-
Medical journals
- Career
Changes in essential and trace elements content in degenerating human intervertebral discs do not correspond to patients’ clinical status
Authors: R. Staszkiewicz 1,2; F. Bolechala 3; J. Wieczorek 4; S. Drewniak 2; W. Strohm 2; J. Miodoński 2; T. Francuz 5; W. Marcol 1
Authors‘ workplace: Department of Physiology, Medical University of Silesia Katowice, Poland 1; Departament of Neurosurgery, 5th Military Hospital with Polyclinic in Cracow, Poland 2; Chair and Department of Forensic Medicine, Jagiellonian University Medical College, Krakow, Poland 3; Department of Agricultural and Environmental Chemistry, University of Agriculture in Krakow, Poland 4; Department of Biochemistry, Medical University of Silesia Katowice, Poland 5
Published in: Cesk Slov Neurol N 2019; 82(2): 203-208
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2019203Overview
Aim: To date, there has been no paper considering the disc degeneration process in respect of the content of essential and trace elements in degenerating discs tissue, clinical status of patients, and imaging analysis. Concentration of essential and trace elements in disc tissue may be a consequence of both environmental and genetic factors. The study aims to analyse and assess the contents of essential and trace elements in intervertebral discs.
Patients and methods: The material of 19 intervertebral discs was obtained from 17 patients during lumbar discectomy. Control was 9 healthy discs obtained from organ donors. Atomic absorption spectrometry was used to assess levels of Cu, Fe, Mn, Pb, Zn, Na, Mg, K, Ca, and P in the tissue, as well as dry weight (d.w.) of the tissue.
Results: All 10 essential and trace elements were detected in all samples. A significant increase of Ca, Mg, Fe, and P, and decrease of Cu and K in operated discs was found; the remaining changes between unhealthy and healthy discs were not significant. There were no age / elements, Pfirrmann grade / elements, or Modic grade changes / elements correlations. A significant positive correlation was found between Mg and Zn, K and Fe, Ca and Zn, Ca and Mg, P and Zn, P and Mg, and P and Ca. A negative correlation was only indicated between age and Na. Ca levels were higher in the degenerating disc group than in the healthy group.
Conclusion: A lack of correlation between the Ca content and the stage of intervertebral disc degeneration as well as the age of patients in the degenerating disc group is an unexpected result.
人椎间盘退变过程中必需元素和微量元素含量的变化与患者的临床情况并不相符
目的:
到目前为止,还没有文献考虑椎间盘退变过程中退变组织中必需和微量元素的含量、患者的临床状况以及影像学分析。椎间盘组织中必需和微量元素的富集可能是环境和遗传因素共同作用的结果。本研究旨在分析和评价椎间盘中必需和微量元素的含量。
患者和方法:
对17例腰椎间盘摘除术患者的19个椎间盘材料进行分析。对照组为9例来自器官捐赠者的健康椎间盘。采用原子吸收光谱法测定组织中铜、铁、锰、铅、锌、钠、镁、钾、钙、磷的含量,以及组织的干重(d.w.)。
结果:
所有样品中均检出10种必需及微量元素。手术椎间盘中Ca、Mg、Fe、P明显升高,Cu、K明显降低;不健康和健康椎间盘之间的剩余变化不显著。年龄/因素、Pfirrmann评分/因素、Modic评分变化/因素之间无相关性。Mg与Zn、K与Fe、Ca与Zn、Ca与Mg、P与Zn、P与Mg、P与Ca呈显著正相关,仅年龄与Na呈负相关。椎间盘退变组Ca水平高于正常组。
结论:
Ca含量与椎间盘退变的分期及退变椎间盘组患者的年龄之间缺乏相关性是一个出乎意料的结果。
关键词:
椎间盘退变-必需和微量元素-腰椎间盘疾病
Keywords:
intervertebral disc degeneration – essential and trace elements – lumbar disc disease
Introduction
The process of degeneration of human intervertebral discs is a problem which has been investigated in a number of ways, and yet there are still many elements which remain unexplained. Many putative factors concerning its aetiology have been suggested, such as genetics, which seem the most important, as well as causes of an environmental origin [1 – 5].
The trigger point in disc degeneration is an injury of the disc, and the changes following the injury are aberrant cell-mediated responses to the structural damage [1].
Factors like age, genetic inheritance, and a history of inadequate transport and loading of metabolites can weaken discs to such an extent that structural failure occurs during daily activities such as physical effort, or even sneezing or coughing [1,2].
At the moment, we are faced with the chicken and egg scenario – which came first, the chicken or the egg? Are the structural changes a cause or an effect of the disc degeneration process?
Many factors and substances were examined in intervertebral discs to answer the following questions: 1. what is disc degeneration; 2. what triggers it, and 3. how does the process continue.
A degenerating intervertebral disc undergoes many changes which are different in nature, such as biomechanical (structural damage) or biochemical (levels of many complex substances as well as chemical elements in the tissue are changing).
In this paper, the author would like to focus attention on the chemical changes in degenerating intervertebral discs, and the relationship of these changes to the clinical status of the patients.
Excessive deposition of selected elements in an avascular adult intervertebral disc can lead to the acceleration of a cascade of adverse metabolic changes that normally occur during ageing; such a situation reflects negatively the stability of the intercellular matrix of disc pulpose.
To date, the correlations between changes in the contents of selected elements in the intervertebral discs and the clinical patients’ status have not been studied. Such examination might provide more light on the pathogenesis of the disc degeneration process and perhaps newer therapeutic approaches.
The first aim of this study was an evaluation of the differences of the essential and trace elements concentration in intervertebral disc tissue between healthy people and patients with degenerative changes. We decided to analyse (according to the other authors [6,7]) the following elements: Cu, Fe, Mn, Pb, Zn, Na, Mg, K, Ca, and P.
The second purpose of our study was to investigate the correlation of the content of these elements with clinical characteristics of the patients with intervertebral disc degeneration.
Patients and methods
The study was approved by the Bioethics Committee of the Medical University of Silesia (decision number KNW/ 0022/ KB/ 42/ 15). Nineteen specimens were obtained from 17 patients (7 women, mean age 41.7 years) during lumbar discectomies. All specimens were prolapsed lumbar intervertebral disc. All patients matched two basic criteria: 1. discectomy was their first spinal surgery; 2. standard pre-operational 2-month pharmacotherapy was not effective. Sixteen samples were collected from the left site, and three from the right side. Seven specimens were obtained from L5/ S1, eleven from L4/ L5, and one from L3/ L4 level. All patients were examined and interviewed; data regarding age, gender, level and site of operation, as well as intensity of the pain measured by means of Visual Analogue Scale (VAS) [5,8] were collected and are presented in Tab. 1.
1. Gender, age, location, level of surgery and complaint level in Visual Analogue Scale in patients in the experimental group 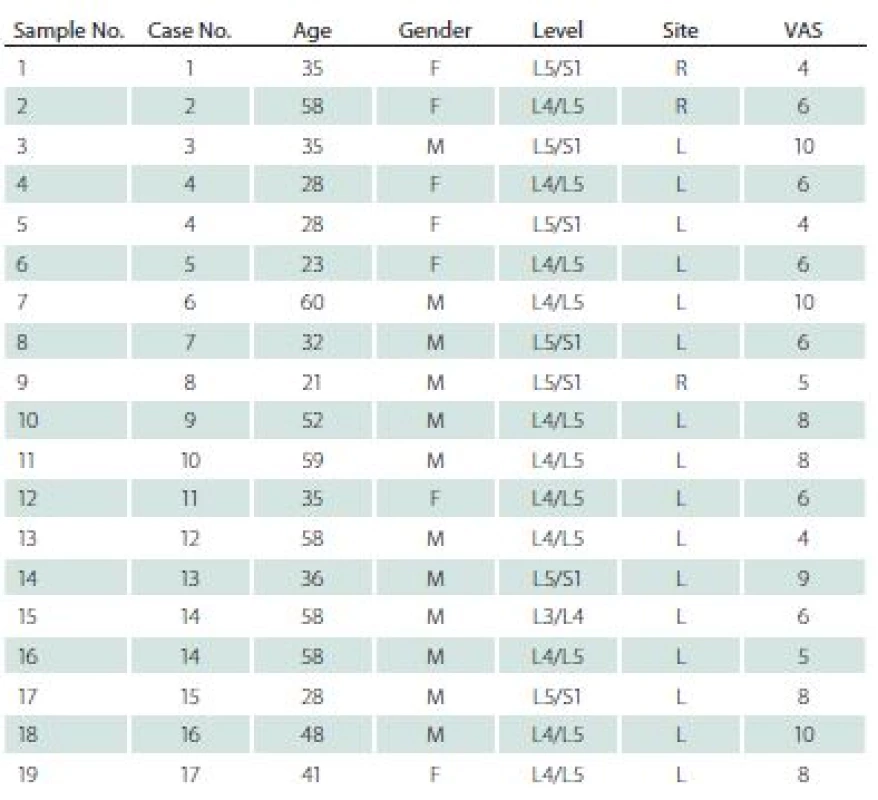
F – female; M – male; L – left; R – right; VAS – Visual Analogue Scale Before surgery, all patients underwent a MRI examination. In this examination, each intervertebral disc and vertebral body were analysed in terms of degeneration stage (Pfirrmann grade) [9], level of surgery, and Modic type endplate changes in adjacent vertebral bodies [10] (Tab. 2).
2. Degeneration changes of discs and adjacent vertebral bodies assessed in Pfirrmann and Modic scales in the presented group. 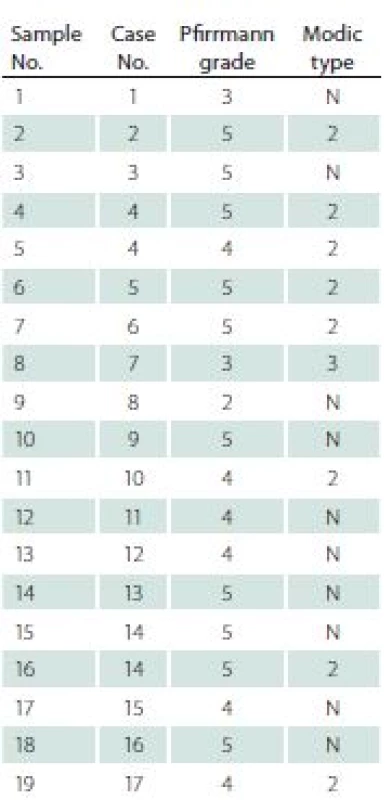
N – no Modic type changes The control group was constituted of nine intervertebral discs collected from three patients diagnosed with brain death, who were referred to be organ donors. All these discs were then evaluated for the presence of degenerative changes by two qualified board--certified pathologists. Only completely healthy discs, with no degenerative changes, were included as controls in the research.
Laboratory analyses
Immediately after surgery, specimens were deep frozen at – 80 °C and stored. All samples of intervertebral discs were determined in terms of: dry matter (d. m.) and contents of Cu, Fe, Mn, Pb, Zn, Na, Mg, K, Ca and P.
The d. m. content of analysed discs was determined by weighing a sample before and after complete drying at 105 °C. On the basis of the difference in sample mass before and after drying, the percentage of dry mass was calculated.
In order to determine the content of elements, material was digested using the wet method in a closed system in a microwave oven (Multiwave 3000, Anton Paar, Graz, Austria). About 0.5 g of d. m. of samples was treated with a 7 cm3 mixture (1 : 6 v/ v) of concentrated acids, HCl and HNO3 (Suprapure, Merck, Darmstadt, Germany). Digestion was conducted in teflon vessels with maximum power of the oven (1,400 W) for 25 min. The concentration of elements in the obtained filtrate was determined with the use of a PerkinElmer Optima 7300 DV (PerkinElmer, Inc., Waltham, MA, USA), inductively coupled plasma optical emission spectrometer. Each analysis was repeated to ensure the accuracy of the result. If the replication analyses results differed from one another by more than 5%, another two analyses were conducted for that same sample. The results were given in mg × kg– 1 d. m.
Statistical analysis
Data were analysed using the Statistica 8.0. computer software (StatSoft, Inc., Tulsa, OK, USA). All variables were tested for normality of distribution using the Shapiro-Wilk test. Statistical analysis was conducted using the nonparametric Kruskal-Wallis / Mann-Whitney U test with post-hoc Dunna test as well as the analysis of variance for parametric data (Analysis of Variance [ANOVA] test with post-hoc RIR Tukey test). The correlation rate was calculated using the Spearman’s test. The Spearman rank correlation coefficient was determined. Statistical significance was set at a P value of less than 0.05.
Results
The content of Fe, Zn, Na, Mg, K, Ca, and P in the discs were detected in high levels in all samples. Trace elements (Cu, Mn, Pb) were considered as present when their concentration exceeded 0.6 mg × kg– 1 d. m.
Mn was detected in 5 (26%) samples, Cu in 8 (42%) samples, and Pb in 4 (21%) samples.
The ranges of concentrations for particular elements were as follows (mean value ± standard deviation, range resp.) (Tab. 3).
3. The contents of elements in intervertebral discs. 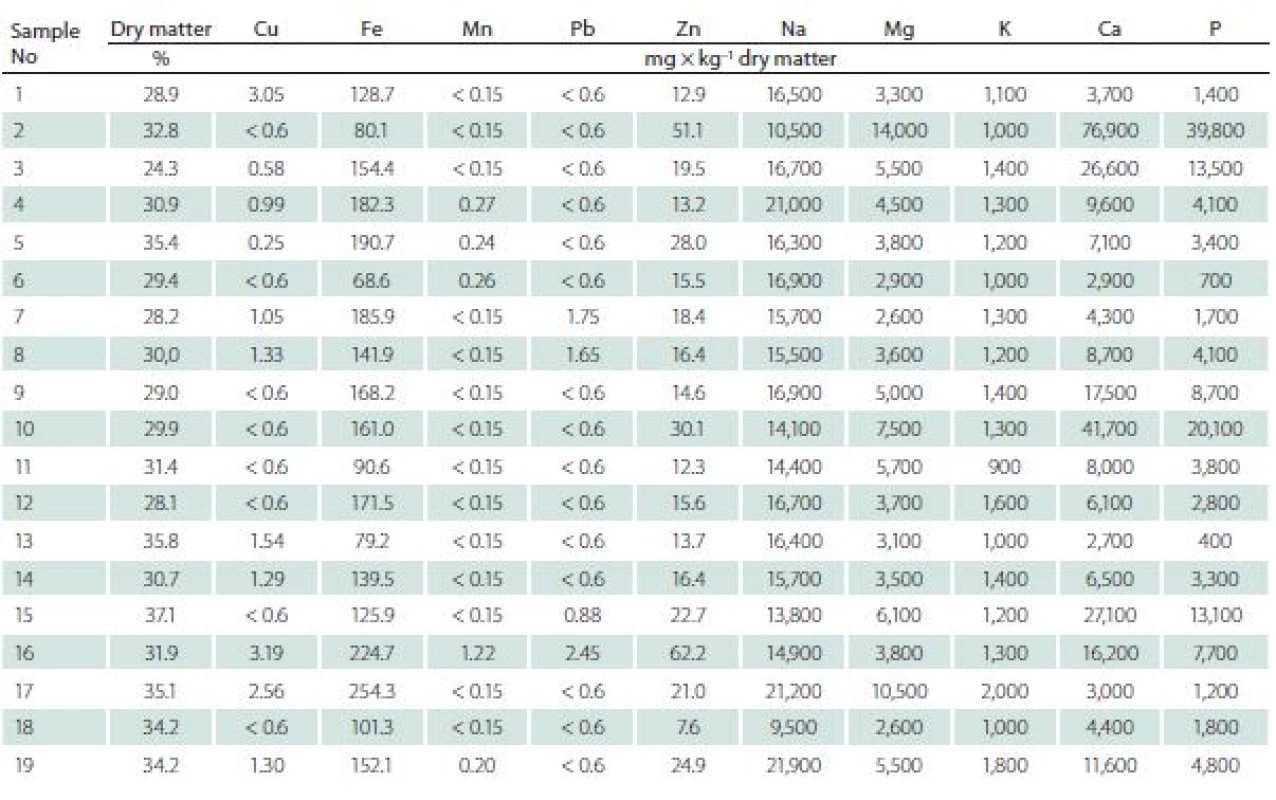
Cu mean was 1.19 ± 0.88 mg × kg–1 d.m., range of 0.25–3.19 mg × kg–1 d.m. in degenerating discs, while in the healthy ones the mean was 5.9 ± 1.51 mg × kg–1 d.m., range of 3.5–9.22 mg × kg–1 d.m.
Ca mean was 1.50 ± 1.82% of d.m., range of 0.27–7.69% of d.m. in degenerating disc, while in healthy ones the mean was 0.04 ± 0.02% of d.m., range of 0.02–0.07% of d.m.
Fe mean was 147.42 ± 49.94 mg × kg–1 d.m., range of 68.60–254.30 mg × kg–1 d.m. in degenerating discs, while in healthy ones the mean was 104.57 ± 16.84 mg × kg–1 d.m. range of 75.00–125.60 mg × kg–1 d.m.
K mean was 0.13 ± 0.03% of d.m., range of 0.09 –0.20% of d.m. in degenerating disc, while in healthy ones the mean was 0.30 ± 0.11% of d. m., range of 0.17–0.45% of d.m.
Mg mean was 0.51 ± 0.29% of d.m., range of 0.26–1.40% of d.m., in degenerating disc, while in healthy ones the mean was 0.03 ± 0.01% of d.m., range of 0.26 –1.4% of d.m.
Mn mean was 0.23 ± 0.24 mg × kg–1 d.m. range of 0.15–1.22 mg × kg–1 d.m. in degenerating disc, while in healthy ones the mean was 0.20 ± 0.16 mg × kg–1 d.m., range of 0.15–0.62 mg × kg–1 d.m.
Na mean was 1.60 ± 0.31% of d.m. range of 0.95–2.16% of d.m. in degenerating disc, while in healthy ones the mean was 1.43 ± 0.20% of d.m., range of 1.08–1.78% of d.m.
P mean was 0.72 ± 0.95% of d.m. range of 0.04–3.98% of d.m., in degenerating disc, while in healthy ones the mean was 0.13 ± 0.03% of d.m., range of 0.08–0.15% of d.m.
Pb mean was 0.83 ± 0.32% of d.m., range of 0.60–1.47% of d.m. in degenerating disc, while in healthy ones the mean was 0.83 ± 0.52% of d.m., range of 0.60 –2.45% of d.m.
Zn mean was 21.90 ± 13.59 mg × kg–1 d.m., range of 7.60–62.20 mg × kg–1 d.m. in degenerating disc, while in healthy ones it was 21.76 ± 13.59 mg × kg–1 d.m., range of 7.60–62.20 mg × kg–1 d.m.
The differences between healthy and degenerating discs appeared significant for Cu, Fe, Mg, Ca, K and P (Tab. 4).
4. The comparison of elements content in healthy and degenerating discs (mg × kg–1 dry matter). 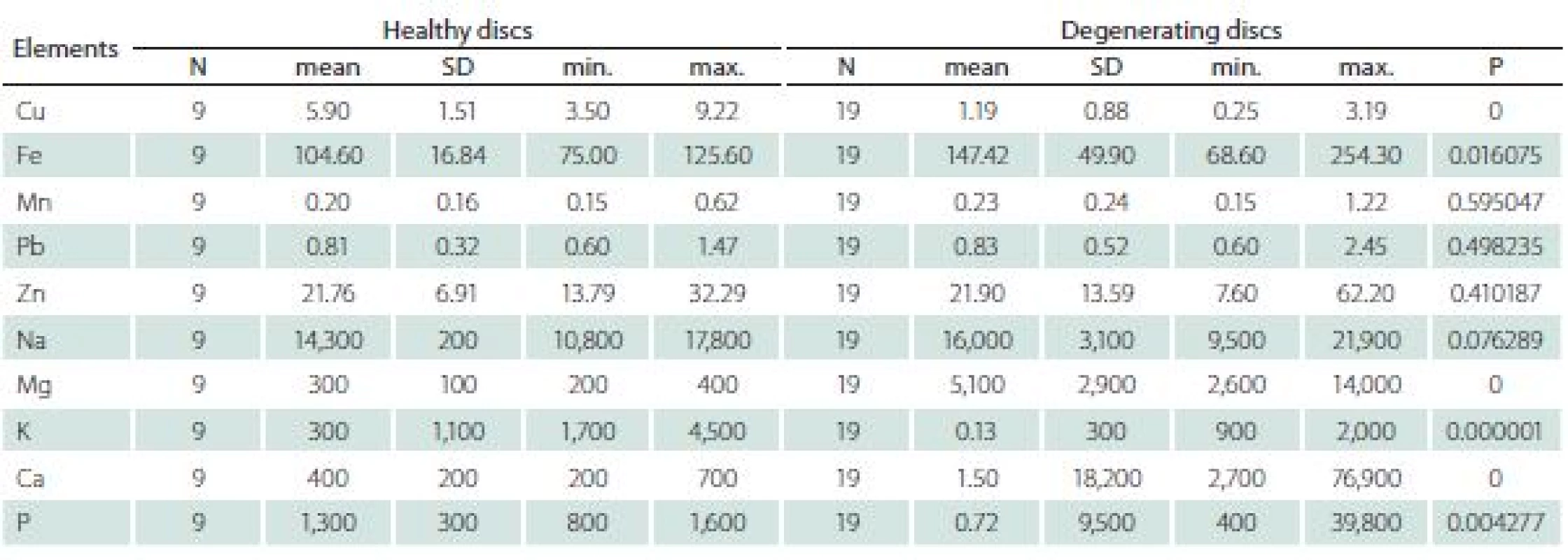
N – number of patients; SD – standard deviation The levels of K and Cu were higher in healthy discs, while levels of P, Ca, Mg and Fe were higher in degenerating discs.
In the surgery group, the correlation analysis revealed a significant negative relationship between age and sodium content. A positive correlation was indicated between VAS and Pfirrmann grade, Fe and K, Zn and Mg, Cu and P, Na and K, Mg and Ca, Mg and P (Tab. 5).
5. Correlation between elements, age, Visual Analogue Scale, and Pfi rrmann grade. 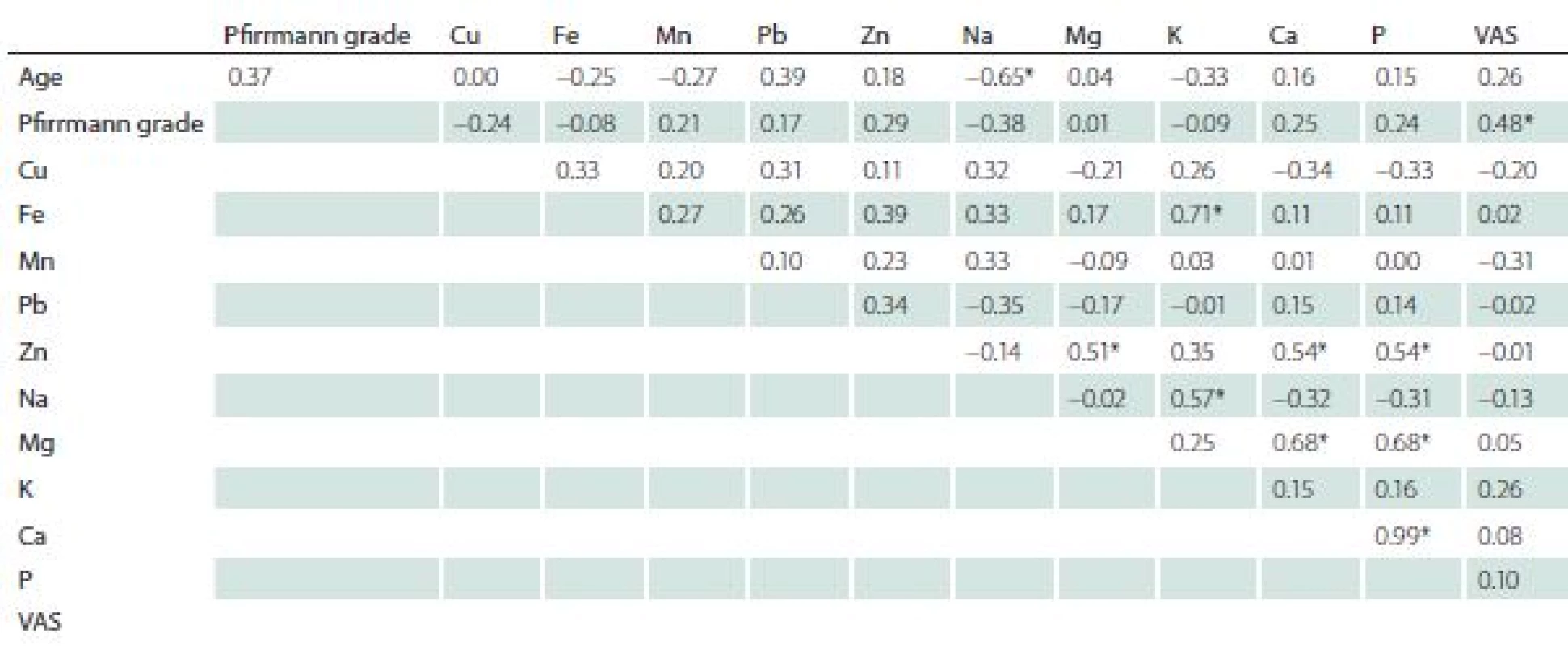
* significant P < 0.05<{r> VAS – Visual Analogue Scale Discussion
Analysis of selected trace elements in degenerating intervertebral discs showed a significant increase of Fe, Mg, Ca and P, and a decrease in Cu and K as compared with healthy ones.
Calcium
Calcium is one of 21 essential elements for humans [11]; it regulates many intracellular and extracellular processes. Dysregulation in production, level, or transport of Ca is always associated with a disease. Ca deposits are well known elements of disc degeneration; however, the role of the deposits in the degeneration process is still unclear. Several papers showed that there was a correlation between the presence of Ca crystals and disc degeneration phenomenon [12 – 14]. Some data advocate that deposits present in degenerated intervertebral discs are made of calcium pyrophosphate dihydrate, and this phenomenon is more associated with the previous history of trauma or surgery [12]. On the other hand, there are data which state that the calcium pyrophosphate dihydrate is both the cause and effect of disc degeneration [13]. In the literature, there is still a lack of quantitative analysis; therefore, data contained in this paper may be helpful in understanding the process of disc degeneration. In the degenerating disc group, there was no correlation between age and Ca levels, as well as between Ca levels and Pfirrmann grade of degeneration. Considering the differences in Ca concentration between healthy and degenerating discs, there was a higher Ca content in degenerating discs than in healthy ones.
Copper
Copper is an active metal characteristic for organisms living in an oxygen-rich environment. It is associated with animal proteins involved in reduction-oxygenation processes [3]. Many enzymes harness the changes in the Cu oxidation stage to catalyse redox reactions in a numerous range of biochemical transformations [15]. Cu plays an important role in cell haemostasis, and cell signalling processes. Moreover, Cu handling and Cu utilising proteins control metabolic changes in cancer cells known as the Warburg effect – the down-regulation of cell respiratory capacity observed in cancer cells [15]. In examined groups, there was a higher concentration of Cu in the healthy group, which may be a consequence of improved oxygenation and improved blood supply to healthy disc tissue [4].
Iron
The role of Fe in many processes is difficult to overestimate. As a very important component of haemoglobin, it plays a significant role in oxygen transport. There are four classes of Fe-related proteins: Fe containing haeme proteins (haemoglobin, myoglobin, cytochromes), iron sulfur enzymes (flavoproteins, haemaflavoproteins), proteins for Fe storage and transport (transferrin, lactoferrin), and other Fe-containing and Fe-activated enzymes. The role of Fe in the disc degeneration process is still unknown, and there are no papers describing this topic [16]. The content of Fe was higher in degenerating discs, and this result was statistically significant.
Sodium and potassium
Sodium and potassium as well as their attendant anions are important components of all body fluids [17]. Na and K play a principal role in maintaining body fluid homeostasis. Levels of these ions are important for water balance, and disc dehydration is one of the components of disc degeneration. These two ions are important in the creation of nerve impulses, using concentration gradients across plasma membrane produced by Na(+), K(+) adenosine triphosphate (ATP)-ase [18].
Our results show higher levels of K in healthy discs, and a negative correlation between age and the content of Na; both results are statistically significant.
Magnesium
Magnesium is the second most abundant intracellular cation, and fourth cation in terms of abundance for the whole body. This cation is essential for the synthesis of nucleic acids and proteins, and plays a role in Ca metabolism by competing with Ca for membrane binding. Mg has many important biological functions, such as intracellular energy metabolism cell replication, and protein synthesis [19]. Levels of Mg were higher in degenerating discs and the difference was statistically significant.
Manganese
Manganese is essential for bone formation. It plays an important role in the metabolism of amino acids, lipids, and carbohydrates. Glycosyltransferases and xylotransferases are important in proteoglycan synthesis and they are very sensitive in the presence of Mn. Thus, the latter can play a role in the disc degeneration process [6]. The levels of Mn in healthy discs and degenerating discs were similar, and the difference was not statistically significant.
Phosphorus
Numerous normal physiologic functions are dependent on P, including skeletal development, cell membrane phospholipid content and function, cell signalling, platelet aggregation, and energy transfer through mitochondrial metabolism [6]. P is essential for the bone mineralisation process [20]. The level of P was higher in degenerating discs, and the difference was statistically significant.
Lead
Lead accumulates in bones and its concentration tends to increase with age, because lead is difficult to remove from the tissue [21]. More than 90% of the body’s Pb burden is found in the skeleton [8]. The biological half--life of lead is about one month for soft tissue, it is longer – years – for trabecular bones, and decades for cortical bones [22]. Pb can cause several adverse health effects, such as neuropathy, encephalopathy, and kidney damage. Pb levels in intervertebral discs should not be high, because most Pb cumulates in bones. This is the case in the presented group, where only four specimens showed Pb levels higher than 0.60 mg × kg–1 ; the difference between healthy and degenerating discs was not significant.
Zinc
Zinc is a component of various enzymes; it forms and helps to maintain the structural integrity of proteins and regulates gene expression [16]. The biological role of Zn can be divided into three categories: structural, catalytic, and regulatory. Zn plays a crucial role in the immune system, and Zn-deficient individuals present increased susceptibility to infection [23]. The inflammatory process in disc degeneration is still to be examined and at the moment we know that it is a part of the whole degeneration process [1,24,25]. Moreover, matrix metalloproteinases are Zn--dependent enzymes, and these enzymes are responsible for extracellular matrix synthesis and degradation. Balance between these two processes is a basic condition to stop the degeneration process [1,24]. Zn levels may indirectly indicate a metalloproteinase concentration and activity in the disc tissue. The difference in Zn levels between healthy and degenerating tissue was not statistically significant.
Conclusions
The study is one of only a few to present elements concentration in vertebral disc tissue; moreover, there are no papers analysing either a healthy control group or the clinical status of the patients analysed (MRI images and elements contents).
The results showing differences between healthy and degenerating discs in terms of Ca levels are particularly important. There is a statistically significant difference between healthy and degenerating discs (the level of Ca is higher in degenerating discs), while there is no correlation between Ca levels and the age of the patient, and Ca levels and disc degeneration stage. The examined group may be too small to demonstrate such correlation, but if this fact is confirmed upon further examination, questions regarding Ca chemistry and its role in disc degeneration process should be formulated.
Levels of other elements may be influenced by diet, and other exogenous factors such as contamination which is associated with technological development of the dwelling location.
Identification of significant factors as well as their influence on the disc degeneration process are both issues still demanding further investigation.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 24. 9. 2018
Accepted for print: 7. 2. 2019
Wiesław Marcol, MD, PhD
Medical University of Silesia
Medyków 18
407 52 Katowice
Poland
e-mail: wmarcol@tlen.pl
Sources
1. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006; 31(18): 2151 – 2161.
2. Downie WW, Leatham PA, Rhind VM et al. Studies with pain rating scales. Ann Rheum Dis 1978; 37(4): 378 – 381.
3. Gutierrez PL. The metabolism of quinone-containing alkylating agents: free radical production and measurement. Front Biosci 2000; 5: D629 – D638.
4. Liang C, Li H, Tao Y et al. New hypothesis of chronic back pain: low pH promotes nerve ingrowth into damaged intervertebral disks. Acta Anaesthesiol Scand 2013; 57(3): 271 – 277. doi: 10.1111/ j.1399-6576.2012.02670.x.
5. Urban JP, Winlove CP. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging 2007; 25(2): 419 – 432.
6. Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 2006; 46(8): 621 – 628.
7. Kepler CK, Ponnappan RK, Tannoury C et al. The molecular basis of intervertebral disc degeneration. Spine J 2013; 13(3): 318 – 330.
8. Berlin K, Gerhardsson L, Borjesson J et al. Lead intoxication caused by skeletal disease. Scand J Work Environ Heal 1995; 21(4): 296 – 300. doi: 10.1016/ j.spinee.2012.12.003.
9. Pfirrmann CW, Metzdorf A, Zanetti M et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001; 26(17): 1873 – 1878.
10. Modic MT, Steinberg PM, Ross JS et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988; 166(1 Pt 1): 193 – 199.
11. Weaver CM, Heaney RP (eds). Calcium in human health. Totowa, NJ: Humana Press 2006.
12. Berlemann U, Gries NC, Moore RJ et al. Calcium pyrophosphate dihydrate deposition in degenerate lumbar discs. Eur Spine J 1998; 7(1): 45 – 49.
13. Gruber HE, Norton HJ, Sun Y et al. Crystal deposits in the human intervertebral disc: implications for disc degeneration. Spine J 2007; 7(4): 444 – 450.
14. Lee RS, Kayser MV, Ali SY. Calcium phosphate microcrystal deposition in the human intervertebral disc. J Anat 2006; 208(1): 13 – 19.
15. Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 2009; 284(2): 717 – 721. doi: 10.1074/ jbc.R800055200.
16. Trumbo P, Yates AA, Schlicker S et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 2001; 101(3): 294-301.
17. Motulsky AG. National Research Council (US) Committee on diet and health. Diet and health: implications for reducing chronic disease risk. Washington (DC): National Academies Press (US) 1989.
18. Clausen MJ, Poulsen H. Sodium/ Potassium homeostasis in the cell. Met Ions Life Sci 2013; 12 : 41 – 67. doi: 10.1007/ 978-94-007-5561-1_3.
19. Swaminathan R. Disorders of magnesium metabolism. CPD Bull Clin Biochem 2000; 2(1): 3 – 12.
20. Moe SM, Daoud JR. Disorders of mineral metabolism: calcium, phosphorus, and magnesium. In: National Kidney Foundation‘s Primer on Kidney Diseases, 6th ed.Elsevier Health Sciences 2013 : 100 – 112.
21. Kubaszewski Ł, Zioła-Frankowska A, Frankowski Met al. Atomic absorption spectrometry analysis of trace elements in degenerated intervertebral disc tissue. Med Sci Monit 2014; 20 : 2157 – 2164. doi: 10.12659/ MSM. 890654.
22. Nilsson U, Attewell R, Christoffersson JO et al. Kinetics of lead in bone and blood after end of occupational exposure. Pharmacol Toxicol 1991; 68(6): 477 – 484.
23. Shankar H. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998; 68(2 Suppl): 447S – 463S. doi: 10.1093/ ajcn/ 68. 2.447S.
24. Hadjipavlou AG, Tzermiadianos MN, Bogduk N et al. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br 2008; 90(10): 1261 – 1270. doi: 10.1302/ 0301-620X.90B10.20910.
25. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta 1981; 673(4): 443 – 453.Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2019 Issue 2-
All articles in this issue
- Intradural extramedullary spinal cord tumors
- Multiple sclerosis and the role of gut microbiota during a harmful inflammatory response
- Genetic and neurobiological aspects of comorbid occurence of autism spectrum disorder and epilepsy
- Extra-intracranial bypass indicated during neurorehabilitation due to cognitive deterioration
- Traumatic pseudoaneurysm of the superficial temporal artery
- Multiple sclerosis, pregnancy, maternity, and breastfeeding
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
- Modern microsurgery as a permanent, safe and gentle solution of unruptured intracranial aneurysms
- Surgical explantation of a vagal nerve stimulator according to the magnetic resonance imaging protocol
- General movements and neurological development of the early age in children with neonatal hypoglycemia
- Comparison of cosmetic effects after short longitudinal and transverse skin incision for carotid endarterectomy
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Analýza dat v neurologii LXXIV. - Neparametrický Spearmanův koeficient korelace
- Recenze knih
- Doc. Vladimír Škorpil, 100 let od narození zakladatele naší elektromyografie
- Does leptin have a role in the development of intracranial meningiomas?
- A comparative study of myasthenic patients in the Czech and Slovak Republics
- Changes in essential and trace elements content in degenerating human intervertebral discs do not correspond to patients’ clinical status
- How extracellular sodium replacement affects the conduction velocity distribution of rats’ peripheral nerves
- Aneurysmal subarachnoid haemorrhage in pregnancy – successful clipping after coiling failure
- Tick-borne meningitis complicated by a cardioembolic intraluminal carotid artery thrombus and stroke
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Intradural extramedullary spinal cord tumors
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

