-
Medical journals
- Career
Preparation and evaluation of indomethacin loaded alginate microspheres
Authors: Penjuri Subhash Chandra Bose; Ravouru Nagaraju; Damineni Saritha; Budumuru Padmasri; Poreddy Srikanth Reddy
Authors‘ workplace: Department of Pharmaceutics, MNR College of Pharmacy, Sangareddy, Telangana, India 1; Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Visvavidyalayam, India 2; Department of Pharmaceutics, Sultan-ul-Uloom College of Pharmacy, Hyderabad, Telangana, India 3
Published in: Čes. slov. Farm., 2016; 65, 104-110
Category: Original Articles
Overview
Indomethacin-loaded alginate microspheres were prepared by the ionic cross linking technique using calcium chloride. The effect of calcium chloride concentration was evaluated with respect to the size, entrapment efficiency and shape (sphericity) of the particles. The entrapment efficiency and in vitro release profiles were found to be altered by changing various formulation parameters. The desired indomethacin in vitro release profile (as per USP specifications for extended release formulations) was obtained from microspheres prepared from gel containing 2% of sodium alginate and 2% of methyl cellulose hardened in 3% calcium chloride solution. The kinetic modeling of the release data indicated that indomethacin release from alginate microspheres followed Higuchi model and the release mechanism was diffusion. FTIR study confirmed the absence of any drug polymer interaction. DSC and XRD studies revealed that the crystallinity of the drug decreased when loaded in the alginate microspheres. The pharmacokinetic parameters were also evaluated in rabbits using HPLC technique and it was found that indomethacin loaded microspheres showed increased t1/2 and AUC values. Ke value was less than that of pure drug. This confirmed controlled release of the drug from microspheres leading to more residence time in the body within the therapeutic range providing longer duration of action which is preferable in chronic treatment of the diseases.
Key words:
indomethacin • controlled release • Fickian diffusion • pharmacokineticsIntroduction
Indomethacin is an important indole acetic acid non-steroidal anti-inflammatory drug which is used in the treatment of rheumatoid arthritis and other severe inflammatory diseases1, 2). Indomethacin is an inhibitor of prostaglandin synthesis and is used for several inflammatory diseases, but in recent years indomethacin has also been recommended as the treatment of choice for low birth weight infants with ductus arteriosus. Patients with Bartter’s syndrome have been treated successfully with indomethacin.
Indomethacin decreases the duration of morning stiffness. It relieves pain, reduces swelling and tenderness of the joints. In these actions it is 10 to 40 times more potent when compared to other salicylates. The main side effects of indomethacin are ulceration of the entire upper GIT, sometimes with perforations and hemorrhage and these associated adverse effects are due to initial high plasma concentration3) and are dose-related4). Occult blood loss may lead to anemia in the absence of ulceration4). In the case of rheumatoid arthritis, the frequent administration of the drug into the intra-articular cavity is very difficult. Oral conventional dosage forms are administered three or four times a day to maintain adequate and effective therapeutic concentration in blood, which is responsible for the occurrence of high initial peak plasma concentrations. However, it fails to protect the patients against morning stiffness5). The incidence and severity of side effects with indomethacin can limit its therapeutic activity. So controlled release is better to avoid the incidence and severity of side effects, moreover indomethacin fulfills many biopharmaceutic characteristics required for controlled release, as apparent partition coefficient of the drug is 1, dissociation constant of indomethacin is 4.5, which is ideal for acidic drugs, stable in the GI environment, it is not absorbed by carrier-mediated transport processes, it is rapidly and almost completely absorbed from the gastrointestinal tract after oral administration6). The aim of this work is to formulate and characterize alginate microspheres for the controlled delivery of indomethacin and to carry out pharmacokinetic evaluation of the optimized formulation.
Materials and methods
Indomethacin (gift sample from Dr. Reddy’s labs, Hyderabad, India), sodium alginate, calcium chloride and methylcellulose (Loba Chemie Pvt. Ltd., Mumbai, India). All the chemicals, solvents and reagents were of analytical grade.
Preparation of alginate microspheres7)
Indomethacin alginate microspheres were prepared by ionotropic external gelation technique. Sodium alginate/methyl cellulose dispersion was prepared by dispersing specified quantity of sodium alginate and methyl cellulose in a specified quantity of distilled water with vigorous stirring and the specific quantity of indomethacin was dispersed with vigorous stirring (Table 1).
1. Formulation chart of gels for indomethacin microspheres 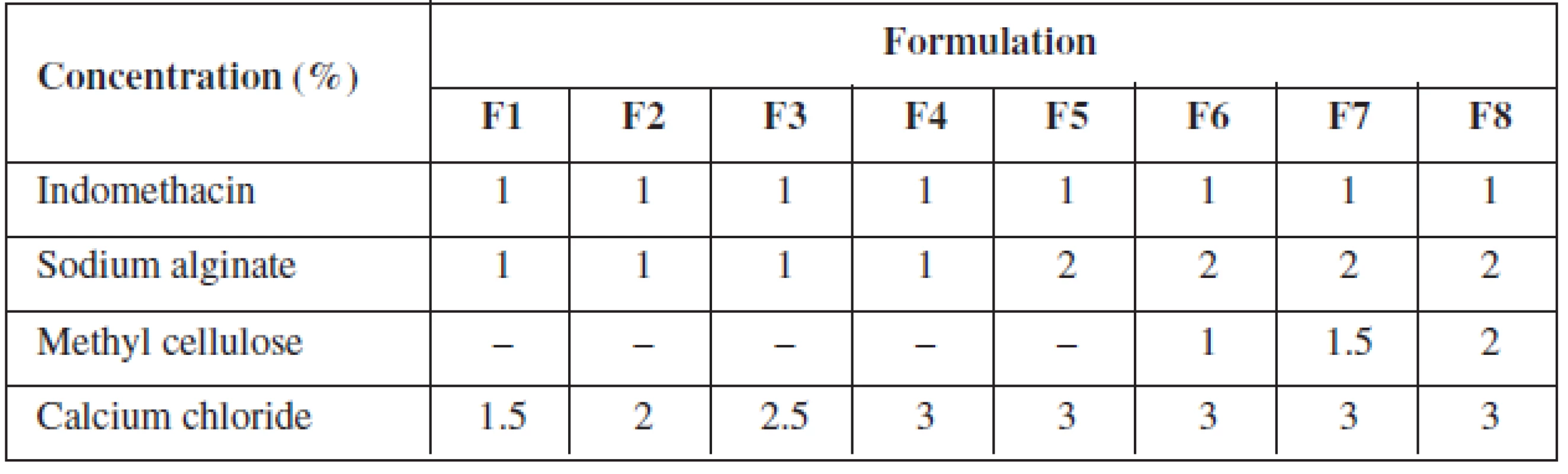
The indomethacin polymer mixture was added slowly drop by drop with the help of a 20-gauge hypodermic needle fitted with a 10 ml syringe into calcium chloride solution, which resulted in the formation of microspheres. The microspheres were left aside for two hours in the calcium chloride solution, filtered and rinsed with distilled water. The microspheres were air-dried for 24 hrs at room temperature.
Entrapment efficiency8)
Microspheres were powdered in a mortar and indomethacin was extracted from microspheres using ethanol, filtered and analyzed UV spectrophotometrically (1601 A, Schimadzu Corporation, Japan) after necessary dilution at 320 nm. Entrapment efficiency was calculated using the formula
Entrapment efficiency =
[(Actual Loading/Theoretical Loading) ⋅ ×100] %.
Particle size measurement9)
Particle size and size distribution of different microsphere formulations were measured using an optical microscope (Olympus, Model HB, India), and the mean particle size was calculated by measuring 200 particles with the help of a calibrated ocular micrometer. The average particle size was expressed as the volume mean diameter in micrometers.
Fourier Transform Infrared Spectroscopy10, 11)
The indomethacin-loaded alginate microspheres were finely ground with KBr to prepare the pellets under a hydraulic pressure of 600 pounds for square inch and spectra were scanned between 400 and 4000 cm–1 (Shimadzu-8400 S, Japan) to confirm the presence of any interaction between the polymers and indomethacin.
Differential Scanning Calorimetry 10, 11)
DSC thermograms of indomethacin and indomethacin-loaded sodium alginate microspheres were recorded using a modulated differential scanning calorimeter (DSC-60, Schimadzu Corporation, Japan). The analysis was performed by heating the 2–3 mg samples on an aluminium crimp pans at a rate of 10 °C/min in a nitrogen atmosphere (50 ml, min–1) to know the thermal behaviour of the samples.
XRD Studies10, 11)
The X-ray diffraction pattern of the microspheres were recorded using a Rigaku Geigerflex diffractometer (Japan), equipped with Ni-filtered CuKα radiation (λ = 1.5418A°), goniometer speed-2°/min voltage – 30 Kv and current – 20 mA to know the crystallinity of the drug dispersed in the microspheres.
Scanning Electron Microscopy12)
SEM images of the indomethacin-loaded microspheres were recorded using a Hitachi S520 scanning electron microscope (Japan) at the required magnification. A working distance of 33.5 mm was maintained and the acceleration voltage used was 10 KV with the secondary electron image (SEI) as a detector to know the shape of the microspheres. Prior to examination, the samples were gold-coated under vacuum to render them electrically conductive.
Determination of sphericity13)
To determine sphericity, the tracings of microspheres (magnification 45× ⋅) were taken on a black paper using a Lucida camera, (Model-Prism type, Rolex, India) and the circulatory factor was calculated using the equation:
S = p2/(12.56 ⋅× A), [1]
where A is area (cm2) and p is perimeter (cm).
Dissolution studies14)
The dissolution studies of indomethacin and indomethacin microspheres were determined using a USP dissolution apparatus type II (Electrolab, Mumbai, India). The dissolution medium used was 900 ml of pH 7.2 phosphate buffer. 5 ml of sample solutions were withdrawn at predetermined time intervals (0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 hours) and then filtered through Whatman filter paper (No. 40). 5 ml of pH 7.2 phosphate buffer were replaced in the dissolution flask to maintain sink conditions. The amount of released indomethacin was analyzed spectrophotometrically at 320 nm.
Evaluation of release kinetics15)
To investigate the mechanism of indomethacin release from optimized formulation, the release data was analyzed for zero order, first order, Higuchi model, Hixon-Crowell and Korsmeyer-Peppas model. The data were presented in the following graphical representation and regression analysis was performed.
Mt is the cumulative % of drug released at time t.
Korsmeyer et al.16) derived a simple relationship which described drug release from a polymeric system:
Mt/M∞ = ktn , [2]
where Mt/M∞ is the fraction of drug released at time t, k is the rate constant and n is the release exponent. Release curve where Mt/M∞ < 0.6 was used to determine the exponent ‘n’ value. The n value was used to characterize different release mechanisms. For example, n = 0.45 for Case I or Fickian diffusion, 0.45 < n < 0.89 for anomalous behaviour or non-Fickian transport, n = 0.89 for Case II transport, and n > 0.89 for Super Case II transport. Fickian diffusional release occurs by the usual molecular diffusion of the drug due to a chemical potential gradient. Case II relaxational release is the drug transport mechanism associated with stresses and state-transition in hydrophilic glassy polymers, which swell in water or biological fluids. This term also includes polymer disentanglement and erosion. The rate constant ‘k’, coefficients of correlation (R2) and ‘n’ of each model were calculated by linear regression analysis.
HPLC analysis17)
Plasma samples analysis were performed using a Schimadzu (Japan) HPLC system equipped with a SPD-20A tunable absorbance detector and a Luna 5u C18 (2) 100 A0 column (250 mm ⋅× 4.6 mm I.D) at ambient temperature (23–27 °C). The mobile phase was a mixture of 400 ml of sodium acetate buffer pH 3.6 and 600 ml of acetonitrile. The solution was filtered through 0.45 μm filter and degassed by sonication. The flow rate was 1 ml per minute. Detection was carried on at 320 nm wavelength. Prepared samples were injected to a HPLC column through a Rheodyne injector (HAMILTON, 702 NR) fitted with a 20 μl loop. A calibration curve was plotted for indomethacin in the range of 1–5 μg/ml. A good linear relationship was observed between the concentration of indomethacin and its peak area (R2 = 0.9972).
Pharmacokinetic evaluation of the optimized formulation18–20)
The optimized indomethacin microspheres were further evaluated for pharmacokinetic parameters. Pharmacokinetic study protocol was approved by the IAEC (Reg. No. IAEC/MNRCOP/CPCSEA/2013). Six male/female adult rabbits weighing between (1.5–2 kg) were used for the study. The animals were housed in individual cages under standard laboratory conditions of light, temperature and relative humidity. The study was conducted as open randomized design in which a single dose 10 mg/kg was administered to rabbits. The animals were divided into 2 groups containing 3 animals in each. For one group pure indomethacin was given as suspension with 1% sodium carboxy methyl cellulose and for another group indomethacin microspheres were given. After clipping off the hair on the ears of the rabbits, the blood sample of 1 ml was withdrawn at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8 and 12 hr intervals, using a 1 ml syringe with a 26 gauze needle (nominal outer diameter, 0.4636 mm, inner diameter, 0.260 mm and wall thickness, 0.101 mm). The withdrawn samples were collected in Eppendorf tubes containing 0.4 ml of 3% sodium citrate solution and centrifuged (Remi equipments, Mumbai, India) at 2,500 rpm for 15 min to separate plasma and analyzed for drug content by using HPLC (Schimadzu, Japan).
Results and discussion
Entrapment efficiency
All formulations showed entrapment efficiency in the range from 64.17% to 86.33% (Table 2). Formulation F1 showed the lowest and F8 showed the highest entrapment efficiency, respectively. The entrapment efficiency increased progressively with increasing alginate concentration in formulations F1 to F4. An increase in the alginate concentration resulted in the formation of larger microspheres entrapping greater amounts of the drug. This may be attributed to the greater availability of the active calcium-binding sites in the polymeric chains and, consequently, the greater degree of cross-linking as the quantity of sodium alginate increased21). The decreased incorporation efficiency in F5 may be due to increased concentration of CaCl2, which could be attributed to the formation of porous beads ensuring the diffusion of the drug out of the beads at the time of curing22). The entrapment efficiency increased progressively with increasing alginate and methyl cellulose concentration in formulations F6 to F7.
2. Entrapment efficiency and particle size of formulation 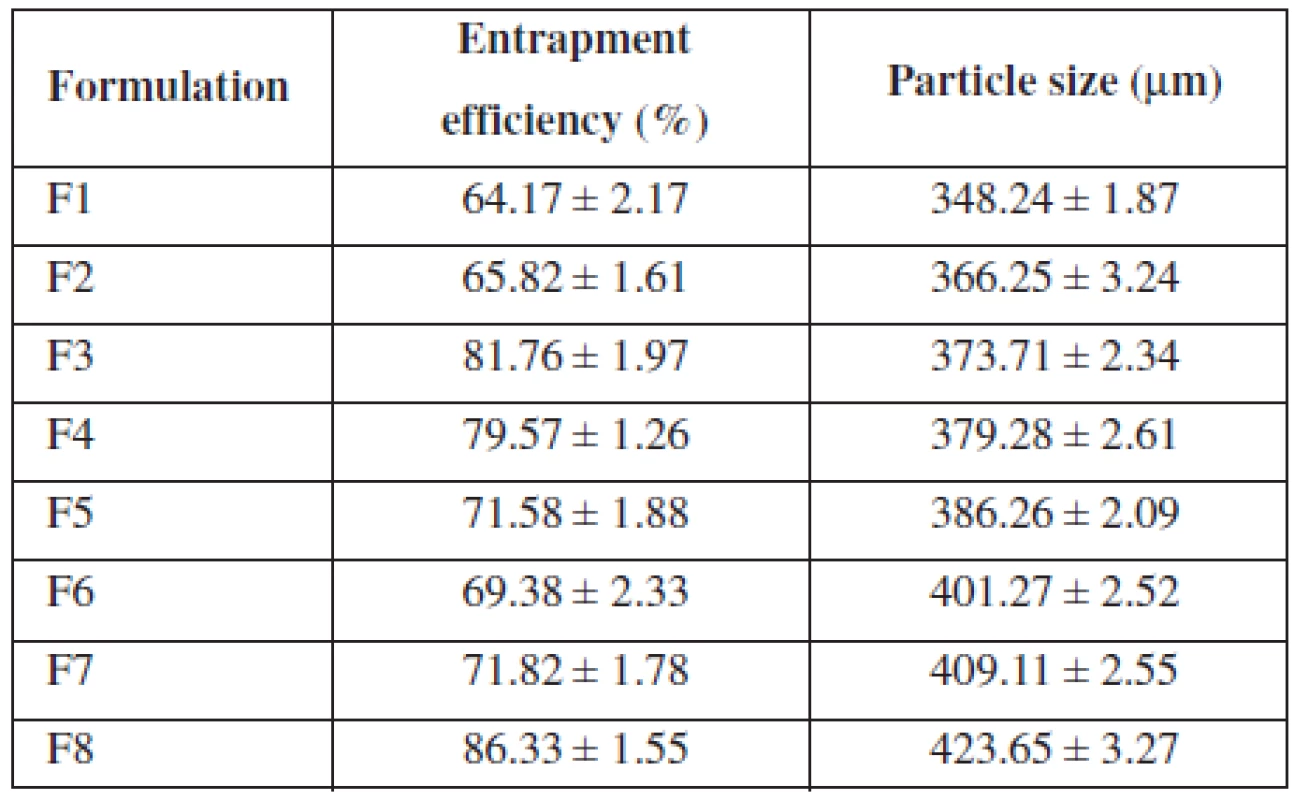
Particle size measurement
The mean particle size of the various formulations of alginate microspheres were between 348.24 ± 1.87 μm to 423.65 ± 3.27 μm. It was found that the particle size distribution of each formulation was within a narrow range but the mean particle size was different among the formulations (Table 2). The results indicated the proportional increase in the mean particle size of the microspheres with increasing amounts of sodium alginate in the formulations F1, F2, F3, F4 and F5 and sodium alginate and methylcellulose in the formulations F6, F7 and F8. This could be probably attributed to an increase in the relative viscosity at higher concentration of sodium alginate and formation of large droplets during addition of the polymer solution to the cross-linking agents23).
Fourier Transform Infrared Spectroscopy
The FTIR spectra for pure indomethacin and indomethacin-loaded alginate microspheres are shown in Figure 1. Pure indomethacin showed characteristic bands at 3400–2500 cm–1 (aromatic C-H stretching), 1716.53 cm–1 and 1689.53 cm–1 (C = O stretching), 1589.23 cm–1 (aromatic C = C stretching), 1454.23 cm–1 (O-CH3 deformation), 1234.36 cm–1 (C-O stretch plus O-H deformation), 925.77 cm–1 (carboxylic O-H out of plane deformation) and 900–600 cm–1 (C-H out of plane deformation for the substituted aromatic ring), where as indomethacin microspheres showed characteristic bands with lower intensity indicating the chemical stability of indomethacin in alginate microspheres24).
Fig. 1. FTIR spectra of pure indomethacin and indomethacin microspheres 
Differential Scanning Calorimetry
The DSC thermograms of pure indomethacin and indomethacin-loaded microspheres are shown in Figure 2. Indomethacin showed a sharp endothermic peak at 159.88 °C indicating the melting of the drug, whereas indomethacin-loaded alginate microspheres showed a small endothermic peak with low intensity at 142 °C which demonstrated the reduction in drug crystallinity and molecular dispersion of the drug in the polymer matrix24, 25).
Fig. 2. DSC thermograms of pure indomethacin and indomethacin microspheres 
XRD Studies
XRD spectra of pure indomethacin and indomethacin-loaded microspheres are shown in Figure 3. Pure indomethacin showed many peaks due to its crystalline nature, whereas XRD spectrum of indomethacin-loaded sodium alginate microspheres did not show any peaks indicating the amorphous molecular dispersion of indomethacin in microspheres24, 26).
Fig. 3. XRD spectra of pure indomethacin and indomethacin microspheres 
Scanning Electron Microscopy
SEM photograph showed that the indomethacin microspheres were spherical in nature with smooth surfaces with inward dents and shrinkage due to the collapse of the wall of the microspheres27) (Fig. 4). The sphericity factor calculated for the microspheres are nearer to value 1 (1.024 ± 0.02, 1 ± 0.007, 0.994 ± 0.005, 1.004 ± 0.005, 0.994 ± 0.005, 0.996 ± 0.005, 1.002 ± 0.004 and 1.016 ± 0.02 for the formulations F1 to F8), which confirms the prepared formulations are spherical in nature.
Fig. 4. SEM photograph of indomethacin microspheres 
Dissolution Studies
Faster release observed in some formulations could be related to the lesser amount of the polymer in formulation as well as small size of microspheres which might be due to the fact that smaller particles offered more surface area to release the drug28, 29). A slower release pattern was observed for formulation containing higher amounts of the polymer and larger size30, 31). The effect of sodium alginate and methylcellulose concentration on indomethacin release from microspheres is shown in Figure 5. It can be observed that with increasing polymer concentration, therate of drug release from the microspheres decreases dramatically. The formulations containing relatively higher polymer contents showed less initial drug release due to the unavailability of drug molecules at the surface of microspheres. Moreover, an increase in the concentration of the polymer in microspheres retarded the formation of pores or channels by drug particles in the matrix. This in turn affected the leaching and diffusion of drug from the matrix, and thus the drug release rate was lowered. Based on controlled drug release (over a period of 12 hr) and good entrapment efficiency, F8 was selected as optimized formulation and was used for pharmacokinetic evaluation.
Fig. 5. In vitro dissolution profile of indomethacin microspheres 
To study the release kinetics of optimized formulation, obtained in vitro release data were fitted in various kinetic models such as zero order, first order, Higuchi model, Hixson-Crowell model and Korsmeyer-Peppas model (Table 3). The in vitro release profile of F8 could be best expressed by Higuchi kinetic model, as the plot showed highest linearity (R2: 0.9029) indicating that the release of indomethacin was diffusion-controlled . During the dissolution studies microspheres did not burst, but extended swelling occurred and thus good conditions for diffusion of the drug from microspheres were created32, 33).
3. Determination coefficient (R2) of different kinetic models of F8 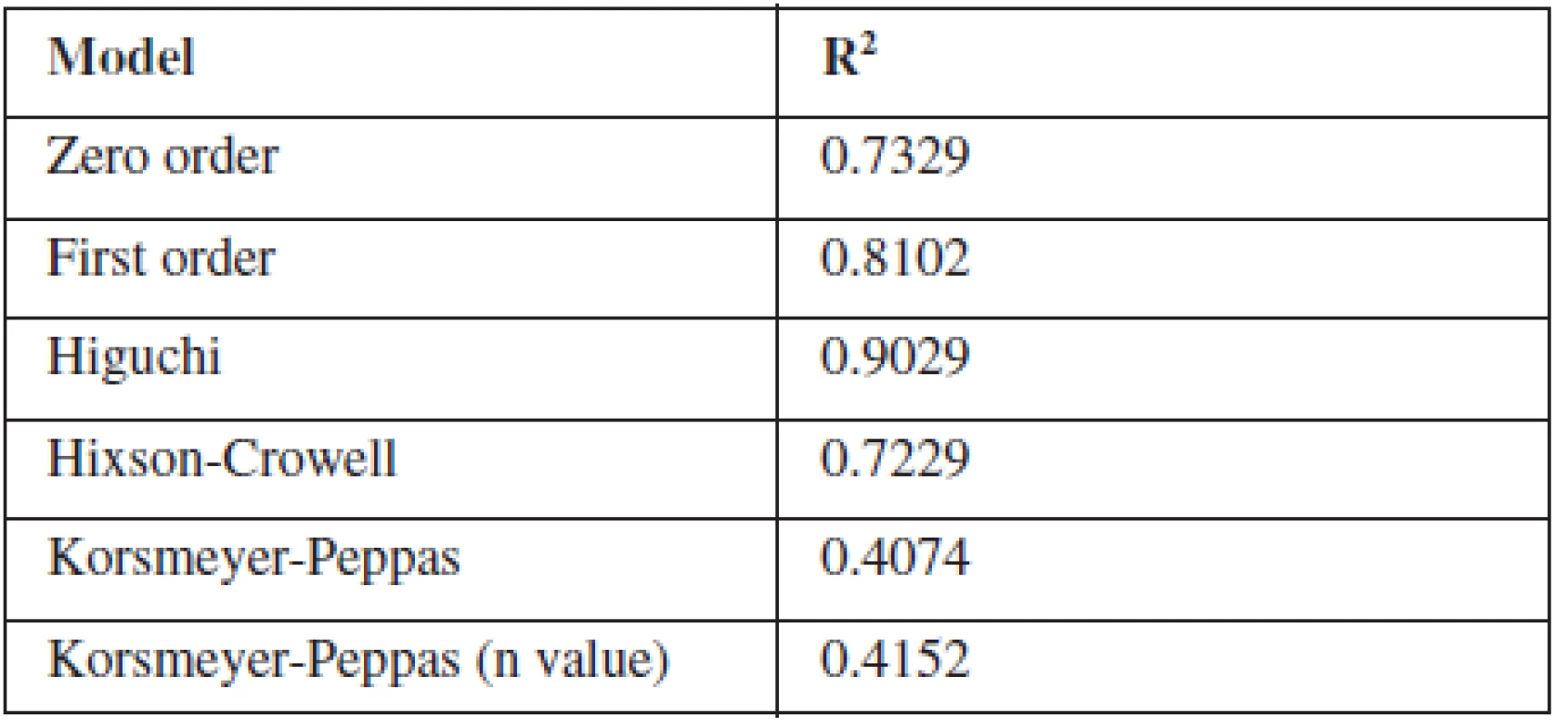
Pharmacokinetic evaluation of the optimized formulation18)
The elimination rate constant Ke of indomethacin when given in microspheres (0.092 hr–1) was found to be decreased in comparison with pure indomethacin (0.184 hr–1) (Table 4). When microspheres were administered, about a two-fold decrease in elimination rate was observed indicating a slow elimination of the drug from the body. The elimination half life (t½1/2) of the indomethacin from microspheres was found to be 6.861 hours indicating a slow elimination and longer residence of the drug in the body which was further evidenced by the increased AUC value of the drug when given in microspheres. Almost a two-fold increase in AUC was observed when the drug was loaded in microspheres. The absorption rate constant Ka of indomethacin when given in microspheres was found to be decreased when comparing to pure indomethacin indicating a slower absorption of the drug because of slow release of the drug from the cross links of the polymer matrix.
4. Pharmacokinetic parameters of indomethacin 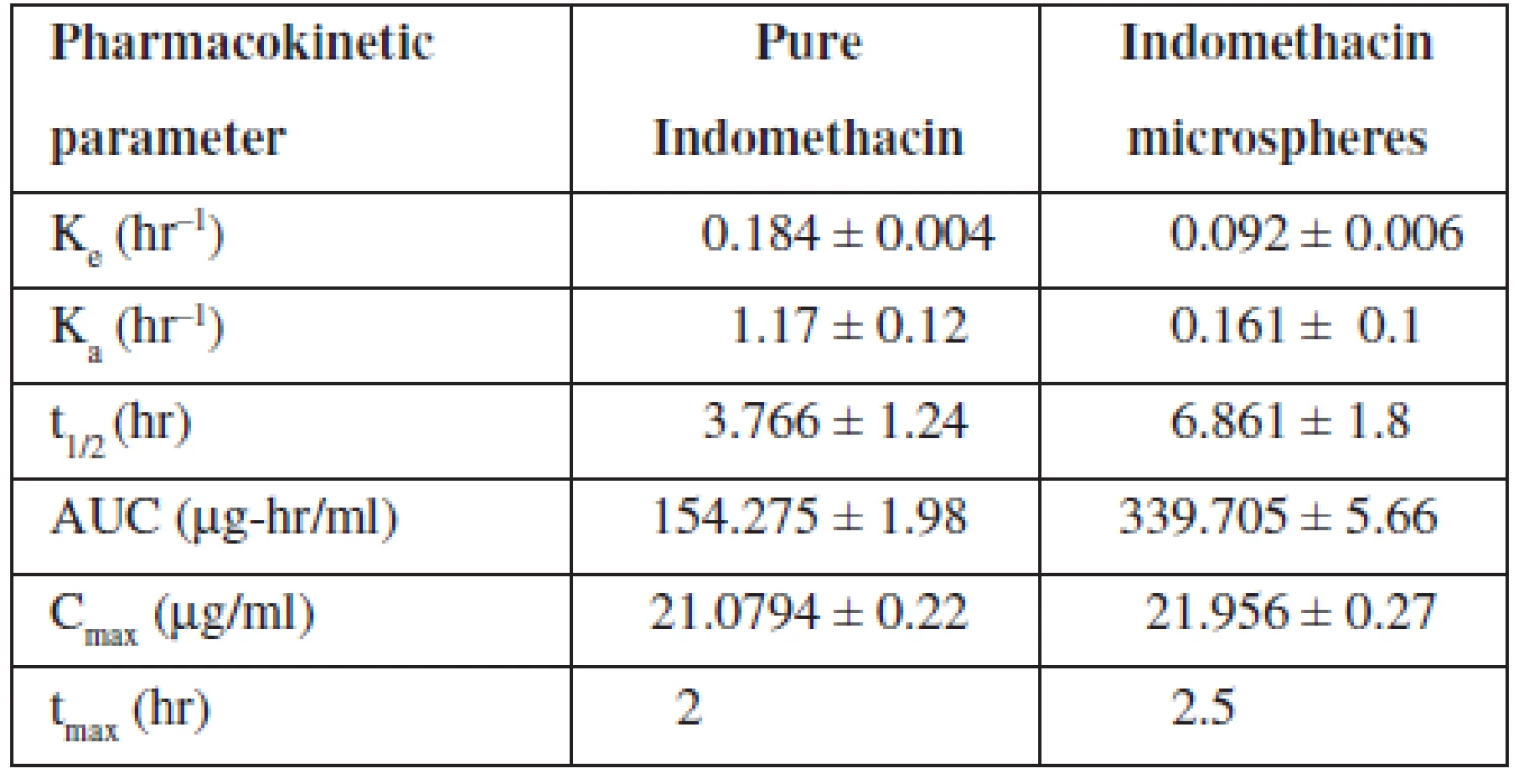
However, there was no significant change in time required to reach the maximum drug concentration in plasma observed between pure indomethacin and indomethacin-loaded microspheres (tmax for pure indomethacin, 2 hours and tmax indomethacin-loaded microspheres, 2.5 hours). But the Cmax was found to be the same for pure indomethacin as well as indomethacin loaded in controlled release Microspheres (Fig. 6). Significant changes in Ke, t1/2, Ka and AUC values of the drug when administered as microspheres clearly indicated that the microspheres developed in the present study showed a controlled release of the drug confirming the results of in vitro studies.
Fig. 6. Mean plasma concentration-time profiles of indomethacin 
Conclusion
From the present study it can be concluded that indomethacin controlled release microspheres can be formulated using sodium alginate, methyl cellulose and calcium chloride. Prepared microspheres exhibited a spherical shape, good entrapment efficiency up to 87% and controlled drug release over a period of 12 hours with no drug-excipient interactions. Based on in vivo pharmacokinetic data, it can be concluded that the indomethacin microspheres released the drug in controlled manner leading to prolonged drug residence and therapeutic effect. Both in vitro and in vivo studies confirmed the advantage of control release of indomethacin in the effective therapeutic management of inflammation and proved to be better than conventional dosage form with substantial decrease in side effects.
Acknowledgements
We thank the MNR educational trust, Sangareddy for their valuable support to carry out this research.
Conflict of interest: none.
Received 11 Januar 2016
Accepted 21 June 2016
Dr. Penjuri Subhash Chandra Bose • Dr. Poreddy Srikanth Reddy
Department of Pharmaceutics, MNR College of Pharmacy
Sangareddy, Telangana, India
e-mail: penjurisubhash@gmail.com
Dr. Ravouru Nagaraju • Budumuru Padmasri
Institute of Pharmaceutical Technology,
Sri Padmavathi Mahila Visvavidyalayam (Women’s University),
Tirupati, Andhra Pradesh, India
Dr. Damineni Saritha
Department of Pharmaceutics
Sultan-ul-Uloom College of Pharmacy
Hyderabad, Telangana, India
Sources
1. Salib S., Donney S., Doyle D. Therapy and drugs in the control of osteoartrities. Prescr. 1996; 8, 41–59.
2. Brahmankar M., Sunil Jaiswal B. Controlled Release Medication. In: Biopharmaceutics and Pharmacokinetics A treatise. 1st ed. New Delhi: Vallabh Prakashan 2005.
3. Jackson Roberts L., Jason D. M. Analgesic antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Joel Hardman G., Lee Limbird E., Alfred Goodman Gilman. The Pharmacological Basis of Therapeutics, 10th ed. London: Mc Graw-Hill 2001.
4. Gowda D. V., Shivakumar H. G. Comparative Bioavailability studies of Indomethacin from Two-controlled release formulations in healthy albino sheep. Ind. J. Pharm. Sci. 2006; 68, 760–763.
5. Monographs. In: Indian Pharmacopoeia 1996, Volume I (A–O), The controller of Publications, Delhi, 393–395.
6. Gunnar A., Orme M., Bertilsson L., Ekstrand R., Palmer L. Pharmacokinetics of Indomethacin. Clin. Pharm. Ther. 1975; 18, 364–373.
7. Mallikarjun Reddy K., Ramesh babu V., Krishna Rao K. S. V., Subha M. C. S., Chowdoji Rao K., Sairam Aminabhavi T. M. Temperature sensitive Semi-IPN microspheres from sodium alginate and N-iso propylacrylamide for controlled release of 5-fluorouracil. J. Appl. Polym. Sci. 2007; 107, 2820–2829.
8. Bodmeier R., Chen H. Preparation and characterization of microspheres containing the anti-inflammatory agents, indomethacin, ibuprofen, and ketoprofen. J. Control. Release. 1989; 10, 167–175.
9. Anande N. M., Jain S. K., Jain N. K. Con-A conjugated mucoadhesive microspheres for the colonic delivery of Diloxanide furoate. Int. J. Pharma. 2008; 359, 182–189.
10. Babay D., Hoffman A., Bentia S. Design and release kinetic pattern evaluation of indomethacin microspheres intended for oral administration. Biomaterials 1988; 9, 482–488.
11. Malamataris S., Avgerinos A. Controlled release indomethacin microspheres prepared by using an emulsion solvent-diffusion technique. Int. J. Pharm. 1990; 62, 105–111.
12. Basu S. K., Kunchu K., Mani R. Evaluation of ketorolac tromethamine microspheres by chitosan/gelatin B complex coacervation. Sci. Pharm. 2010; 78, 79–92.
13. Paradhkar A. R., Pawar A. P., Chordia J. K., Patil V. B., Ketkar A. R. Spherical crystallization of celecoxib. Drug Dev. Ind. Pharm. 2002; 28, 1213–1220.
14. Indian Pharmacopoeia, Ministry of Health and Family welfare, The Indian Pharmacopoeia commission, Ghaziabad, 6th edition 2010, PP 559, 560, 1482, 1495, 1758.
15. Brazel C. S., Peppas N. A. Modelling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000; 49, 47–58.
16. Peppas N. A. Analysis of Fickian and non - Fickian drug release from polymers. Pharm. Acta. Helv. 1985; 60, 110–111.
17. Boon V., Glass B., Nimmo A. High-performance liquid chromatographic assay of indomethacin in porcine plasma with applicability to human levels. J. Chromatogr. Sci. 2006; 44, 41–44.
18. Riew K. D., Long J., Rhee J., Lewis S., Kuklo T., Kim Y. J., Yukawa Y., Zhu Y. Time-dependent inhibitory effects of indomethacin on spinal fusion. J. Bone. Joint. Surg. Am. 2003; 85, 632–634.
19. Ahrengart L., Lindgren U., Reinholt F. P. Comparitive study of the effects of radiation, Indomethacin, prednisolone, and ethane – 1--hydroxy-1, 1-diphosphonate (EHDP) in the prevention of ectopic bone formation. Clin. Orthop. Relat. Res. 1988; 229, 265–273.
20. Johnson A. G., Ray J. E. Improved high-performance liquid chromatographic method for the determination of indomethacin in plasma. Ther. Drug. Monit. 1991; 14, 61–65.
21. El-Kamel A. H., Al-Ghoray O. M. N., Hosny. Alginate-diltiazem hydrochloride beads: Optimization of formulation factors, in vitroand in vivo availability. J. Microencapsulation 2003; 2, 211–225.
22. Das M. K., Senapati P. C. Evaluation of furosemide-loaded alginate microspheres Prepared by ionotropic external gelation technique. Acta Pol. Pharm. 2007; 64, 253–262.
23. Das M. K., Senapati P. C. Furosemide-loaded Alginate Microspheres Prepared by Ionic Cross-linking Technique: Morphology and Release Characteristics. Ind. J. Pharm. Sci. 2008; 70, 77–84.
24. Madani F., Jean-claude C. Coating of indomethacin-loaded embolic microspheres for a successful embolization therapy. J. Microencapsul 2008; 25, 121–133.
25. Ofokansi K. C., Okorie O., Adikwu M. U. Biodegradable microspheres based on gelatin–porcine mucin admixtures: in vitro and in vivo delivery studies. Biol. Pharm. Bull. 2009; 32, 1754–1759.
26. Hafner A., Filipovic J., Voinovich D., Jalsenjak I. Development and in vitro characterization of chitosan-based microspheres for nasal delivery of promethazine. Drug Dev. Ind. Pharm. 2007; 33, 427–436.
27. Gowda D. V., Shivakumar H. G. Preparation and evaluation of waxes/fat microspheres loaded with indomethacin for controlled release. Ind. J. Pharm. Sci. 2007; 69, 251–256.
28. Kilicarslan M., Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int. J. Pharm. 2003; 252, 99–109.
29. Gibaud S., Bonneville A., Astier A. Preparation of 3,4-diaminopyridinemicroparticles by solvent-evaporation methods. Int. J. Pharm. 2002; 242, 197–201.
30. Venkatesan P., Muralidharan C., Manavalan R., Valliappan K. Selection of better method for the preparation of microspheres by applying analytic hierarchy process. J. Pharm. Sci. Res. 2009; 1, 64–78.
31. Sahin S., Selek H., Ponchel G., Ercan M. T., Sargon M, Hincal A. A., Kas HS. Preparation, characterization and in-vivo distribution of terbutaline sulfate loaded albumin microspheres. J. Cont. Rel. 2002; 82, 345–358.
32. Sanghvi S. P., Nairn J. G. Effect of viscosity and interfacial tension on particle size of cellulose acetate trimellitate microspheres. J. Microencapsulation 1992; 9, 215–227.
33. Perumal D. Microencapsulation of ibuprofen & Eudragit RS 100 by the emulsion solvent diffusion technique. Int. J. Pharm. 2001; 218, 1–11.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2016 Issue 3-
All articles in this issue
- Rhodiola rosea and its neuropsychotropic effects
- Ageing and Alzheimer disease – system dynamics model prediction
- Preparation and evaluation of indomethacin loaded alginate microspheres
- 44th Conference drug synthesis and analysis – Part 4*
- The influence of hyaluronan addition on thickness, weight, uniformity of mass and water content of mucoadhesive films
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Rhodiola rosea and its neuropsychotropic effects
- Ageing and Alzheimer disease – system dynamics model prediction
- Preparation and evaluation of indomethacin loaded alginate microspheres
- The influence of hyaluronan addition on thickness, weight, uniformity of mass and water content of mucoadhesive films
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


