-
Medical journals
- Career
Tick-borne meningitis complicated by a cardioembolic intraluminal carotid artery thrombus and stroke
Authors: M. Čierny 1; M. Škorňa 2; S. Peška 2; P. Polák 3; S. Voháňka 2
Published in: Cesk Slov Neurol N 2019; 82(2): 227-228
Category: Letters to Editor
doi: https://doi.org/10.14735/amcsnn2019227Overview
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Dear Editor,
Intraluminal carotid thrombus (ILCT) is present in less than 2% of patients presenting with acute ischaemic stroke, and 75–81% of ILCT cases are associated with atherosclerosis [1,2]. We present a rare case of acute stroke with ILCT on bare carotid artery without a plaque.
A 48-year-old man with a history of hypertension presented with 6 days of fever, headache, dizziness and episodes of mild cough and throat pain. He denied smoking or any recent tick bites. On exam, patient had fever of 40 °C and pharyngeal erythema. Initial testing revealed leukocytosis (11 × 109/L), elevated C-reactive protein (CRP) (13 mg/L) and normal procalcitonin level and chest X-ray. Cerebrospinal fluid analysis showed 36 mononuclears, 20 polymorphonuclears, elevated protein (0.95 g/L) and positive antibodies against European tick-borne encephalitis virus (TBEV), diagnostic of meningitis due to TBEV. A course of dexamethasone was initiated and patient’s symptoms were slowly improving. On the evening of the 6th day, the patient developed sudden global aphasia and severe right-sided hemiparesis, National Institute of Health Stroke Scale (NIHSS) was 13. He was last seen normal 3 hours prior. CT was normal, without early ischaemic changes. Supra-aortic CTA revealed left middle cerebral artery (MCA) thrombus (Fig. 1), and severe stenosis of extracranial left internal carotid artery (ICA) (Fig. 2) due to an elongated filling defect, concerning for ILCT. Intravenous tissue plasminogen activator (tPA, 70 mg) was administered 120 min after symptoms were recognized (300 min after last seen normal). Endovascular thrombectomy was not performed due to perceived high risk of fragmentation and distal embolization of carotid thrombus. Within 24 hours after tPA administration, patient’s deficit worsened to NIHSS 19, and a repeat head CT showed a new area of ischaemia in the distribution of left M2 MCA without bleeding. Clopidogrel, low molecular weight heparin and a statin was started. Carotid duplex ultrasonography (CUS) confirmed a recent thrombus in the left ICA producing a 75% stenosis, with preserved flow speed (Fig. 3 A, B). Further investigations revealed hypertriglyceridemia (7.8 mmol/L) and hypercholesterolemia (5.3 mmol/L). Transthoracic echocardiogram showed ejection fraction of 68%, insignificant grade I mitral regurgitation and slight left atrial enlargement (LAE). Transesophageal echocardiography and blood cultures did not reveal any new findings. Screening for thrombophilias showed low protein S activity (57%, reference range 65–140). Other tests were negative, including thyroid stimulation hormone, fasting glucose and extensive thrombophilia workup. A week later, the patient developed atrial fibrillation (AF) with rapid ventricular response, which converted to sinus rhythm after amiodarone and betaxolol. Nine days after the stroke, repeat CUS showed complete resolution of the thrombus with no residual stenosis (Fig. 3 C, D). The patient was discharged three weeks later with NIHSS 15, modified Rankin score (mRS) 4. After 6 months, the patient was readmitted for a secondarily generalized tonic-clonic seizure. Levetiracetam was initiated. Patient's expressive aphasia and distal paresis of right hand remained severe, NIHSS was 11, mRS 3.
1. CTA in coronal section (left) and 3D reconstruction (right) shows short intraluminal filling defect in the M2 segment of the left MCA (arrow). MCA – middle cerebral artery
Obr. 1 CTA ve frontálním řezu (vlevo) a 3D rekonstrukce (vpravo) ukazuje krátký intraluminální defekt v úseku M2 levé ACM (šipka). ACM – arteria cerbri media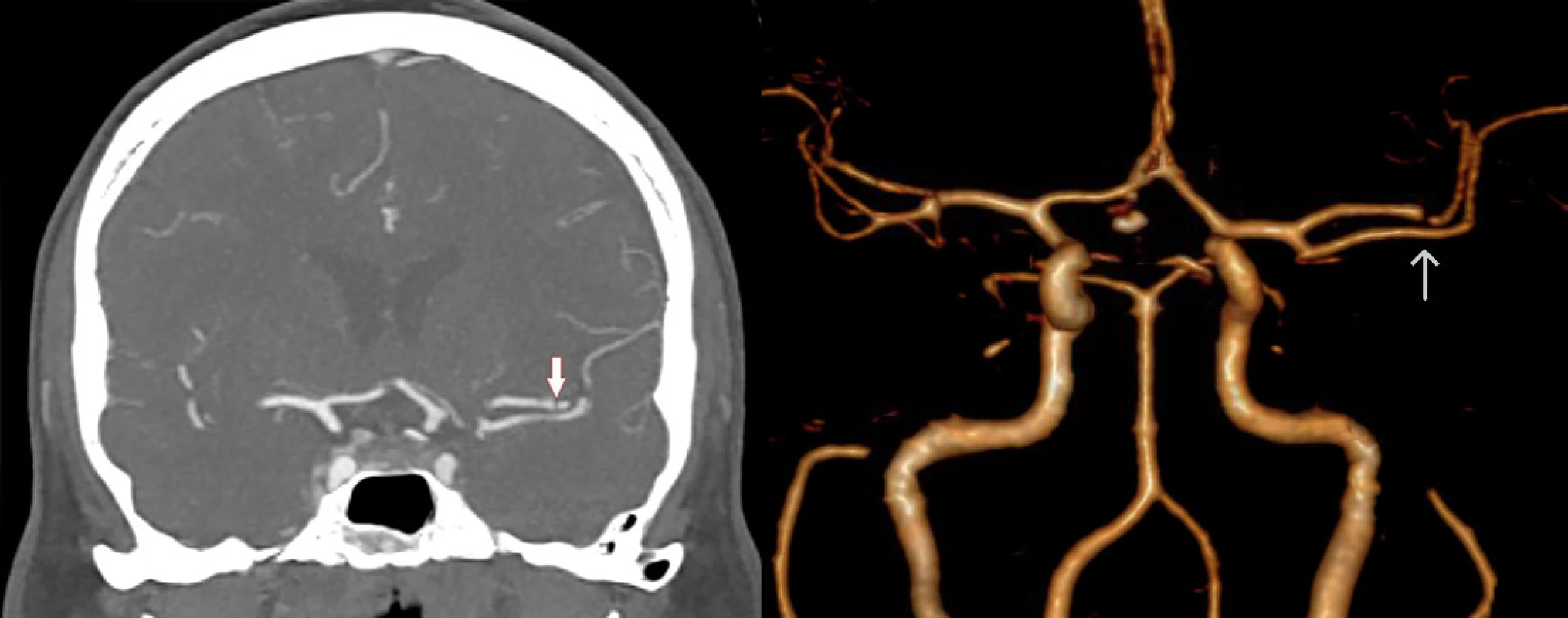
2. CTA in coronal section (left) and 3D reconstruction (right) shows short intraluminal filling defect in the M2 segment of the left MCA (arrow). MCA – middle cerebral artery
Obr. 1 CTA ve frontálním řezu (vlevo) a 3D rekonstrukce (vpravo) ukazuje krátký intraluminální defekt v úseku M2 levé ACM (šipka). ACM – arteria cerbri media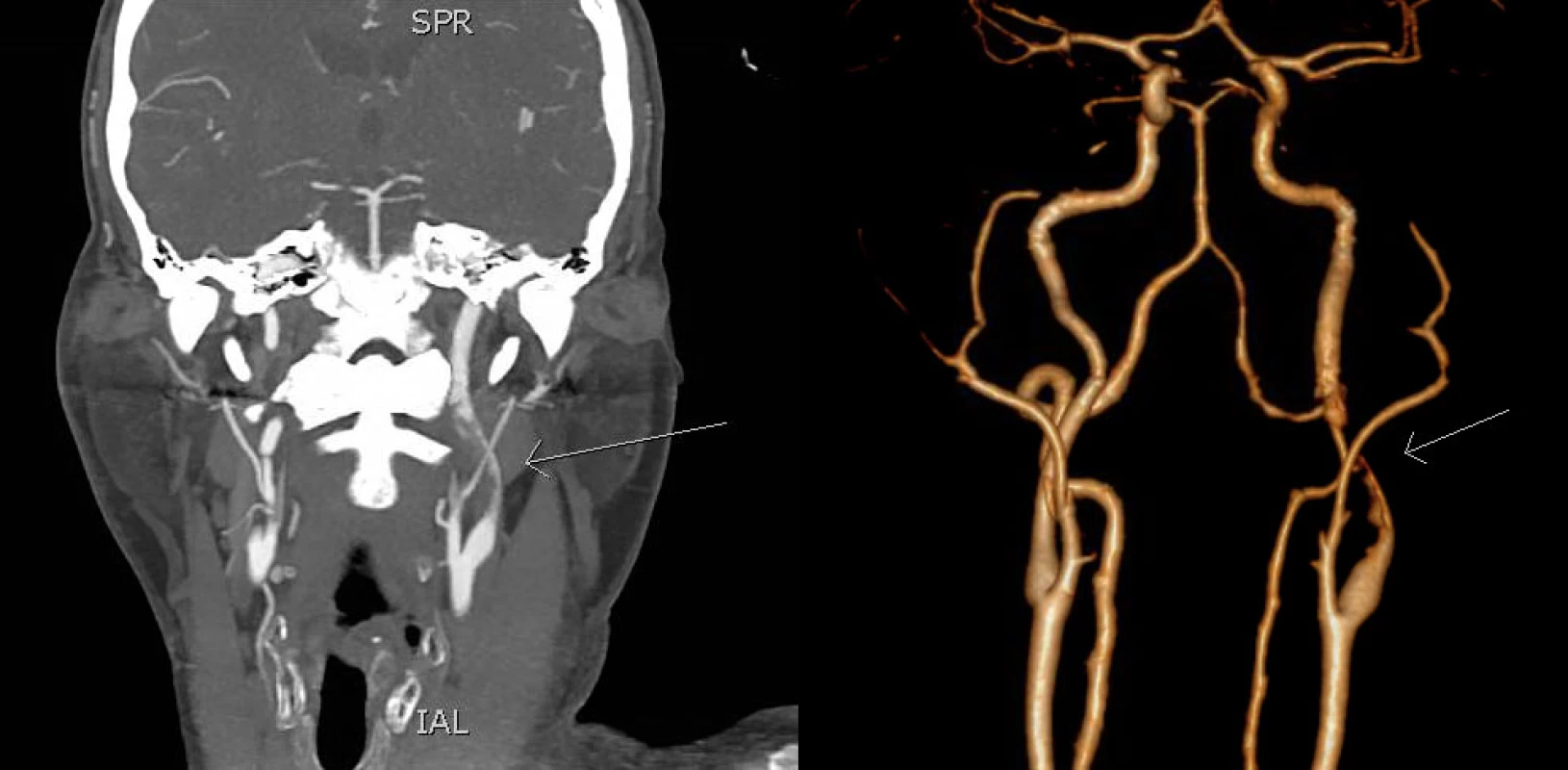
3. CUS of left ICA demonstrates thrombus and 75% stenosis on longitudinal (A) and transverse (B) views. After nine days, repeated CUS shows complete thrombus resolution on longitudinal (C) and transverse (D) views. CUS – carotid ultrasonography; ICA – internal carotid artery
Obr. 3. CUS levé ACI prokazuje trombus a 75% stenózu v longitudinálním (A) a transverzálním (B) zobrazení. CUS opakované po devíti dnech ukazuje kompletní vymizení trombu v longitudinálním (C) a transverzálním (D) zobrazení. CUS – ultrasonografie karotid; ACI – arteria carotis interna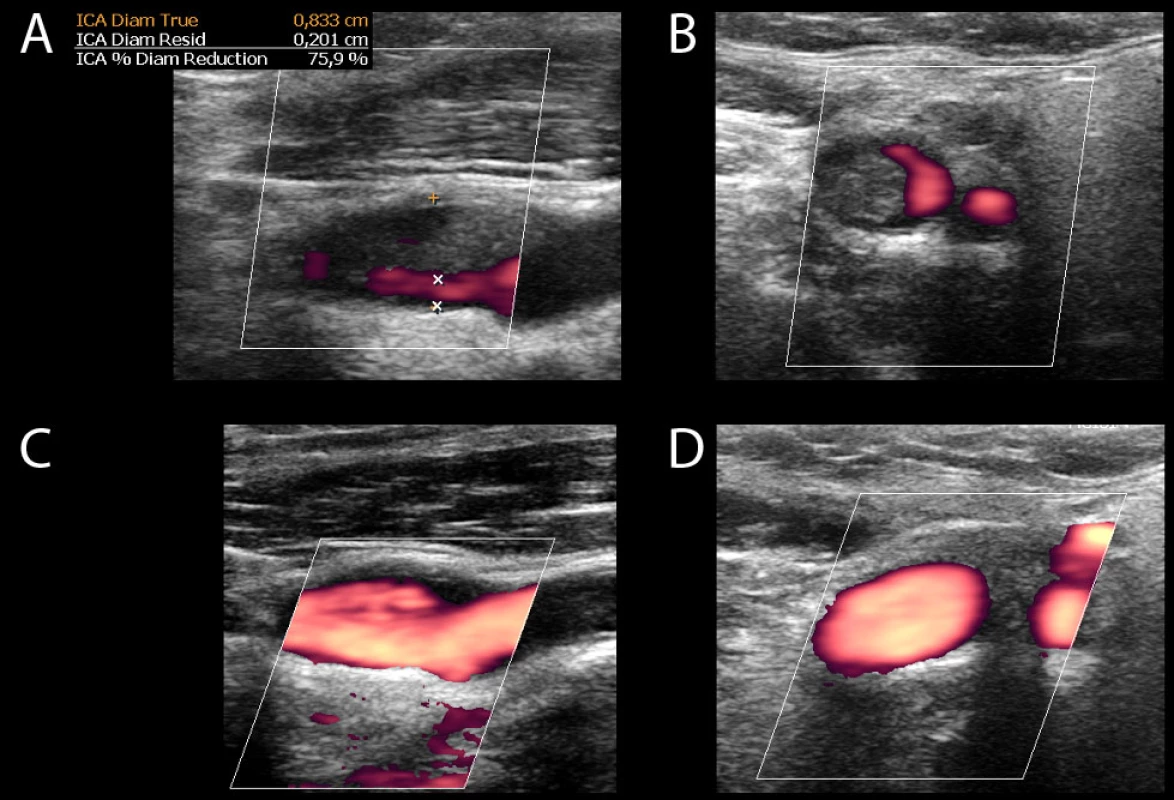
ILCT without atherosclerosis has been associated with hypercoagulable state or malignancy in 4–50% of patients [2–4], severe iron deficiency anaemia and thrombocytosis, cisplatin chemotherapy, and patent foramen ovale. Cardioembolism due to AF is associated with intracranial [4] and, in our patient, intracarotid thrombus. Respiratory, urinary tract or CNS infection in the preceding week increases the risk of stroke by approximately 2–3 times even in young patients or those without vascular risk factors. Pre-stroke infection may be associated with large-vessel cardioembolic strokes and higher stroke severity [5,6]. Meningoencephalitides have been associated with ischaemic stroke due to vasculitis, [7] however, as with any other systemic infection, meningoencephalitis may also contribute to stroke through triggering AF [5] or inflammation-induced procoagulant status, causing an embolic stroke. This report illustrates a rare pathophysiologic sequence of recent meningoencephalitis, AF, and symptomatic ILCT.
The hyperacute management of acute ischaemic stroke due to M2 occlusion should include rapid diagnosis, decision on tPA administration, and mechanical thrombectomy (MT). Ischaemic strokes in the inpatient setting, as compared to the emergency department, commonly experience longer times to neurology consultation, transport, imaging, and tPA administration [8]. Delay in contacting neurologist, initial suspicion for postictal paralysis, overnight campus-wide cross-coverage and transport to CT scanner contributed to the delay in tPA administration in our patient. While seizures are a common complication of encephalitis due to Herpes simplex virus, meningoencephalitis due to TBEV causes seizures in only about 0.3–3.3% of cases [9]. Training in stroke recognition, stroke-specific alerts, and other system-based quality-improvement efforts can expedite evaluation and management of acute stroke in hospitalized patients [10].
The presence of a tandem (extracranial and intracranial) thrombus may represent a risk for distal embolization, but guidelines (published a few years after this patient’s stroke) suggest benefit of MT in this subgroup as well. It is unknown which approach to the proximal lesion (no intervention vs. angioplasty vs. stenting) is superior [11].
Historically, the secondary stroke prevention in patients with ICA stenosis due to ILCT included emergent carotid endarterectomy or carotid artery stenting. More recent case series suggests that endovascular aspiration or stenting with reversed-flow system and proximal or distal protection are beneficial [1]. Surgical intervention should be considered in patients with significant underlying atherosclerotic stenosis, recurrent symptoms, thrombus progression, inability to undergo anticoagulation, or crescendo transient ischaemic attack. Recently, initial treatment with anticoagulation has been recommended for ILCT. A review of 145 cases concluded that medical treatment has lower risk and less benefits when compared to surgery, and leads to thrombus dissolution in 86% of patients [2]. In one serie, 24 patients were successfully treated with initial anticoagulation: of those, 14 cases with medical therapy alone, and 10 with delayed surgery [3]. Another serie reported 20/23 cases were successfully treated with one week of initial anticoagulation, while 3/23 suffered new neurologic deficit or carotid occlusion, only 2/3 patients had diffusion weighted imaging changes on MRI at one week follow-up [4]. If medical therapy does not lead to thrombus dissolution within 1–4 weeks, surgery should be considered.
In conclusion, timely CT and CTA for in-house acute ischaemic stroke is crucial to guide decisions on tPA and MT. Tandem (extracranial and intracranial) occlusion is not a contraindication for MT in acute stroke with large vessel thrombosis. Patients with ILCT without atheromatous changes can be treated with acute thrombolysis, MT, anticoagulation, acute or delayed carotid endarterectomy or endovascular intervention. Identification of recent infection as a temporary risk factor for stroke and careful consideration of abnormal, but insignificant findings (such as grade I mitral regurgitation and slight LAE), provided clues to cardioembolism as a cause of this patient’s stroke and ILCT.
Accepted for review: 28. 6. 2018
Accepted for print: 13. 2. 2019
The authors would like to thank MUDr. Jaroslav Boudny, PhD. (University Hospital Brno, Department of Radiology, Brno) for a valuable consultation regarding this case.
This work was supported by MH CZ – DRO (FNBr, 65269705).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manu script met the ICMJE “uniform requirements” for biomedical papers.
MUDr. Marek Čierny
Medical College of Wisconsin
9200 W Wisconsin Ave, Milwaukee
532 26 Wisconsin
USA
e-mail: m.cierny@mail.muni.cz
Sources
1. Iwata T, Mori T, Tajiri H et al. Safety and effectiveness of emergency carotid artery stenting for a high-grade carotid stenosis with intraluminal thrombus under proximal flow control in hyperacute and acute stroke. J Neurointerv Surg 2013; 5(1): 40–44. doi: 10.1136/neurintsurg-2011-010147.
2. Bhatti AF, Leon LR Jr, Labropoulos N et al. Free-floating thrombus of the carotid artery: literature review and case reports. J Vasc Surg 2007; 45(1): 199–205. doi: 10.1016/j.jvs.2006.09.057.
3. Vellimana AK, Kadkhodayan Y, Rich KM et al. Symptomatic patients with intraluminal carotid thrombus: outcome with a strategy of initial anticoagulation. J Neurosurg 2013; 118(1): 34–41. doi:10.3171/2012.9.JNS12406.
4. Gülcü A, Gezer NS, Men S et al. Management of free-floating thrombus within the arcus aorta and supra-aortic arteries. Clin Neurol Neurosurg 2014; 125 : 198–206. doi: 10.1016/j.clineuro.2014.08.008.
5. Paganini-Hill A, Lozano E, Fischberg G et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke 2003; 34(2): 452–457. doi: 10.1161/01.STR.0000053451.28410.98.
6. Grabska K, Gromadzka G, Członkowska A. Infections and ischemic stroke outcome. Neurol Res Int 2011; 2011(1): 1–8. doi: 10.1155/2011/691348.
7. Ionita CC, Siddiqui AH, Levy EI et al. Acute ischemic stroke and infections. J Stroke Cerebrovasc Dis 2011; 20(1): 1–9. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.011.
8. Vera R, Lago AB, Fuentes B et al. In-hospital stroke: a multi-centre prospective registry. Eur J Neurol 2011; 18(1): 170–176. doi: 10.1111/j.1468-1331.2010.03105.x.
9. Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371(9627): 1861–1871. doi: 10.1016/S0140-6736(08)60800-4.
10. Cumbler E. In-hospital ischemic stroke. Neurohospitalist 2015; 5(3): 173–181. doi: 10.1177/1941874415588319.
11. Powers WJ, Rabinstein AA, Ackerson T et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association 2018. Stroke 2018 : 49(3): e46–e110. doi: 10.1161/STR.0000000000000158.Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2019 Issue 2-
All articles in this issue
- Intradural extramedullary spinal cord tumors
- Multiple sclerosis and the role of gut microbiota during a harmful inflammatory response
- Genetic and neurobiological aspects of comorbid occurence of autism spectrum disorder and epilepsy
- Extra-intracranial bypass indicated during neurorehabilitation due to cognitive deterioration
- Traumatic pseudoaneurysm of the superficial temporal artery
- Multiple sclerosis, pregnancy, maternity, and breastfeeding
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
- Modern microsurgery as a permanent, safe and gentle solution of unruptured intracranial aneurysms
- Surgical explantation of a vagal nerve stimulator according to the magnetic resonance imaging protocol
- General movements and neurological development of the early age in children with neonatal hypoglycemia
- Comparison of cosmetic effects after short longitudinal and transverse skin incision for carotid endarterectomy
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Analýza dat v neurologii LXXIV. - Neparametrický Spearmanův koeficient korelace
- Recenze knih
- Doc. Vladimír Škorpil, 100 let od narození zakladatele naší elektromyografie
- Does leptin have a role in the development of intracranial meningiomas?
- A comparative study of myasthenic patients in the Czech and Slovak Republics
- Changes in essential and trace elements content in degenerating human intervertebral discs do not correspond to patients’ clinical status
- How extracellular sodium replacement affects the conduction velocity distribution of rats’ peripheral nerves
- Aneurysmal subarachnoid haemorrhage in pregnancy – successful clipping after coiling failure
- Tick-borne meningitis complicated by a cardioembolic intraluminal carotid artery thrombus and stroke
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Intradural extramedullary spinal cord tumors
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

