-
Medical journals
- Career
Can we prevent ovarian cancer?
Authors: Ali Tariq Aus
Authors‘ workplace: Department of Chemical Pathology, NHLS, Tygerberg Hospital and Stellenbosch Faculty of Medicine and Health Sciences, Cape Town, South Africa
Published in: Ceska Gynekol 2020; 85(1): 49-58
Category: Review Article
Overview
Introduction: An ovarian cancer prevention program must encourage the application of factors associated with decreased risk that include both surgical and non-surgical approaches. Non-surgical preventive approaches include oral contraceptives, parity, multiparity and breastfeeding. In addition, approaches that decrease inflammation and oxidative stress such as regular exercise and a healthy diet are also important. Surgical approaches include tubal ligation, hysterectomy and prophylactic bilateral salpingo-oophorectomy.
Objective: To highlight protective approaches for the prevention of ovarian cancer in order to increase awareness among women of the general population and too find out whether or not these approaches are enough to prevent the disease.
Design: Review article.
Setting: Department of Chemical Pathology, NHLS, Tygerberg Hospital and Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Methods: Literary sources related to the topic were used. Articles were selected primarily based on PubMed and Google searches.
Conclusion: Ovarian cancer cannot be prevented completely, however the application of preventive approaches may decrease the risk significantly. Although, multiparity followed by long periods of breastfeeding may not seem feasible for most today women, it is the most pronounced preventive approach for women in the general population. Tubal ligation, hysterectomy also reduce the risk significantly. Opportunistic salpingectomy may provide better prevention for women at average risk, while women at high risk (BRCA mutation and family with history of ovarian cancer) are advised to undergo risk-reducing salpingo-oophorectomy. Highlighting these approaches may increase women's awareness towards decreasing risk and decrease the incidence of ovarian cancer and potentially increase the five-year survival rate.
Keywords:
Physical activity – breastfeeding – ovarian cancer – Contraceptives – Aspirin – parity – prophylactic surgeries
INTRODUCTION
Ovarian cancer is the fifth leading cause of all cancer related deaths in women in developed countries and is the most lethal of all gynaecological cancers. Ovarian cancer risk increases with age progression and is more prevalent in westernised compared to non-westernised societies. This difference can be explained by reproductive and environmental factors including number of births, breastfeeding and diet. Less than 15% of epithelial ovarian cancer is due to genetic predisposition which include breast cancer 1 and breast cancer 2 (BRCA1/2) gene mutation carriers [34]. Early ovarian cancer is an asymptomatic disease and despite the development in screening technology, surgical procedures and chemotherapy, ovarian cancer remains the most lethal gynaecologic cancer worldwide. A longer survival time is influenced by younger age at the time of diagnosis, stage of cancer at diagnosis, type of mutation (i.e. BRCA vs. KRAS), body mass index (BMI), low-grade and nonserous histology [13]. Although the pathophysiology of ovarian cancer is well understood and assisted technologies in this field have been improved, up till today there is no specific marker or technique that enables the early detection of ovarian cancer. Thus, the five-year survival rate is lower compared with other gynaecological cancers as most patients are diagnosed at an advanced stage [13, 34].
Previous epidemiological studies have shown that the risk of ovarian cancer mainly decreases with surgical and non-surgical approaches. Non-surgical approaches include oral contraceptives [17, 21, 32, 33], parity [17, 44, 70], breastfeeding [21, 52, 37, 38, 64], anti-inflammatory medicines such as with aspirin [2, 30, 54, 68, 71, 77], healthy diet [50, 69, 80, 81] and regular exercise [51, 60]. Surgical approaches involve tubal ligation [40, 58, 59, 63], salpingectomy [42, 75] hysterectomy [58, 59], hysterectomy with unilateral oophorectomy [59] and bilateral salpingo-oophorectomy [16, 57]. For women with a family history of ovarian cancer or those with a genetic predisposition (BRCA mutations), bilateral salpingo-oophorectomy could be the most effective approach. Although, this surgery is usually performed to prevent certain types of gynecologic cancers, like ovarian cancer, it might also prevent breast cancer in women with a strong family history or genetic link. Despite the former, a previous study has reported a slight increase in peritoneal cancer following bilateral salpingo-oophorectomy [16]. This article attempts to answer the question of whether prevention of ovarian cancer is feasible and to outline what a prevention program of ovarian cancer might look like, and what might be the potential challenges.
METHODS
Appropriate articles were selected primarily based on PubMed and Google searches using the following keywords as search terms: ovarian cancer, preventive approaches, preventive surgeries and ovarian cancer, reproductive factors and ovarian cancer. In order to prevent missing any article covering patients with BRCA1/2, the following sentence“BRCA1/2 and ovarian cancer“ was used both in PubMed and Google. Later on, another precise selection was carried out to exclude articles with small sample size and those that did not control for confounders. Case reports were not included in this review.
RESULTS AND DISCUSSION
From a public health perspective, challenges facing any prevention program for ovarian cancer may include; firstly, the majority of patients are diagnosed at late stages (stage III and IV), secondly, the recurrence of the disease that is often chemo-resistant and lastly, most ovarian cancer tumors are detected in women without family history and/or falling under identifiable high-risk groups [62, 67].
INFLAMMATION AND OVARIAN CANCER
Inflammation is a multifaceted state that involves a variety of changes in tissues associated with ongoing immune responses. Obesity is associated with higher circulating levels of inflammatory markers [7]. Increasing the daily intake of saturated fatty acids (SFA) is a risk factor for obesity and ovarian cancer and it worsen inflammation in overweight and obese individuals [56]. At a cellular level, transforming normal cells into highly malignant cells is a multistep process that involves genetic alterations. Genetic alterations produce oncogenes with a dominant gain of function and tumour suppressor genes with a recessive loss of function. In humans, p53 is considered to be one of the most relevant human oncosuppressor genes and the most frequently mutated gene. It is regulated by inflammation and its precise function is to inhibit the generation and the maintenance of cancer stem cells. In the absence of p53, enhanced inflammatory reactions cause cells to be continuously exposed to inflammatory cytokines, chemokines and growth factors, resulting in accumulation of DNA damage induced by oxidative stress and enhanced energy metabolism [24, 49]. These conditions might cause reprogramming of the cells to facilitate the differentiation of cancer stem cells, tumour development and invasion.
On the other hand, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) binds to consensus DNA sequences at promoter regions of responsive genes regulating various cellular processes [74]. In normal, unstimulated cells all NF-κB members are held in an inactive form, bound to one of a family of inhibitory proteins called NF-κB inhibitor (IκBs), which is involved in propagating the cellular response to inflammation. However, when stimulated, NF-κB promotes tumor cells proliferation, suppresses apoptosis and enhances angiogenesis. Thus, the inhibition of NF-κB leads to tumour regression [74]. While down regulation of p53 enhance cancer initiation, the activation of NF-κB signaling is associated with advanced cancer and promotes tumour metastasis by influencing tumour cell migration and angiogenesis (Figure 1).
1. Dietary fat, Obesity, hormonal imbalance and the role of inflammation in increasing ovarian cancer risk
Chronic inflammation is associated with alteration in glucose metabolism and may increase the chances for mutation, via decreasing cell ability to detect and repair DNA damage. In addition chronic inflammation increases cyclo-oxidase enzyme which is associated with increased p53 loss and activate NF-κB. Increased cyclo-oxidase is also associated with an increased conversion rate of Arachidonic acid into prostaglandins which then leads to increased oestrogen. Increased oestrogen is associated with an increased risk of ovarian cancer.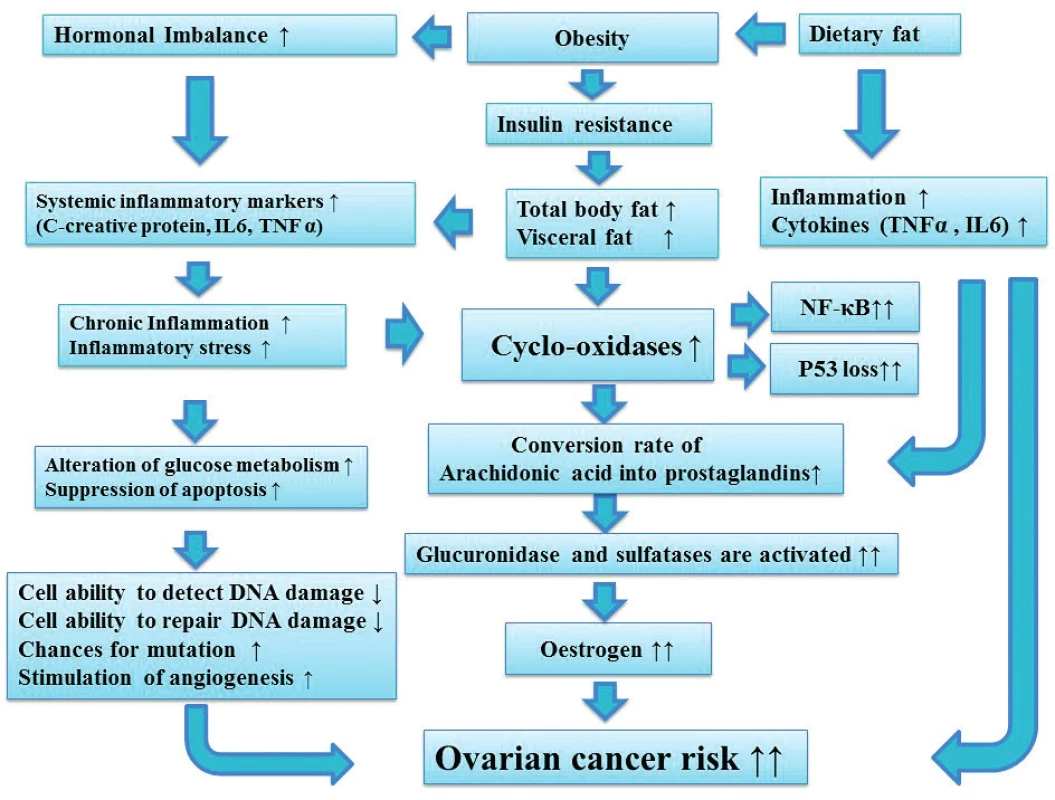
As a result of this, and in order to reduce the risk of ovarian cancer, decreasing inflammation is an important approach. Inflammation can be targeted using anti-inflammatory medications (i.e. aspirin and ibuprofen) and/or lifestyle interventions (i.e. healthy diet and regular exercise) as described in figure 2.
2. The importance of targeting inflammation on ovarian cancer risk
Regular physical activity, healthy diet and anti-inflammatory medications (i. Aspirin) are three important aspects to reduce inflammation, which has a significant impact on ovarian cancer risk. Maintaining normal weight is associated with a decrease in different types of cancer and other diseases including Diabetes type2, CVD, arthritis and stroke. Ultimately, this will improve quality of life and the five years survival rate.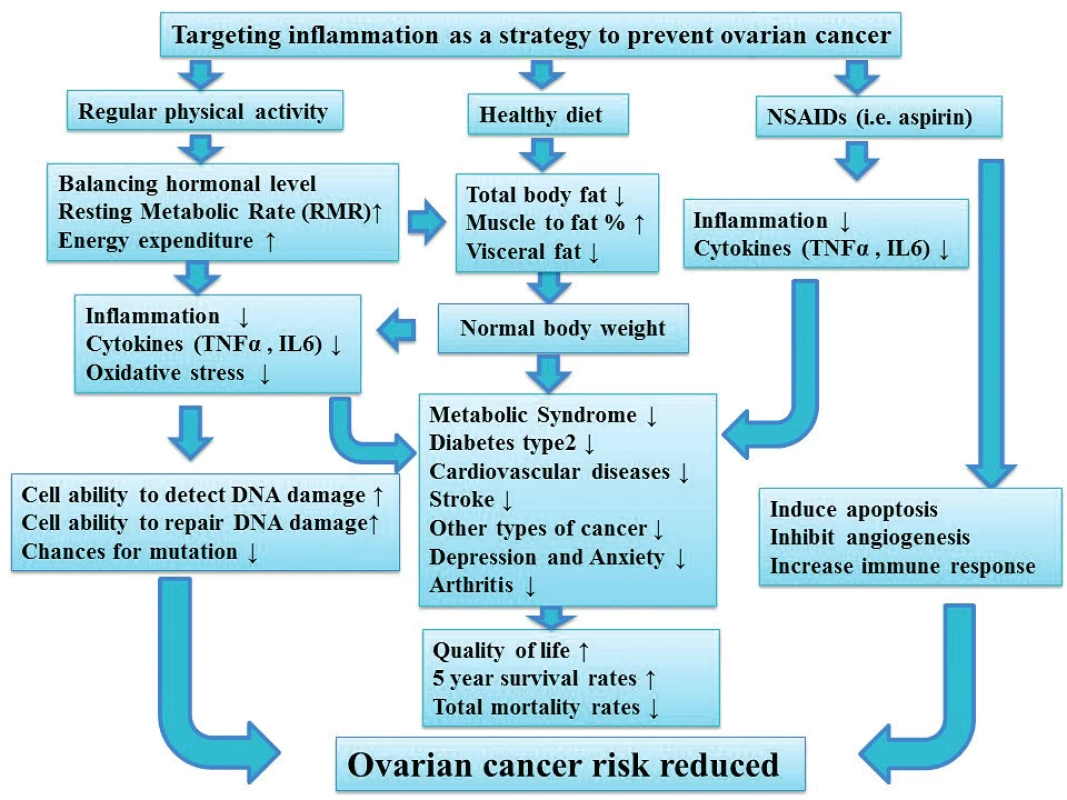
Aspirin is associated with modest [1] to very significant reduction in ovarian cancer risk [2, 30, 54, 68, 71], and regular aspirin intake is associated with 44% reduction in ovarian cancer risk [2, 54]. However, side effects such as gastrointestinal bleeding was reported among aspirin users [76]. In addition, aspirin users without history of cardiovascular disease (CVD) had a higher mortality rate when compared to controls [29].
Lifestyle modifications including regular exercise and healthy diet is another proven approach to reduce inflammation. Regular exercise reduces the risk of cancer development [51, 60, 66, 79] and the risk of ovarian cancer continued to decrease with increasing the duration of strenuous activity [79]. Regular exercise also improves quality of life and decreases the prevalence of metabolic diseases including CVD and type 2 diabetes (Figure 2). This is because regular exercise and physical activity boost health, help to maintain normal BMI, increases muscle mass and decreases fat mass including visceral fat. The former is a sign of insulin resistance, increase inflammatory markers, abnormal lipid profiles. Visceral fat is also a predictor of impaired glucose tolerance, type 2 diabetes and CVD development. In contracts, inactivity or sedentary lifestyle is associated with an increased ovarian cancer risk [9] and death rate [8]. Excess weight or abnormal BMI not only increases ovarian cancer risk [3, 39], but also decreases five-year survival rate [46, 61]. Compared with the general population, women who include vegetables and fruits in their diet are less prone to develop cancer in general [6]. In addition, a significant reduction in dietary fat including animal fat, red meat, processed food in general and increase the intake of vegetables, fruits and white meat (i.e. poultry, fish and other sea food) may reduce the risk of ovarian cancer even further [4, 35, 36, 69, 78, 80, 81] and usually have a positive impact on general health (Figure 2). Although other studies showed no support towards the former results [56, 65, 23], however, the consumption of white meat is associated with decrease the risk of breast and colon cancer [23]. Thus, targeting inflammation must be encouraged in any prevention program for ovarian cancer (Figure 2).
PARITY, BREASTFEEDING AND CONTRACEPTIVES
Parity is an effective preventive factor against ovarian cancer development. Historically, multiparity had protected women against breast, endometrial and ovarian cancer. Parturition used to be followed by prolonged periods of breastfeeding. Although, women in developed and most developing countries are satisfied with small family size, women in many regions of the developing world (especially rural areas) are still having large numbers of children. A 65% decrease in the risk of ovarian cancer was achieved after the sixth delivery [26]. With further reduction gained from prolonged periods of breastfeeding [21, 22], women who are not at high risk may prevent the disease with the more children they have. Although breastfeeding is a well-known preventive factor against ovarian cancer [21, 52, 37, 38, 64], however, women in westernized societies rarely practice breastfeeding [72]. Multiparity and prolonged breastfeeding are among the main factors explaining the significant differences in prevalence of ovarian cancer between westernised and non-westernised societies. An increase in parity is associated with a significant inverse relationship with ovarian cancer risk among BRCA1 but not BRCA2 mutation carriers [37, 45]. Although, multiparity reduces the risk of ovarian cancer significantly among women with BRCA1 mutations carriers [45], however, it is difficult to predict outcomes similar to multiparous women without genetic predisposition. Breastfeeding for more than 12 months was associated with a 38% and 50% reduction in risk among BRCA1 and BRCA2 mutation carriers, respectively.
Hundreds of millions of women around the world have been using oral contraceptives. This application was associated with significant reduction in the risk of ovarian cancer [21, 32, 33], and other types of cancer [33]. Among ever users of oral contraceptives the risk reduced by 33% for ovarian cancer 34% for endometrial cancer, 19% for colorectal cancer and 26% for lymphatic and hematopoietic cancer [33]. Among current or recent users the relative risk has decreased with increased duration of use from 18% [RR=0.82, (95% CI: 0.59-1.12)] with ? 1year use to 74% [RR=0.26, (0.16-0.43)] when the duration of contraceptives use exceed 10 years [32]. The use of oral contraceptives for five or more years was associated with a 50% reduction in ovarian cancer risk among BRCA1 mutation carriers. Amongst women with BRCA2 mutation, the use of oral contraceptives for three or more years was associated with a 58% reduction in the risk of ovarian cancer [37].
Unfortunately, there is a significant increase in the risk of breast [19, 33] and cervical cancer [33] among women using oral contraceptives and the risk was more pronounced amongst recent [19, 33] and current users [33]. Although, the risk increased significantly among women with genetic mutations (BRCA1/2) and amongst those with a strong family history of breast cancer [12, 20, 47]. However, among women of the general population, the risk appeared to be lost within approximately 5 years of stopping oral contraception [33]. Table 1 displays some of the preventive approaches.
1. The role of protective approaches on the risk of ovarian cancer 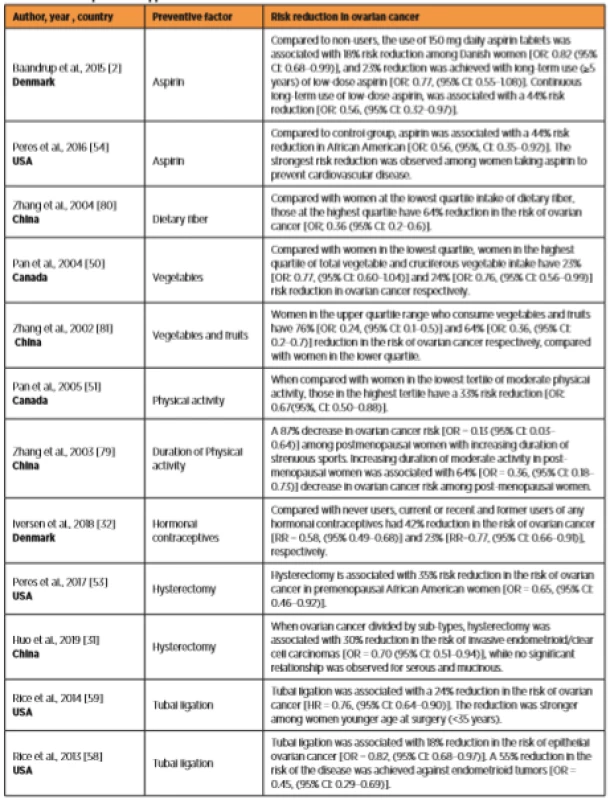
PROPHYLACTIC SURGERIES
When compared with controls, a significant reduction in the risk of ovarian cancer was reported amongst women who underwent one or more of the prophylactic surgeries as shown in table 1.
Amongst the general population, tubal ligation was associated with 20 to 34% risk reduction in ovarian cancer [11, 18]. It was suggested that some cells that cause ovarian cancer come from the fallopian tubes. Thus, when fallopian tubes are blocked off (i.e. by tubal ligation), the risk of potential cancer cells moving from the fallopian tubes to the ovary is reduced dramatically if not eliminated. As a result, the risk of ovarian cancer can be reduced by up to 50%. This is because different sub-types of the cancer can evolve through different pathophysiology (i.e. serous tumours). In addition, the age at which the tubal ligation was performed was found to be crucial [43]. McNamara and colleagues observed an inverse association between tubal ligation and the risk of ovarian cancer amongst women who had this surgery at age 35 years or later but not amongst those who had it before the age of 35 years. This risk reduction varied by type of tumours and the most pronounced reduction was observed for endometrioid tumors in which the risk was reduced by 69% [OR: 0.31, (95% CI 0.14-0.70)]. Whereas no significant reduction were detected in mucinous and serous tumors [43]. A previous study reported that women with BRCA1 who performed tubal ligation will gain further reduction in ovarian cancer risk when they are exposed to oral contraceptives [48].
Worldwide, there is an increase in number of women performing opportunistic salpingectomy [10, 14, 42, 75]. Opportunistic salpingectomy is a safe, cost effective approach for decreasing ovarian cancer risk when done concurrently with hysterectomy instead of tubal ligation [58]. Hysterectomy is associated with a significant reduction in ovarian cancer risk [58, 59]. Women in the general population who completed their childbearing may go for hysterectomy with bilateral salpingectomy. Comparing the previous surgical approaches, Falconer and colleagues found that bilateral salpingectomy was associated with a 65% risk reduction in comparison to 21% and 28% risk reduction following hysterectomy and tubal ligation, respectively [15]. Nevertheless, the risk of ovarian cancer was increased significantly among patients with hysterectomy who used hormone replacement therapy [12, 55]. In such patients ovaries most probably are still able to secret significant amount of oestrogen, thus using hormone replacement therapy may disturb the estrogen-progesterone balance and can trigger the initiation of ovarian cancer. The previous results provide evidence for clinicians to re-evaluate the prescription of hormone replacement therapy for women with hysterectomy. In addition, and in order to weigh the benefits and associated risks, future research must target the possibility of bilateral oophorectomy at the time of hysterectomy for benign conditions for women within the general population [16, 73].
Bilateral salpingo-oophorectomy is performed as a preventive approach in individuals with high risk of ovarian and breast cancer. A woman‘s family history of ovarian cancer and breast cancer and those with genetic perception (BRCA1/2) may preform bilateral salpingo-oophorectomy, preferably after completing childbearing, as it is the definitive method for preventing ovarian cancer [16, 42]. Although, bilateral salpingo-oophorectomy may protect women at high risk, the application of this approach faces many challenges. These challenges include; firstly, the absence of a screening method to identify individuals at risk, secondly, the willingness of the patient/family to go through such surgery and lastly, the possible development of health consequences such as sexual dysfunction, premature menopause, bone mineral loss and CVD [5, 25, 28].
Overall, ovarian cancer cannot be prevented completely, however, it can be reduced significantly. Women of the general population who are not at high risk may prevent the disease with the more children they have and consider prolonged breastfeeding (Fig 3). Those who are not willing to have children or are satisfied with a small family size may consider contraceptives and prophylactic surgeries. Women with family history of BRCA mutations and other genetic disorders related to ovarian cancer may benefit from the previous preventive approaches, however bilateral salpingo-oophorectomy is the most effective surgical preventive approach.
3. Steps to decrease ovarian cancer risk among women of general population
Education towards risk and preventive factors for ovarian cancer should be the first step in any preventive program. Since obesity is a risk factor for ovarian cancer, maintaining normal weight is crucial and this can be achieved through a healthy diet and physical activity. Oral contraceptives is an ideal option for nulliparous women, women satisfied with small family size and women who do not practice breastfeeding. In addition, prophylactic surgeries (i.e. Tubal ligation, hysterectomy and opportunistic salpingectomy) are other options to decrease ovarian cancer risk among these women. For women who consider having children, the current guidelines from the Institute of Medicine for weight gain during gestation must be considered. Thus, a total weight gain of 12.7–18.1 kg (28–40 lb) for underweight women (BMI less than 18.5), 11.34–15.9 (25-35 lb) for normal weight women ( BMI between 18.5 and 24.9), 6.8–11.34 kg (1525 lb) for overweight women (BMI between 25 and 29.9), and 4.5-9 kg (11–20 lb) for all obese women (BMI more than 30). Being physically active with a healthy diet is important to maintain normal weight.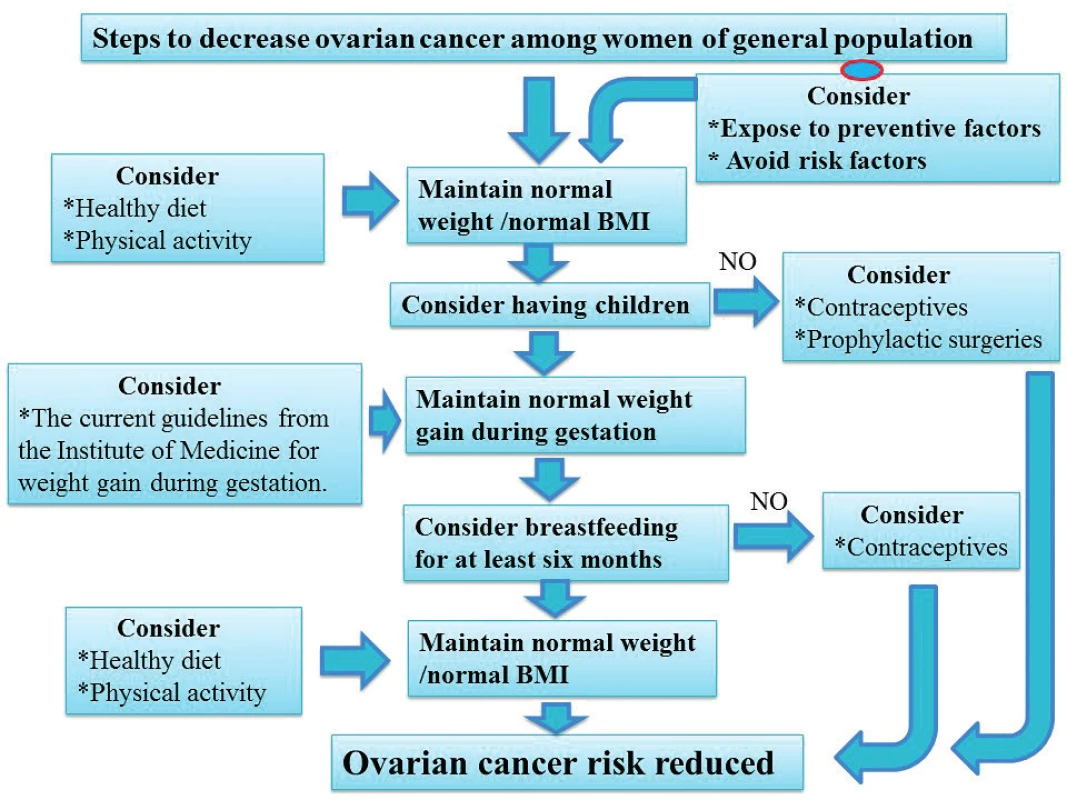
CONCLUSION
Upon reviewing the literature, the author found that a prevention program for ovarian cancer should start with educating women in general in order to improve their awareness towards risk factors and prevention possibilities. Although ovarian cancer can be reduced drastically among women in the general population, it cannot be prevented completely. Women are encouraged to maintain a normal BMI and consider lifestyle interventions. In addition, parous women should be encouraged to practice breastfeeding for at least 6 months, while longer periods of breastfeeding must be encouraged if feasible. Nulliparous women and those satisfied with a limited number of children (1 and/or 2) and those who abandon breastfeeding should be encouraged to take oral contraceptives and/or undergo prophylactic surgeries (tubal ligation and opportunistic salpingectomy). Combining these approaches may reduce the risk of the disease even further. Although hysterectomy is associated with a decreased risk of ovarian cancer, there is a significant increase in the risk of the disease amongst women who used hormone therapy after surgery. Women at a high risk of ovarian cancer (BRCA1/2 mutations carriers) can also benefit from these approaches, however, the most definitive preventive strategy is bilateral salpingo-oophorectomy. The former should not be performed without calculating risks and benefits, and preferably performed when childbearing is complete.
Acknowledgement:
I would like to thank doc. MUDr. Vít Weinberger,Ph.D., the head of the Department of Gynecology and Obstetrics, University Hospital Brno, Czech Republic for his valuable comments.
Correspondence:
Dr. Aus Tariq Ali
Department of Pathology
Tygerberg Hospital and Stellenbosch Medical School
Po Box 19113
Tygerberg 7505
Cape Town
South Africa
e-mail: atali@sun.ac.za
Sources
1. Baandrup, L., Faber, MT., Christensen, J., et al. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: systematic review and meta-analysis of observational studies. Acta Obstet Gynecol Scand, 2013, 92(3), p. 245–255.
2. Baandrup, L., Kjaer, SK., Olsen, JH., et al. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann Oncol, 2015, 26, p. 787–792.
3. Bhaskaran, K., Douglas, I., Forbes, H., et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet, 2014, 384(9945), p. 755–765.
4. Blank, MM., Wentzensen, N, Murphy, MA., et al. Dietary fat intake and risk of ovarian cancer in the NIH-AARP Diet and Health Study. Br J Cancer., 2012, 106(3), p. 596–602.
5. Bober, SL., Recklitis, CJ., Bakan, J., et al. Addressing sexual dysfunction after risk-reducing salpingooophorectomy: effects of a brief, psychosexual intervention. J Sexual Med, 2015, 12(1), p. 189–197.
6. Boyle, P., Maisonneuve, P., Autier, P. Update on cancer control in women. Int J Gynaecol Obstet, 2000, 70, p. 263–303.
7. Calder, PC., Ahluwalia, N., Brouns, F., et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr, 2011, 106(Suppl. 3), p. S5–78.
8. Cannioto, RA., LaMonte, MJ., Kelemen, LE., et al. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. Br J Cancer, 2016, 115(1), p. 95–101.
9. Cannioto, R., LaMonte, MJ., Risch, HA., et al. Chronic recreational physical inactivity and ovarian cancer risk: Evidence from the Ovarian Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev, 2016, 25(7), p. 1114–1124.
10. Chen, Y., Du, H., Bao, L., Liu, W. Opportunistic salpingectomy at benign gynecological surgery for reducing ovarian cancer risk: a 10-year single centre experience from China and a literature review. J Cancer, 2018, 9(1), p. 141–147.
11. Cibula, D., Widschwendter, M., Májek, O., Dusek, L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update, 2011, 17(1), p. 55–67.
12. Cibula, D., Zikan, M., Dusek, L., Majek, O. Oral contraceptives and risk of ovarian and breast cancers in BRCA mutation carriers: a meta-analysis. Expert Rev, Anticancer Ther, 2011, 11(8), p. 1197–1207.
13. Cress, RD., Chen, YS., Morris, CR., et al. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol, 2015, 126(3), p. 491–497.
14. Dilley, SE., Havrilesky, LJ., Bakkum-Gamez, J., et al. Cost-effectiveness of opportunistic salpingectomy for ovarian cancer prevention. Gynecol Oncol, 2017, 146(2), p. 373–379.
15. Falconer, H., Yin, L., Grönberg, H., Altman, D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst, 2015, 107(2).
16. Finch, A., Beiner, M., Lubinski, J., et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA, 2006, 296(2), p. 185–192.
17. Friebel, TM., Domchek, SM., Rebbeck, TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst, 2014, 106(6), dju091.
18. Gaitskell, K., Coffey, K., Green, J., et al. Tubal ligation and incidence of 26 site-specific cancers in the Million Women Study. Br J Cancer, 2016, 114(9), p. 1033–1037.
19. Gierisch, JM., Coeytaux, RR., Urrutia, RP., et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev, 2013, 22(11), p. 1931–1943.
20. Grabrick, DM., Hartmann, LC., Cerhan, JR., et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA, 2000, 284(14), p. 1791–1798.
21. Greggi, S., Parazzini, F., Paratore, MP., et al. Risk factors for ovarian cancer in central Italy. Gynecol Oncol, 2000, 79(1), p. 50–54.
22. Gronwald, J., Byrski, T., Huzarski, T., et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat, 2006, 95(2), p. 105–109.
23. Grosso, G., Bella, F., Godos, J., et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev, 2017, 75(6), p. 405–419.
24. Gudkov, AV., Gurova, KV., Komarova, EA. Inflammation and p53: A tale of two stresses. Genes Cancer, 2011, 2(4), p. 503–516.
25. Guidozzi, F. Hormone therapy after prophylactic risk reducing bilateral salpingo-oophorectomy in women who have BRCA gene mutation. Climacteric, 2016, 19(5), p. 419–422.
26. Hankinson, SE., Colditz, GA., Hunter, DJ., et al. A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer, 1995, 76(2), p. 284–290.
27. Herder, C., Peltonen, M., Koenig, W., et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes, 2006, 55(8), p. 2340–2346.
28. Hibler, EA., Kauderer, J., Greene, MH., et al. Bone loss after oophorectomy among high-risk women: an NRG oncology/gynecologic oncology group study. Menopause, 2016, 23(11), p. 1228–1232.
29. Huang, WY., Daugherty, SE., Shiels, MS., et al. Aspirin use and mortality in two contemporary U.S. cohorts. Epidemiology, 2018, 29(1), p. 126–133.
30. Huang, Y., Lichtenberger, LM., Taylor, M., et al. Antitumor and antiangiogenic effects of Aspirin-PC in ovarian cancer. Mol Cancer Ther, 2016, 15(12), p. 2894–2904.
31. Huo, X., Yao, L., Han, X., et al. Hysterectomy and risk of ovarian cancer: a systematic review and meta-analysis. Arch Gynecol Obstet, 2019, 299(3), p. 599–607.
32. Iversen, L., Fielding, S., Lidegaard, C., et al. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ, 2018, 362, p. k3609.
33. Iversen, L., Sivasubramaniam, S., Lee, AJ., et al. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners‘ Oral Contraception Study. Am J Obstet Gynecol, 2017, 216(6), p. 580.e1–580.e9.
34. Köbel, M., Kalloger, SE., Lee, S., et al. Ovarian Tumor Tissue Analysis consortium. Biomarker-based ovarian carcinoma typing: a histologic investigation in the ovarian tumor tissue analysis consortium. Cancer Epidemiol Biomarkers Prev, 2013, 22(10), p. 1677–1686.
35. Kolahdooz, F., Ibiebele, TI., van der Pols, JC., Webb, PM. Dietary patterns and ovarian cancer risk. Am J Clin Nutr, 2009, 89(1), p. 297–304.
36. Kolahdooz, F., van der Pols, JC., Bain, CJ., et al. Meat, fish, and ovarian cancer risk: Results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am J Clin Nutr, 2010, 91(6), p. 1752–1763.
37. Kotsopoulos, J., Lubinski, J., Gronwald, J., et al. Hereditary Breast Cancer Clinical Study Group. Factors influencing ovulation and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Int J Cancer, 2015, 137(5), p. 1136–1146.
38. Li, DP., Du, C., Zhang, ZM., et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pac J Cancer Prev, 2014, 15(12), p. 4829–4837.
39 Liu, Y., Warren Andersen, S., Wen, W., et al. Prospective cohort study of general and central obesity, weight change trajectory, and risk of major cancers among Chinese women. Int J Cancer, 2016, 139(7), p. 1461–1470.
40. Madsen, C., Baandrup, L., Dehlendorff, C., Kjaer, SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand, 2015, 94(1), p. 86–94.
41. Marchetti, C., De Felice, F., Palaia, I., et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all-cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health, 2014, 14, p. 150.
42. McAlpine, JN., Tone, AA., Hanley, GE. Opportunistic salpingectomy: We chose to act, not wait. J Obstet Gynaecol Can, 2016, 38(5), p. 425–427.
43. McNamara, C., Abbott, SE., Bandera, EV., et al. Tubal ligation and ovarian cancer risk in African American women. Cancer Causes Control, 2017, 28(10), p. 1033–1041.
44. Merritt, MA., De Pari, M., Vitonis, AF., et al. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum Reprod, 2013, 28(5), p. 1406–1417.
45. Milne, RL., Osorio, A., Ramón y Cajal, T., et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat, 2010, 119(1), p. 221–232.
46. Nagle, CM., Dixon, SC., Jensen, A., et al. Ovarian Cancer Association Consortium. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer, 2015, 113(5), p. 817–826.
47. Narod, SA., Dubé, MP., Klijn, J., et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst, 2002, 94(23), p. 1773–1779.
48. Narod, SA., Sun, P., Ghadirian, P., Lynch, H., et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet, 2001, 357(9267), p. 1467–1470.
49. Pal, S., Bhattacharjee, A., Ali, A., et al. Chronic inflammation and cancer: potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J Inflamm (Lond), 2014, 11, p. 23.
50. Pan, SY., Ugnat, AM., Mao, Y., et al. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev, 2004, 13(9), p. 1521–1527.
51. Pan, SY., Ugnat, AM., Mao, Y. Physical activity and the risk of ovarian cancer: a case control study in Canada. Int J Cancer, 2005, 117(2), p. 300–307.
52. Park, B., Park, S., Shin, HR., et al. Population attributable risks of modifiable reproductive factors for breast and ovarian cancers in Korea. BMC Cancer, 2016, 16, p. 5.
53. Peres, LC., Alberg, AJ., Bandera, EV., et al. Premenopausal hysterectomy and risk of ovarian cancer in African-American women. Am J Epidemiol, 2017, 186(1), p. 46–53.
54. Peres, LC., Camacho, F., Abbott, SE., et al. Analgesic medication use and risk of epithelial ovarian cancer in African American women. Brit J Cancer, 2016, 114, p. 819–825.
55. Purdie, DM., Bain, CJ., Siskind, V., et al. Hormone replacement therapy and risk of epithelial ovarian cancer. Br J Cancer, 1999, 81(3), p. 559–563.
56. Qiu, W., Lu, H., Qi, Y., Wang, X. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget, 2016, 7(24), p. 37390–37406.
57. Rebbeck, TR., Kauff, ND., Domchek, SM. Meta-analysis of risk reduction estimates associated with risk reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst, 2009, 101, p. 80–87.
58. Rice, MS., Murphy, MA., Vitonis, AF., et al. Tubal ligation, hysterectomy and epithelial ovarian cancer in the New England Case-Control Study. Int J Cancer, 2013, 133(10), p. 2415–2421.
59. Rice, MS., Hankinson, SE., Tworoger, SS. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses‘ Health Studies. Fertil Steril, 2014, 102(1), p. 192–198.e3.
60. Riman, T., Dickman, PW., Nilsson, S., et al. Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol, 2004, 19(11), p. 1011–1019.
61. Rodriguez, C., Calle, EE., Fakhrabadi-Shokoohi, D., et al. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev, 2002, 11(9), p. 822–828.
62. Siegel, R., Ma, J., Zou, Z., Jemal, A. Cancer statistics, 2014. CA Cancer J Clin, 2014, 64(1), p. 9–29.
63. Sieh, W., Salvador, S., McGuire, V., et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol, 2013, 42(2), p. 579–589.
64. Sung, HK., Ma, SH., Choi, JY., et al. The effect of breastfeeding duration and parity on the risk of epithelial ovarian cancer. A systemic review and meta-analysis. J Prev Med Public Health, 2016, 49(6), p. 349–366.
65. Tabung, FK., Huang, T., Giovannucci, EL., et al. The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer, 2017, 117(6), p. 907–911.
66. Tavani, A., Gallus, S., La Vecchia, C., et al. Physical activity and risk of ovarian cancer: an Italian case-control study. Int J Cancer, 2001, 91, p. 407–411.
67. Tinelli, A., Malvasi, A., Leo, G., et al. Hereditary ovarian cancers: from BRCA mutations to clinical management. A modern appraisal. Cancer Metastasis Rev, 2010, 29(2), p. 339–350.
68. Trabert, B., Ness, RB., Lo-Ciganic, WH., et al. Ovarian Cancer Association Consortium. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst, 2014, 106(2), djt431.
69. Tzonou, A., Hsieh, CC., Polychronopoulou, A., et al. Diet and ovarian cancer: a case-control study in Greece. Int J Cancer, 1993, 55(3), 4, p. 11–414.
70. Vachon, CM., Mink, PJ., Janney, CA., et al. Association of parity and ovarian cancer risk by family history of breast or ovarian cancer in a population-based study of postmenopausal women. Epidemiology, 2002, 13(1), p. 66–71.
71. Vaughan, LE., Prizment, A., Blair, CK., et al. Aspirin use and the incidence of breast, colon, ovarian, and pancreatic cancers in elderly women in the Iowa Women‘s Health Study. Cancer Causes Control, 2016, 27(11), p. 1395–1402.
72. Victora, CG., Bahl, R., Barros, AJ., et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet, 2016, 387(10017), p. 475–490.
73. Whiteman, MK., Hillis, SD., Jamieson, DJ., et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol, 2008, 198(1), 34.e1–7.
74. Xia, L., Tan, S., Zhou, Y., et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther, 2018, 11, p. 2063–2073.
75. Yoon, SH., Kim, SN., Shim, SH., et al. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: A meta-analysis. Eur J Cancer, 2016, 55, p. 38–46.
76. Yuhara, H., Corley, DA., Nakahara, F., et al. Aspirin and non-aspirin NSAIDs increase risk of colonic diverticular bleeding: a systematic review and meta-analysis. J Gastroenterol, 2014, 49, p. 992–1000.
77. Zhang, D., Bai, B., Xi, Y., et al. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol Oncol, 2016, 142(2), p. 368–377.
78. Zhang, M., Holman, CD., Binns, CW. Intake of specific carotenoids and the risk of epithelial ovarian cancer. Br J Nutr, 2007, 98(1), p. 187–193.
79. Zhang, M., Lee, AH., Binns, CW. Physical activity and epithelial ovarian cancer risk: a case-control study in China. Int J Cancer, 2003, 105(6), p. 838–843.
80. Zhang, M., Lee, AH., Binns, CW. Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol Oncol, 2004, 92(1), p. 320–326.
81. Zhang, M., Yang, ZY., Binns, CW., Lee, AH. Diet and ovarian cancer risk: a case-control study in China. Br J Cancer, 2002, 86(5), p. 712–717.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2020 Issue 1-
All articles in this issue
- Assisted reproduction trends in Czech Republic National Assisted Reproduction Register 2007–2017
- Transabdominal punction of follicular fluid in IVF cyclus in a patient after ovarian transposition
- Bilateral simultaneous tubal pregnancy
- Thrombotic microangiopathy and pregnancy
- Severe thrombotic microangiopathy accompanied by liver rupture and multiorgan failure at week 26 of pregnancy
- Echinococcosis with the image of peritoneal carcinomatosis
- Lactobacillus crispatus dominant vaginal microbita in pregnancy
- The effects of prenatal, perinatal and neonatal factors on academic performance in primary school age children
- Noninvasive prenatal testing: benefits and limitations of the available tests
- Can we prevent ovarian cancer?
- Patients experiences after hospitalization at Gynecologic Obstetrics Department: Identifying parts for improvement
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Thrombotic microangiopathy and pregnancy
- Lactobacillus crispatus dominant vaginal microbita in pregnancy
- Noninvasive prenatal testing: benefits and limitations of the available tests
- Can we prevent ovarian cancer?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


