-
Medical journals
- Career
Clinical characteristics of newly diagnosed Parkinson’s disease patients included in the longitudinal BIO-PD study
Authors: P. Dušek 1; O. Bezdíček 1; H. Brožová 1; I. Dall’antonia 1; S. Dostálová 1; P. Havránková 1; J. Klempíř 1; J. Mana 1; J. Mašková 1; J. Nepožitek 1; J. Roth 1; P. Peřinová 1; F. Růžička 1; T. Serranová 1; J. Trnka 2; O. Ulmanová 1; D. Zogala 2; R. Jech 1; K. Šonka 1; E. Růžička 1
Authors‘ workplace: Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic 1; Institute of Nuclear Medicine, First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic 2
Published in: Cesk Slov Neurol N 2020; 83/116(6): 633-639
Category: Original Paper
doi: https://doi.org/10.48095/cccsnn2020633Overview
Aim: The aim of this study was to describe the protocol of the longitudinal observational study “Biomarkers of Parkinson‘s disease” (BIO-PD) and provide clinical characteristics of newly diagnosed Parkinson‘s disease (PD) patients enrolled in the BIO-PD study to date.
Methods: Treatment-naive PD patients diagnosed according to the Movement Disorders Society clinical diagnostic criteria with abnormal finding on dopamine transporter SPECT as well as healthy controls were enrolled in the study. Baseline examination included brain MRI, structured interview, Movement Disorders Society-Unified PD Rating Scale (MDS-UPDRS), video-polysomnography, transcranial sonography, olfactory testing, visual color sensitivity, a battery of neurophysiological tests, detailed cognitive testing, a comprehensive set of questionnaires as well as sampling of biofluids.
Results: As of July 2020, 95 PD patients (37 females, mean age 61.0 [SD = 12.4] years, mean symptom duration 1.9 [1.7] years) and 57 healthy controls (15 females, mean age 62.2 [9.4] years) were included in the study. Compared to controls, PD patients were more likely to be never smokers (P = 0.02) and to have a history of anxiety or depression disorder (P = 0.02), and constipation (P = 0.002). In addition, PD patients had worse scores in the visual color sensitivity (Farnsworth-Munsell Hue 100) test (P = 0.002), MDS-UPDRS motor score, Beck depression inventory-II, State-Trait Anxiety Inventory X1/X2, Scales for Outcomes in PD-Autonomic questionnaire (SCOPA-AUT), University of Pennsylvania Smell Identification Test, and more frequent substantia nigra hyperechogenicity on transcranial sonography (P < 0.001 for all tests). The frequencies of symptoms and abnormal findings in PD patients were as follows: hyposmia 82.4%, substantia nigra hyperechogenicity 75.4%, history of anxiety or depression disorder 29.5%, constipation 22.1%, and REM (rapid eye movement) sleep behavior disorder 22%.
Conclusion: Baseline characteristics of patients enrolled in the BIO-PD study are comparable to other de novo PD cohorts, indicating that it is a representative sample of PD patients. Hyposmia is the most prevalent non-motor abnormality in newly diagnosed PD patients and is thus a suitable diagnostic marker for early PD.
Keywords:
Parkinson disease – cohort studies – Longitudinal studies – biomarkers – non-motor symptoms – Neuroimaging – phenotype
Introduction
Identification of robust biomarkers could greatly improve clinical management and understanding of disease mechanisms in medicine. Several longitudinal cohorts of de novo Parkinson’s disease (PD) patients have been followed world-wide in order to find and validate diagnostic and progression biomarkers in phenotypically well-characterized patients [1,2]. Parkinson’s Progression Markers Initiative (PPMI) is one of the largest international studies aiming at discovering new biomarkers [3]. Although several potential biomarkers have been identified, e. g., synuclein aggregation properties in the cerebrospinal fluid (CSF) [4], replication studies are still needed. Additionally, large longitudinal cohorts with de novo patients enable better understanding of the phenotypic variety and prevalence of motor and non-motor symptoms in different stages of PD. This will ultimately improve diagnostic accuracy in the prodromal and manifest disease as well as patient stratification into clinical trials through combined clinical and biomarker-driven phenotyping [5]. Capturing full phenotypic spectrum of PD requires pooling data from different populations with varying genetic backgrounds and lifestyles. However, most longitudinal studies in PD take place in western Europe and the United States [1]. This led us to set up the longitudinal observational study “Biomarkers of Parkinson’s disease” (BIO-PD) in the Czech Republic. The ultimate aims of this single center project are: 1) to identify clinical, imaging, neurophysiological, genetic, and biochemical markers of disease severity and progression and 2) describe associations among these markers and PD phenotypes.
Herein, we describe the BIO-PD study protocol and summarize elementary demographic and clinical findings of the subjects enrolled in this cohort to date.
Methods
Subjects
Treatment-naive PD patients newly diagnosed between June 2015 and July 2020 at our tertiary center were invited to participate. The diagnosis was confirmed by a movement disorders specialist (PD) according to the Movement Disorders Society (MDS) clinical diagnostic criteria [6]. The exclusion criteria were treatment with antiparkinsonian medication before baseline examination, clinical, imaging or laboratory signs of atypical parkinsonism, and normal finding on dopamine transporter single-photon emission CT (DAT-SPECT) examination.
The control subjects were recruited from the general community through advertisements. To be eligible for the study, controls had to be free of major neurologic disorders, active oncologic illness, and abuse of psychoactive substances. In all control subjects, REM (rapid eye movement) sleep behavior disorder (RBD) was excluded by thorough history and video-polysomnography. The target sample size is 130 PD patients and 70 controls.
Study protocol
All subjects undergo a comprehensive longitudinal protocol involving clinical, neurophysiological, and neuroimaging assessments. The schedule of evaluations comprise screening/baseline examinations and then lifelong yearly follow-up visits at 1-year intervals. The baseline examination include brain MRI, DAT-SPECT, structured interview, MDS-Unified Parkinson‘s Disease Rating Scale (MDS-UPDRS), video-polysomnography, transcranial sonography (TCS), olfactory testing, visual color sensitivity examination, a battery of neurophysiological tests, detailed cognitive testing, a set of questionnaires as well as sampling and biobanking of serum, plasma, DNA, and optionally CSF. In healthy controls, DAT-SPECT and CSF sampling are not performed. After the baseline examination, dopaminergic medication is initiated in PD patients when needed. The follow-up examinations include MDS-UPDRS, Montreal Cognitive Assessment (MoCA) [7], a battery of neurophysiological tests, and a set of questionnaires (Fig. 1). The study data are collected and managed using REDCap electronic data capture tools hosted at General University Hospital in Prague. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies [8,9]. Large data files are stored at the CESNET storage facilities [10].
1. Protocol of the BIO-PD study.
Obr. 1. Protokol studie BIO-PD.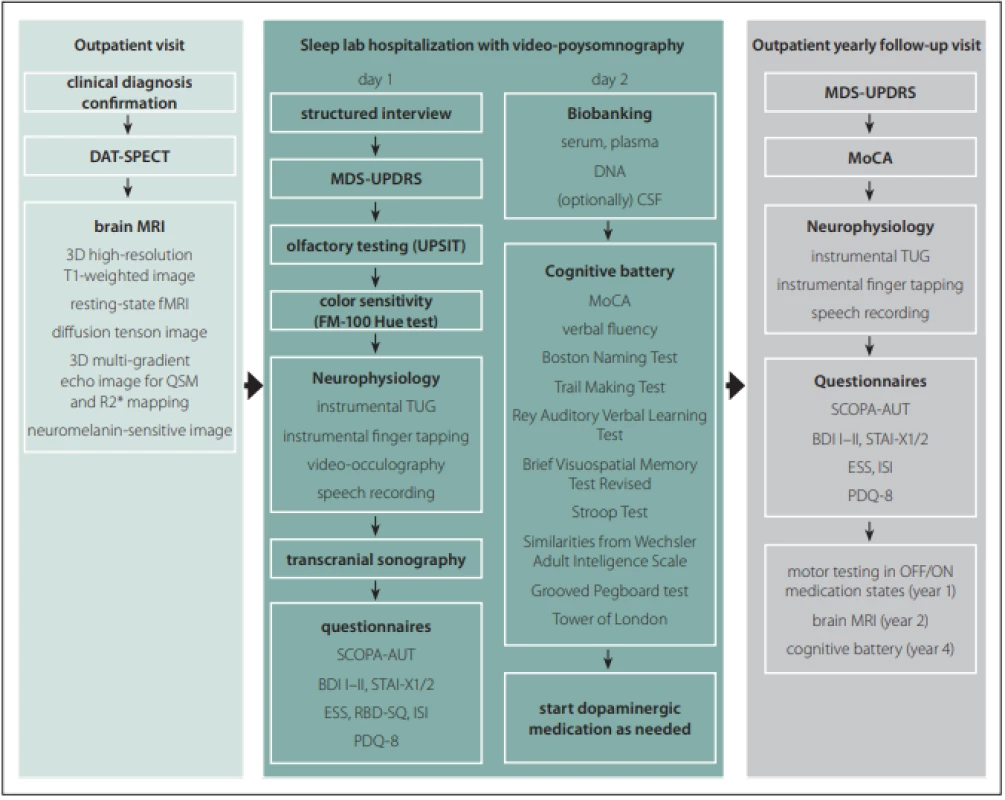
BDI – Beck depression inventory; CSF – cerebrospinal fl uid; DAT-SPECT – dopamine transporter single-photon emission CT; ESS – Epworth sleepiness scale; FM – Farnsworth-Munsell; fMRI – functional MRI; ISI – insomnia severity index; MDS-UPDRS – Movement Disorders SocietyUnifi ed Parkinson‘s Disease Rating Scale; MoCA – Montreal Cognitive Assessment; PDQ – Parkinson‘s Disease Questionnaire; QSM – quantitative susceptibility mapping; RBD-SQ – REM Sleep Behavior Disorder Screening Questionnaire; SCOPA-AUT – Scales for Outcomes in Parkinson Disease-Autonomic; STAI – State-Trait Anxiety Inventory; TUG – Timed up and go; UPSIT – University of Pennsylvania Smell Identifi cation Test
BDI – Beck depression inventory; CSF – mozkomíšní mok; DAT-SPECT – jednofotonová emisní CT; ESS – Epworth sleepiness scale; FM – Farnsworth-Munsell; fMRI – funkční MRI; ISI – index závažnosti nespavosti (insomnia severity index); MDS-UPDRS – Movement Disorders Society – Unifi ed Parkinson‘s Disease Rating Scale; MoCA – Montreal Cognitive Assessment; PDQ – Parkinson‘s Disease Questionnaire; QSM – quantitative susceptibility mapping; RBD-SQ – REM Sleep Behavior Disorder Screening Questionnaire; SCOPA-AUT – Scales for Outcomes in Parkinson Disease-Autonomic; STAI – State-Trait Anxiety Inventory; TUG – Timed up and go; UPSIT – University of Pennsylvania Smell Identifi cation TestData acquisition and processing
In this descriptive work, we present elementary demographic, clinical, and imaging data in PD and control groups acquired at baseline. During a structured interview, the information about family history of PD, history of unconscious head trauma, pesticide exposure, anxiety or depression disorder, smoking status, subjective hyposmia, constipation, and orthostasis was retrieved. Pesticide exposure was regarded positive in case of regular exposure for at least 1 year. The history of anxiety or depression was acknowledged if their severity required psychiatric examination or pharmacotherapy. The subjects were regarded as smokers in case of regular smoking for at least 1 year. Constipation was diagnosed in the subjects who scored ≥ 1 point in the MDS-UPDRS item 1.11 and referred ≤ 3 bowel movements per week for most of the time during last 6 months. Orthostasis was diagnosed in the subjects who scored ≥ 1 point in the MDS-UPDRS item 1.12 and confirmed regular orthostasis during last 6 months in the interview. MDS-UPDRS scoring was performed by a single MDS-certified rater (PD). For level 1 cognitive evaluation, MoCA was used. The subjects with MoCA score lower than 1.5 SD below the normative mean were classified as those with mild cognitive impairment (MCI) [11]. Mood, anxiety, and sleepiness were assessed using the Beck depression inventory II (BDI-II), State-Trait Anxiety Inventory (STAI) X1/X2 [12], and Epworth sleepiness scale (ESS) [13]. Olfaction was investigated using the 40-item University of Pennsylvania Smell Identification Test (UPSIT) and the subjects were classified as normal (normosmic or mild microsmic according to the UPSIT manual), hyposmic (moderate or severe microsmic according to the manual) or anosmic [14]. Color vision was examined using the Farnsworth-Munsell Hue 100 test (FMT) [15]. The total FMT error scores were compared to the normative data published, using the following cutoffs for abnormal color sensitivity: age 30–39: > 80; age 40–49: > 100; age 50–59: > 130; age 60–69: > 170; age 70–79 > 195 [16]. Autonomic functions were assessed using the Scales for Outcomes in PD-Autonomic (SCOPA-AUT) questionnaire [17,18]. Video-polysomnography was performed over one night according to the American Academy of Sleep Medicine (AASM) recommendation with the supplementary recording of superficial EMG of bilateral flexor digitorum superficialis muscles; the presence of RBD was established according to the International Classification of Sleep Disorders, third edition (ICSD-3) [19].
DAT-SPECT
DAT-SPECT was performed using the [123I]-2-b--carbomethoxy-3b - (4-iodophenyl) -N - (3-fluoropropyl) nortropane ([123I]FP-CIT, DaTscan®, GE Healthcare, Chicago, IL, USA) tracer according to the European Association of Nuclear Medicine (EANM) procedure guidelines [20]. The scans were acquired 3 hours after 185 MBq [123I]FP-CIT injection on a dual-head camera system (Infinia, GE Healthcare, Chicago, IL, USA) using common acquisition and reconstruction parameters described in detail elsewhere [21]. Automated semi-quantitative analysis was performed using the BasGan V2 software [22] and specific binding ratios (SBR) in the caudate nucleus and putamen in each hemisphere relative to background binding were calculated. The SBR values below 95% prediction interval constructed according to a reference database from 129 healthy subjects were considered abnormal [21]. The DAT-SPECT findings in each patient were classified as normal, unilaterally abnormal, or bilaterally abnormal for the caudate and putamen, respectively.
Transcranial sonography
Transcranial sonography (TCS) examination was performed by a single experienced sonographer (JM) using a commercial ultrasound device (Aplio 300, Toshiba, Tokyo, Japan) with a 2.5 MHz phased array transducer and commonly used settings. Substantia nigra (SN) echogenic areas on both sides were manually encircled and the values above our internal, previously determined, optimal cut off value 0.126 cm2 were considered abnormal. Detailed description of the methodology is described elsewhere [23].
Statistics
Groupwise comparisons were performed between the PD and control groups. The Student t-test or the Mann-Whitney U test were used depending on whether the data distribution had a normal or non-normal shape. The categorical variables are described as frequency percentages and were analyzed using the Fisher’s exact or Chi square tests. Graphpad Prism version 6.07 (Graphpad software, San Diego, CA, USA) was used for statistical analysis.
Results
A total of 95 treatment-naive PD patients and 57 healthy controls with comparable age and gender distributions were enrolled to the longitudinal study. The flow diagram of patient inclusion is shown in Fig. 2. The comparison of demographic and clinical variables in patients and controls is shown in Tab. 1.
2. Flow diagram of patient inclusion.
Obr. 2. Vývojový diagram procesu zařazení pacientů.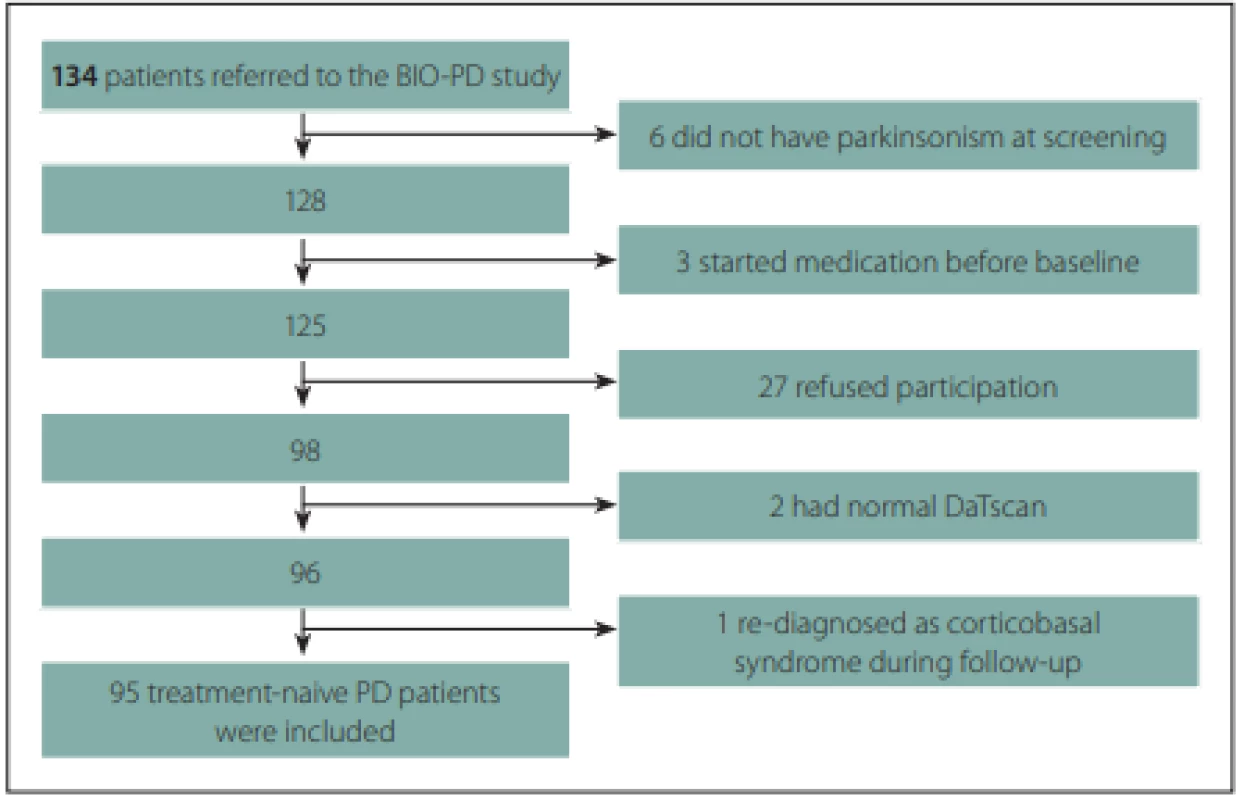
1. Demographic and clinical variables in patients and controls. 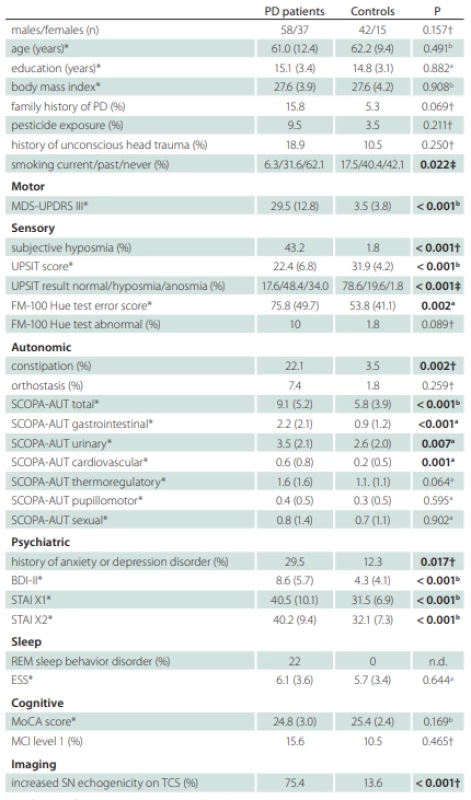
Signifi cant diff erences are marked with bold text.
*values reported as mean (SD); †Fisher’s exact test; ‡Chi square test; a Mann-Whitney U test; b Student t-test
BDI – Beck depression inventory; ESS – Epworth Sleepiness Scale; FM – Farnsworth-Munsell; MCI – mild cognitive impairment; MDS-UPDRS – Movement Disorders Society-Unifi ed Parkinson‘s Disease Rating Scale; MoCA – Montreal Cognitive Assessment; n – number; n.d. – not done; PD – Parkinson‘s disease; REM – rapid eye movement; SCOPA-AUT – Scales for Outcomes in Parkinson Disease-Autonomic; STAI – State-Trait Anxiety Inventory; UPSIT – University of Pennsylvania Smell Identifi cation TestThe mean subjective motor symptom duration in PD patients at baseline was 1.9 (SD = 1.7; range 0.3–11.3) years. Eleven (11.6%) patients fulfilled the criteria for early-onset PD, i.e., symptoms onset at the age below 40 years. First motor symptoms noticed by patients were tremor in 59 (62%) subjects, impaired hand motor function in 16 (17%) subjects, slow, shuffling or unsteady gait in 11 (12%) subjects, arm stiffness in 5 (5%) subjects, low-volume voice in 2 (2%) subjects and arm dysesthesia/pain in 2 (2%) subjects.
Compared to controls, PD patients were more likely to be never smokers and had a history of anxiety or depression disorder, constipation and subjective hyposmia. In addition, PD patients had worse scores in MDS-UPDRS III, UPSIT, FMT, BDI-II, STAI X1/2, and SCOPA-AUT (Tab. 1). The difference between PD patients and controls in the SCOPA-AUT score was mostly driven by higher gastrointestinal, urinary, and cardiovascular autonomic region sub-scores in the former group. The odds ratio for having constipation was 7.8 (95% CI 1.9–34.6) in PD relative to controls. Nine patients were not able to determine the onset of constipation. Median latency from constipation onset to first motor symptoms was one (IQR 0–7.8) year in remaining 12 patients whereby constipation paralleled the occurrence of motor symptoms in six of them. Subjective hyposmia in PD was more common than in controls yielding the odds ratio 42.5 (95% CI 6.8–440.2). The median latency from subjective hyposmia onset to first motor symptom was 5 (IQR 1–15) years whereby seven patients reported the onset of hyposmia paralleling or succeeding the occurrence of first motor symptoms and two patients were not able to determine the hyposmia onset. The prevalence of hyposmia/anosmia objectively diagnosed according to the UPSIT test, as compared to subjective hyposmia, was higher in both groups. The odds ratio for being hyposmic or anosmic was 17.2 (95% CI 7.5–38.3) for PD patients compared to controls. The mean FMT total error score was larger in the PD group, but only 10% of patients had abnormal color sensitivity based on normative data. The history of anxiety or depression disorder was also more frequent in PD patients compared to controls with the odds ratio 3.0 (95% CI 1.9–34.6). Anxiety or depression preceded motor symptoms by a median 4.5 (IQR 0.25–17.5) years whereby it paralleled or succeeded motor symptoms in six patients. RBD was diagnosed in 20 (22%) PD patients after the exclusion of four patients in whom REM sleep was not captured during video-polysomnography. RBD was an exclusion criterion in the control group and resulted in the exclusion of one subject.
DAT-SPECT was performed in all PD patients, since a decreased putaminal binding was an inclusion criterion. It was abnormal unilaterally in 17 (17.9%) and bilaterally in 78 (82.1%) patients. In the caudate nucleus, a bilaterally decreased binding was observed in 30 (31.6%), a unilaterally decreased binding in 31 (32.6 %) and a normal finding in 34 (35.8%) patients. TCS was performed in 86 PD and 47 control subjects whereby 21 (24.4%) PD and 3 (6.3%) control subjects did not have bilaterally permeable temporal window. From the analyzed subjects, 75.4% PD and 13.6% controls had at least unilaterally enlarged SN hyperechogenity area, yielding the diagnostic odds ratio 19.4 (95% CI 6.8–54.3). Seven (10.7%) PD patients had bilaterally enlarged SN hyperechogenity area.
Discussion
We present the protocol and baseline clinical data of patients included in the BIO-PD longitudinal cohort study. Compared to the controls, the PD patients were more likely to be never smokers and had more frequent history of anxiety/depression disorders, hyposmia, constipation, and enlarged SN echogenic area. The PD patients also had worse scores in the tests assessing motor, olfactory, and autonomic functions, as well as color sensitivity and anxiety/depression symptoms.
The protocol of this study is compatible with the set of biomarker assessments recommended for longitudinal PD trials [24]. Its strong points include video-polysomnography, detailed objective neurophysiological assessment of speech, gait, finger and eye movements, and extensive MRI protocol enabling multi-parametric quantitative analysis.
The frequencies of family history of PD, pesticide exposure, unconscious head trauma, and never smoker status were slightly higher in PD patients compared to controls, but the differences, except for never smoker status, did not reach statistical significance. Although this project was not designed for the analyses of risk factors, their frequencies are similar to those observed in large population studies [25–27], indicating that patients enrolled to the BIO-PD study are a representative PD sample.
The reduced putaminal DAT-SPECT binding was an inclusion criterion for the PD group chosen to enrich the cohort [28]. Interestingly, majority of de novo PD patients already had bilaterally decreased binding in the putamen and at least unilaterally decreased binding in the caudate as was already shown in previous studies [29,30]. These findings suggest that synuclein-related pathology affects entire nigrostriatal pathways from both hemispheres simultaneously, yet asymmetrically.
In both groups, objective olfactory testing revealed more subjects with olfactory impairments than a structured interview. The existence of a substantial number of patients who are not aware of olfactory dysfunction warrants the use of objective methods for olfactory testing and questions the validity of subjective estimates of hyposmia onset. The frequency of olfactory impairment in our PD patients was 82%, which is comparable to previous studies that have shown 70–90% prevalence of hyposmia or anosmia in de novo PD patients [31,32].
Increased SN echogenicity was present in 75.4% PD patients and only in 13.6% controls, which is in agreement with previous studies that reported 50–90% sensitivity of TCS in PD diagnosis [32,33]. The usefulness of TCS is hampered in subjects with impermeable temporal bone windows. Nevertheless, even if insonable patients were included in the analysis, SN hyperechogenicity would still be present in 57.0% PD patients.
RBD was diagnosed only in 22% of PD patients, using video-polysomnography. This finding confirms results from a previous study on a smaller number of subjects [34] and is in agreement with another cohort of de novo PD patients [32]. In the latter study, milder REM sleep abnormalities, not fulfilling diagnostic criteria for RBD, were observed in 51% patients and 15% controls. It is thus likely that the quantitative analysis of REM sleep muscle atonia will offer more sensitive sleep biomarkers of PD than the presence of fully developed RBD. Similar prevalence, i.e., 22%, was observed for constipation. Although constipation prevalence in the PPMI study was slightly higher, reaching 33%, probably due to different diag-nostic criteria, both studies confirm that constipation represents a clinical problem only in a minority of patients [35].
In control subjects, a history of depression or anxiety disorder was positive in 12.3% patients which is close to lifetime depression prevalence in the population [36]. In PD patients, a history of depression or anxiety was almost three-times higher compared to controls, which was paralleled by significantly higher BDI-II and STAI scores in the former group. These results are in agreement with 33% prevalence of depression in de novo PD patients in the PPMI study [37].
The MoCA score and frequency of MCI were not significantly different in PD and control groups. This is most likely explained by only subtle cognitive changes in de novo PD patients on one side and low sensitivity of MoCA test for these minor disturbances on the other side. The usage of level 1 MCI criteria also likely explains the relatively low prevalence (15.6%) of MCI compared to other de novo cohorts, where MCI prevalence ranged between 18.9–41.5% [38–41]. The finding of impaired color discrimination in PD is also consistent with previous studies [42] but the frequency of patients with abnormal color sensitivity based on normative data was lower than previously published in more advanced patients [43].
In conclusion, the baseline demographic and clinical data of BIO-PD cohort are comparable to previous studies in newly diag-nosed PD patients, indicating that the patient group enrolled to date is a representative PD sample. Besides the obvious motor and DAT-SPECT findings, we have identified hyposmia as the most prevalent abnormality in newly diagnosed PD patients and thus confirmed its suitability as diagnostic marker of prodromal and early symptomatic PD. Limited by the impermeability of temporal bone window in a quarter of patients, SN hyperechogenicity was the second most prevalent abnormality in PD.
Ethical principles
Ethics approval was obtained from the institutional review board of the General University Hospital in Prague on 24th July 2014 (111/14). All participants gave a written informed consent.
Acknowledgements
The study was supported by the Czech Ministry of Health, grant number 15-25602A. We would like to thank all collaborating neurologists who referred patients to the study as well as patients and control subjects for participation. Access to CESNET storage facilities provided under the program “Projects of Large Research, Development, and Innovations Infrastructures” (CESNET LM2018140), is greatly appreciated.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Petr Dušek, MD, PhD
Department of Neurology 1st Faculty of Medicine General
University Hospital in Prague
Kateřinská 30,
120 00, Prague
Czech Republic
e-mail: pdusek@gmail.com
Přijato k recenzi: 7. 8. 2020
Přijato do tisku: 3. 12. 2020
Sources
1. Heinzel S, Lerche S, Maetzler W et al. Global, yet incomplete overview of cohort studies in Parkinson‘s disease. J Parkinsons Dis 2017; 7 (3): 423–432. doi: 10.3233/JPD-171100.
2. Lerche S, Liepelt-Scarfone I, Alves G et al. Methods in neuroepidemiology characterization of European longitudinal cohort studies in Parkinson‘s disease--report of the JPND Working Group BioLoC-PD. Neuroepidemiology 2015; 45 (4): 282–297. doi: 10.1159/000439221.
3. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011; 95 (4): 629–635. doi: 10.1016/j.pneurobio.2011.09.005.
4. Ning H, Wu Q, Han D et al. Baseline concentration of misfolded alpha-synuclein aggregates in cerebrospinal fluid predicts risk of cognitive decline in Parkinson‘s disease. Neuropathol Appl Neurobiol 2019; 45 (4): 398–409. doi: 10.1111/nan.12524.
5. Espay AJ, Schwarzschild MA, Tanner CM et al. Biomarker-driven phenotyping in Parkinson‘s disease: a translational missing link in disease-modifying clinical trials. Mov Disord 2017; 32 (3): 319–324. doi: 10.1002/ mds.26913.
6. Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinson‘s disease. Mov Disord 2015; 30 (12): 1591–1601. doi: 10.1002/mds.26424.
7. Nasreddine ZS, Phillips NA, Bedirian V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53 (4): 695–699. doi: 10.1111/j.1532-5415.2005.53221.x.
8. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap) --a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42 (2): 377–381. doi: 10.1016/j.jbi.2008.08.010.
9. Harris PA, Taylor R, Minor BL et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95 : 103208. doi: 10.1016/j.jbi.2019.103208.
10. www.cesnet.cz
11. Kopecek M, Stepankova H, Lukavsky J et al. Montreal cognitive assessment (MoCA): Normative data for old and very old Czech adults. Appl Neuropsychol Adult 2017; 24 (1): 23–29. doi: 10.1080/23279095.2015.1065261.
12. Spielberger C. Manual for the State-Trait AnxietyInventory; rev. ed. Palo Alto (CA): Consulting Psychologists Press 1983.
13. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14 (6): 540–545. doi: 10.1093/sleep/14.6.540.
14. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32 (3): 489–502.
15. Farnsworth D. The Farnsworth-Munsell 100-Hue and dichotomous tests for color vision. J Optic Soc Am 1943; 33 (10): 568–578. doi: 10.1364/josa.33.000568.
16. Kinnear PR, Sahraie A. New Farnsworth-Munsell 100 Hue test norms of normal observers for each year of age 5–22 and for age decades 30–70. Br J Ophthalmol 2002; 86 (12): 1408–1411. doi: 10.1136/bjo.86.12.1408.
17. Kaiserova M, Opavsky J, Maertin JJ et al. Czech Version of the Autonomic Scale for Outcomes in Parkinson‘s Disease (SCOPA-AUT) – questionnaire to assess the presence and severity of autonomic dysfunction in patients with Parkinson‘s disease. Cesk Slov Neurol N 2014; 77 (1): 96–99.
18. Visser M, Marinus J, Stiggelbout AM et al. Assessment of autonomic dysfunction in Parkinson‘s disease: the SCOPA-AUT. Mov Disord 2004; 19 (11): 1306–1312. doi: 10.1002/mds.20153.
19. American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine 2014.
20. Darcourt J, Booij J, Tatsch K et al. EANM procedure guidelines for brain neurotransmission SPECT using (123) I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 2010; 37 (2): 443–450. doi: 10.1007/s00259-009-1267-x.
21. Dusek P, Ibarburu V, Bezdicek O et al. Relations of non-motor symptoms and dopamine transporter binding in REM sleep behavior disorder. Sci Rep 2019; 9 (1): 15463. doi: 10.1038/s41598-019-51710-y.
22. Calvini P, Rodriguez G, Inguglia F et al. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging 2007; 34 (8): 1240–1253. doi: 10.1007/s00259-006-0357-2.
23. Maskova J, Skoloudik D, Stofanikova P et al. Comparative study of the substantia nigra echogenicity and (123) I-Ioflupane SPECT in patients with synucleinopathies with and without REM sleep behavior disorder. Sleep Med 2020; 70 : 116–123. doi: 10.1016/j.sleep.2020.02.012.
24. Lerche S, Heinzel S, Alves GW et al. Aiming for study comparability in parkinson‘s disease: proposal for a modular set of biomarker assessments to be used in longitudinal studies. Front Aging Neurosci 2016; 8 : 121. doi: 10.3389/fnagi.2016.00121.
25. Malek N, Swallow DM, Grosset KA et al. Tracking Parkinson‘s: study design and baseline patient data. J Parkinsons Dis 2015; 5 (4): 947–959. doi: 10.3233/JPD-150662.
26. Noyce AJ, Bestwick JP, Silveira-Moriyama L et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012; 72 (6): 893–901. doi: 10.1002/ana.23687.
27. van der Mark M, Nijssen PC, Vlaanderen J et al. A case-control study of the protective effect of alcohol, coffee, and cigarette consumption on Parkinson disease risk: time-since-cessation modifies the effect of tobacco smoking. PLoS One 2014; 9 (4): e95297. doi: 10.1371/journal.pone.0095297.
28. Conrado DJ, Nicholas T, Tsai K et al. Dopamine transporter neuroimaging as an enrichment biomarker in early Parkinson‘s disease clinical trials: a disease progression modeling analysis. Clin Transl Sci 2018; 11 (1): 63–70. doi: 10.1111/cts.12492.
29. Filippi L, Manni C, Pierantozzi M et al. 123I-FP-CIT semi-quantitative SPECT detects preclinical bilateral dopaminergic deficit in early Parkinson‘s disease with unilateral symptoms. Nucl Med Commun 2005; 26 (5): 421–426. doi: 10.1097/00006231-200505000-00005.
30. Bauckneht M, Chincarini A, De Carli F et al. Presynaptic dopaminergic neuroimaging in REM sleep behavior disorder: A systematic review and meta-analysis. Sleep Med Rev 2018; 41 : 266–274. doi: 10.1016/j.smrv.2018.04.001.
31. Rossi M, Escobar AM, Bril A et al. Motor features in Parkinson‘s disease with normal olfactory function. Mov Disord 2016; 31 (9): 1414–1417. doi: 10.1002/mds.26687.
32. Mollenhauer B, Trautmann E, Sixel-Doring F et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013; 81 (14): 1226–1234. doi: 10.1212/WNL.0b013e3 182a6cbd5.
33. Gaenslen A, Unmuth B, Godau J et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson‘s disease: a prospective blinded study. Lancet Neurol 2008; 7 (5): 417–424. doi: 10.1016/S1474-4422 (08) 70067-X.
34. Buskova J, Klempir J, Majerova V et al. Sleep disturbances in untreated Parkinson‘s disease. J Neurol 2011; 258 (12): 2254–2259. doi: 10.1007/s00415-011-6109-7.
35. Krogh K, Ostergaard K, Sabroe S et al. Clinical aspects of bowel symptoms in Parkinson‘s disease. Acta Neurol Scand 2008; 117 (1): 60–64. doi: 10.1111/j.1600-0404.2007.00900.x.
36. Lim GY, Tam WW, Lu Y et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018; 8 (1): 2861. doi: 10.1038/s41598-018-21243-x.
37. Honsey BN, Erickson LO, Wyman-Chick KA. Neuropsychological test performances and depression in early-stage de novo Parkinson‘s disease. Arch Clin Neuropsychol 2019; acz 067. doi: 10.1093/arclin/acz067.
38. Yarnall AJ, Breen DP, Duncan GW et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 2014; 82 (4): 308–316. doi: 10.1212/WNL.0000000000000066.
39. de la Riva P, Smith K, Xie SX et al. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 2014; 83 (12): 1096–1103. doi: 10.1212/WNL.0000000000000801.
40. Pedersen KF, Larsen JP, Tysnes OB et al. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013; 70 (5): 580–586. doi: 10.1001/jamaneurol.2013.2110.
41. Aarsland D, Bronnick K, Williams-Gray C et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010; 75 (12): 1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e.
42. Büttner T, Kuhn W, Müller T et al. Distorted color discrimination in ‚de novo‘ parkinsonian patients. Neurology 1995; 45 (2): 386–387. doi: 10.1212/wnl.45.2.386.
43. Brandt AU, Zimmermann HG, Oberwahrenbrock T et al. Self-perception and determinants of color vision in Parkinson‘s disease. J Neural Transm (Vienna) 2018; 125 (2): 145–152. doi: 10.1007/s00702-017-1812-x.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2020 Issue 6-
All articles in this issue
- Progressive supranuclear palsy
- The international classification of orofacial pain (ICOP) – the official Czech translation of the 1st edition
- Suprascapular neuropathy
- Flexible endoscopic evaluation of swallowing vs. screening tests for dysphagia and their effect on the final outcome in post-acute stroke patients
- Comparison of in vivo and in vitro measurements of flow parameters in carotid stenosis – pilot results of hemodynamic phantom testing
- COVID-19 associated encephalopathy responding to treatment with intravenous immunoglobulins
- Patient with slowly progressive symptoms from cervico-cranial region
- Syndrome of inappropriate antidiuretic hormone secretion as the first symptom of esthesioneuroblastoma
- Coincidence of multiple sclerosis and myasthenia gravis
- Early revascularization after combined surgery in moyamoya disease
- Endoscopic lavage of intraventricular haemorrhage in premature newborns
- Czech Neurological Society CMA JEP expert opinion for primary care management of acute low back pain in adults
- Guidelines for nutritional support in stroke
- Odešel doc. MUDr. Roman Schröder, CSc.
- Zemřel doc. MUDr. Edgar Lukáš, DrSc. (1932–2020)
- MUDr. Michal Králik, CSc. Spomienka na vzácneho človeka.
- Prof. MUDr. Ľubomír Lisý, DrSc.
- Rozloučení s prof. MUDr. Valentinou Stýblovou, DrSc. (1922–2020)
- MUDr. Hanuš Baš, CSc., devadesátiletý
- Prof. MUDr. Peter Kukumberg, PhD. – 75-ročný
- Active secondary progressive multiple sclerosis treatment with a selective sphingosine-1-phosphate receptor modulator
- Hemodynamic changes in four aneurysms leading to their rupture at follow-up periods
- Clinical characteristics of newly diagnosed Parkinson’s disease patients included in the longitudinal BIO-PD study
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Progressive supranuclear palsy
- Suprascapular neuropathy
- Flexible endoscopic evaluation of swallowing vs. screening tests for dysphagia and their effect on the final outcome in post-acute stroke patients
- COVID-19 associated encephalopathy responding to treatment with intravenous immunoglobulins
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

