, Germany) at a total dose of 1 mg/h (fig. 2b). Half of the dose is administered via the straight catheter, with the other half via a sheath inserted to the popliteal vein. Unfractionated heparin at a dose increasing activated thromboplastin time (aPTT) to 60-90 s is administered together with the thrombolytic agent. Supportive therapy includes an elastic compressive bandage and elevation of the affected limb [8]. The thrombus dissolved after 62 hours of therapy in our patient (fig. 2c). PTA with stent implantation was subsequently undertaken because of residual stenosis in the vena cava inferior, most likely due to older thrombi or ligament lesions (fig. 2d). Treatment resulted in the remission of the right lower limb edema and an improvement in the patient‘s status. The patient was discharged to receive aspirin at a dose of 100 mg and warfarin.
Fig. 2 Endovascular treatment of iliofemoral thrombosis. The arrows depict the progress of thrombus recanalisation due to local thrombolytic therapy. 
At the next clinical follow-up scheduled for the autumn of 2010, the right lower limb was free of edema and showed no signs of venous hypertension. Ultrasound confirmed patency of the iliac vein stent. However, the patient complained of recent claudication experienced in the left thigh and calf. His claudication distance was 150 meters. Ultrasound revealed occlusion of the previously treated left CFA with an ABI of 0.70. To get detailed visualization of the pelvic arterial bed, the patient was scheduled for a new lower limb DSA. Although he should have been admitted to the hospital after a week, he came a few days before his scheduled appointment with surprising extensive edema of the entire left lower limb. Upon discontinuation of warfarin prior to elective DSA, the patient forgot to self-administer low-molecular-weight heparin as recommended, leaving him with no anticoagulation medication for 5 days. Ultrasound showed left iliofemoral venous thrombosis. Hence, the patient was scheduled for interventional treatment of iliofemoral venous thrombosis instead of the originally scheduled DSA. The thrombus dissolved after 30 hours of treatment and a stent had to be implanted into the left common iliac vein because of residual stenosis (fig. 3). DSA of the pelvic and lower limb arteries, performed before re-instituting warfarin therapy, documented occlusion of the left CFA. Given the relatively good collateralization of the occlusion via the internal iliac artery (fig. 1b), the patient was recommended to receive conservative therapy.
Fig. 3 Endovascular treatment of iliofemoral thrombosis. a – before implantation of the stent, b – after the implantation 
Laboratory Results
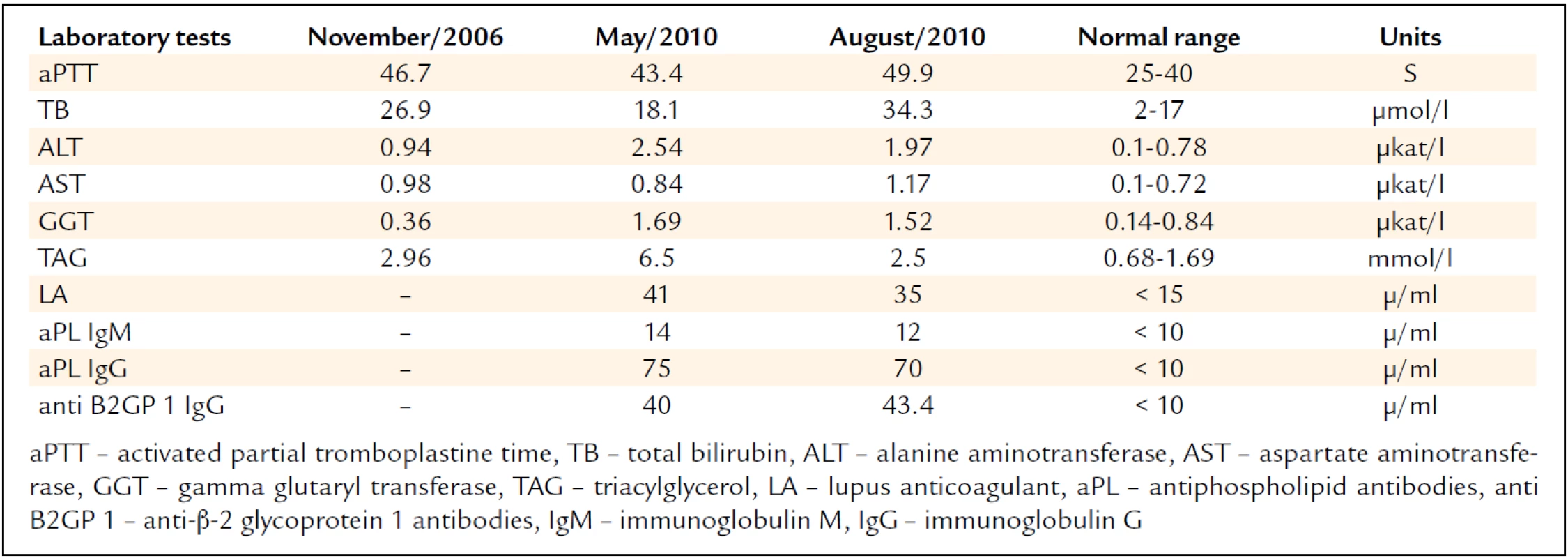
Besides the hepatopathy and triglyceridemia, we also repeatedly noted increased aPTT levels (tab. 1). In 2011, the patient had a new liver biopsy using the transjugular approach; the procedure again confirmed the presence of steatosis while ruling out inflammatory alterations, cirrhosis, or intrahepatic vein thrombosis. The examination included also portal pressure measurement, excluding the presence of portal hypertension, as suspected on the basis of the preceding CT examination. Because of laboratory tests showing signs of liver disease, the anti-hepatocyte antibodies were tested. Their negativity ruled out autoimmune hepatitis.
The examination repeatedly showed high antiphospholipid antibody titers (tab. 1) thus supporting the diagnosis of antiphospholipid syndrome (APS). Laboratory investigations excluded the presence of other thrombophilic conditions as well as a systemic autoimmune disease such as systemic lupus erytematosus (SLE).
Clinical Status
The latest clinical and ultrasound follow-up demonstrated good patency of stents in the vena cava inferior and left common iliac vein. Both lower limbs show no edema or signs of severe venous hypertension. After 9 months of intensive training, the claudication distance is currently more than 1 km, with the patient no longer limited by claudication in his everyday activities.
Discussion
For the diagnosis of antiphospholipid syndrome (APS) to be established, the patient has to meet clinical and laboratory criteria that were last updated at the 11th International Congress on Antiphospholipid Syndrome held in Sydney, Australia, in 2006 [2]. The diagnosis is based on the evidence of at least one thromboembolic event or loss of fetus in women, and antiphospholipid antibody positivity in two blood samples obtained at an interval of 12 weeks to 5 years. Commonly tested antiphospholipid antibodies (aPL) are lupus anticoagulant (LA), anti--cardiolipin antibodies (aCL), and anti-B2-glycoprotein I (anti-B2-GPI) [9]. Our case report confirms that spontaneously increased aPTT is yet another diagnostic tool, possibly alerting the physician to the presence of APS. The increase in aPTT is due to the binding of LA to phospholipids in the reagents used for aPTT determination. Thus, while not signaling reduced coagulability, it does indicate an increased risk for thrombosis [10].
The mechanism whereby antiphospholipid antibodies result in thrombosis and atherosclerosis acceleration is still poorly understood. Phospholipids make an integral part of the cell membranes of endothelial cells and thrombocytes. It has been shown that the main antigenic target of aPL are plasma proteins such as B2-glycoprotein I, prothrombin, protein C, protein S, tissue plasminogen activator, and a variety of other proteins involved in the process of coagulation. The involvement of aPL in the auto-regulation of hemostatic processes results in a prothrombotic state. The proatherosclerotic effect of aPL has been suggested to be due to the direct action of antibodies on endothelial cells, thrombocyte and monocyte membranes leading to upregulation of adhesion molecules, proinflammatory cytokines and local complement activation. The result is enhanced thrombocyte aggregability, impaired fibrinolysis, and inhibition of prostacyclin release by endothelial cells. These processes eventually lead to endothelial dysfunction and atherosclerotic lesions [11]. Major laboratory predictor of the risk for thrombosis is positivity of LA, or combination of antiphospholipid antibodies [5,12].
The high susceptibility to thrombotic events of our patient can be explained by the positivity of all three types of aPL (LA, aCL, anti-B2-GPI). Obesity, lipid metabolism, smoking, and a sedentary lifestyle are risk factors that surely played an important role for atherothrombosis.
Acute treatment of venous thrombosis in APS patients is not different from patients without this condition. The turbulent symptomatology of iliofemoral venous thrombosis in our patient required local thrombolytic therapy, which in fact had a bilateral effect. The final phlebographic picture with residual venous stenoses required implantation of stents. The literature contains case reports documenting an increased risk for early thrombosis in stents implanted into the coronary territory in patients with aPL positivity [10,14]. Our patient did not develop this complication. This case report illustrates the efficacy and safety of this therapeutic modality in APS patients.
Long-term therapy as part of secondary prevention of thrombosis in paftients with a combination of venous and arterial disease has not been clearly defined. In the event of a thromboembolic etiology, patients are scheduled for chronic therapy with warfarin with a target INR of 2–3. In two prospective studies, use of higher doses of warfarin did not result in a significant reduction of the thromboembolic risk compared with a standard dose [15,16]. In patients with the APS and cerebrovascular disease, treatment with aspirin has been shown to be as effective as warfarin in reducing the risk for recurrent stroke [17]. Aspirin is also recommended for prevention of cardiovascular events in patients with aPL positivity in the presence of SLE [18]. While the addition of aspirin to warfarin in patients with venous thrombosis and overt atherosclerosis seems to be a most logical measure, no data from clinical trials supporting combination therapy in APS patients is currently available.
Current guidelines recommend anticoagulation therapy for at least 12 months. However, a number of authors advocate life-long therapy in patients without contraindications or at high risk for bleeding. The rationale for this is the high likelihood of thrombosis recurrence following withdrawal of therapy. Prospective data show the risk is 50–67 % [18,19]. In our case report, recurrent thrombosis was documented after a 5-day discontinuation period of warfarin administration. This experience suggests that even a short discontinuation of therapy is associated with some risk.
In many cases, antiphospholipid syndrome is associated with symptoms of internal organ involvement. Most of these symptoms are caused by thrombosis or embolism into the venous system of the organs, most commonly the liver. Antiphospholipid syndrome has also been linked to autoimmune hepatitis, nodular regenerative hyperplasia, liver cirrhosis, and idiopathic portal hypertension [3]. All these conditions were ruled out in our patient. The mild elevation of liver enzymes may have been due to hepatic steatosis commonly associated with obesity.
Conclusion
Our paper presents the case of recurrent venous thrombosis and unusual arterial lesions in a young individual with a positivity of aPL due to primary APS. In addition to the levels of specific antibodies, the presence of APS may also be signaled by spontaneously increased aPTT. This kind of patient should receive chronic anticoagulation therapy, often in combination with antiplatelet therapy. Our suggestion is to co-administer warfarin and aspirin on a long-term basis. Even a short discontinuation of therapy within the first months of an acute event is associated with a high risk of disease recurrence.
MUDr. David Ručka
http://int2.lf1.cuni.cz/
e-mail: ruckad@centrum.cz
Doručeno do redakce: 3. 12. 2012
Přijato po recenzi: 15. 1. 2013
Sources
1. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346 : 752–763.
2. Miyakis S et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4 : 295–306.
3. Uthman I, Khamashta M. The abdominal manifestations of the antiphospholipid syndrome. Rheumatology (Oxford) 2007; 46 : 1641–1647.
4. Ames PR et al. Atherosclerosis in thrombotic primary antiphospholipid syndrome. J Thromb Haemost 2009; 7 : 537–542.
5. Farmer-Boatwright MK, Roubey RA. Venous thrombosis in the antiphospholipid syndrome. Arterioscler Thromb Vasc Biol 2009; 29 : 321–325.
6. Ruiz-Irastorza G, Khamashta MA. Stroke and antiphospholipid syndrome: the treatment debate. Rheumatology (Oxford) 2005; 44 : 971–974.
7. Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology 1994; 191 : 487–494.
8. Varejka P, Chochola M, Jirat S et al. Cathetrization treatment of deep venous thrombosis, early and long-term outcomes. Interní Med 2004; 12 : 576–580.
9. Hirmerová J, Ulcová-Gallová Z, Seidlerová J et al. Laboratory evaluation of antiphospholipid antibodies in patients with venous thromboembolism. Clin Appl Thromb Hemost 2010; 16 : 318–325.
10. Arnout J. Antiphospholipid syndrome: diagnostic aspects of lupus anticoagulants. Thromb Haemost 2001; 86 : 83–91.
11. Espinosa G, Cervera R. Antiphospholipid syndrome. Arthritis Res Ther 2008; 10 : 230.
12. Buliková A, Zavřelová J, Penka M. Antiphospholipid syndrome in the year 2009. Vnitř Lék 2009; 55 : 253–262.
13. Weissman A, Coplan NL. Antiphospholipid antibody syndrome and acute stent thrombosis. Rev Cardiovasc Med 2006; 7 : 244–246.
14. Middlebrooks EH, Panda M. Multiple recurrent stent thrombosis in a patient with coexisting clopidogrel resistance and increased anticardiolipin antibodies: a case report. Case Report Med 2010 : 974149.
15. Kearon C et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133: (6 Suppl.): 454S–545S.
16. Crowther MA et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349 : 1133–1138.
17. Mohr JP et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345 : 1444–1451.
18. Wajed J et al. Prevention of cardiovascular disease in systemic lupus erythematosus – proposed guidelines for risk factor management. Rheumatology (Oxford) 2004; 43 : 7–12.
19. Lim W et al. Phospholipid antibody syndrome. Hematology Am Soc Hematol Educ Program 2009; 233–239.
Labels
Diabetology Endocrinology Internal medicine
Article was published inInternal Medicine

2013 Issue 2-
All articles in this issue
- Current trends in treatment and follow-up of patients with differentiated thyroid carcinoma – experience with the use of recombinant human thyrotropin
- Practice guideline and trends for immunosuppressive treatment of glomerulonephritides according to KDIGO (Clinical Practice Guideline for Glomerulonephritis)
- Syncope as first and only sign of left atrial myxoma
- Heart transplantation and the subsequent treatment of AL amyloidosis
- Opinion of the Czech Atherosclerosis Society‘s committee (CSAT) on the ESC/EAS guidlines related to the diagnostics and treatment of dyslipidemias issued in 2011
- Aortic regurgitation
- Ischemic peripheral arterial disease and recurrent iliofemoral venous thrombosis in a 24-year-old man with antiphospholipid syndrome
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Aortic regurgitation
- Practice guideline and trends for immunosuppressive treatment of glomerulonephritides according to KDIGO (Clinical Practice Guideline for Glomerulonephritis)
- Current trends in treatment and follow-up of patients with differentiated thyroid carcinoma – experience with the use of recombinant human thyrotropin
- Heart transplantation and the subsequent treatment of AL amyloidosis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career