-
Medical journals
- Career
Tick ‑ borne Encephalitis: Course and Complications – Our Observations from 2009 to 2012
Authors: M. Pýchová 1; J.strakova 2; P. Husa 1
Authors‘ workplace: Department of Infectious Diseases, Faculty of Medicine, Masaryk University and University Hospital Brno 1; Department of Nursing, Faculty of Medicine, Masaryk University 2
Published in: Cesk Slov Neurol N 2014; 77/110(3): 339-342
Category: Short Communication
Overview
Objective:
Description of the course, complications and permanent sequelae of tick ‑ borne encephalitis in patients hospitalized at the Department of Infectious Diseases of the Faculty of Medicine, Masaryk University and University Hospital Brno.Patients and methods:
Prospective monitoring of epidemiological, clinical and laboratory data of 107 patients diagnosed with tick ‑ borne encephalitis (TBE) and hospitalized between 2009 and 2012.Results:
The study set comprised 107 patients. The disease presented as meningitis in 50 patients, meningoencephalitis in 45 patients, and meningoencephalomyelitis in 12 patients (46.7%, 42.1% and 11.2%, respectively). Paresis developed in 14 patients. Permanent paresis was observed in 5.6% of patients. Two patients died of the disease (lethality 1.9%). Ninety seven patients completed six ‑ month follow‑up and 90 patients 12 – month follow‑up after discharge from the hospital. More than one third of patients presented with symptoms of post‑encephalitic syndrome one year after the acute phase of the disease subsided. Concomitant Lyme disease was recorded in 12 patients (11.2%). A statistically significant difference was not found between single and dual infections in severity of the disease and its complications.Conclusion:
Even when its course is uncomplicated, TBE requires several weeks of convalescence. Paresis frequently has permanent sequelae. Following the acute phase of the disease, more than one ‑ third of patients has long‑term complaints associated with the post‑encephalitic syndrome that significantly affect their quality of life. The risk of complications increases with age.Key words:
tick-borne encephalitis – post-encephalitic syndromeIntroduction
Tick ‑ borne encephalitis (TBE) is a viral disease affecting the nervous system. Approximately 3,000 cases are reported in Europe annually. The highest incidence is in Russia. The Czech Republic takes the second place with its 500 – 800 annual cases [1]. A virus belonging to the Flaviviridae family is the causative agent of the disease. It is a naked RNA virus, 20 – 50 nm in size. The disease affects a broad region reaching from the Central Europe to the Far East. There are three viral subtypes – European, Siberian, and Far ‑ Eastern subtype [2 – 4].
Just like in other Flaviviridae, transmission is via a blood ‑ sucking vector. In the Czech Republic, this is the sheep ‑ tick (Ixodes ricinus). The virus is contained in the tick’s salivary glands and its transmission commences at the first instances of sucking. Small mammals, particularly rodents, are the natural source of the virus. Humans and other mammals (goats, cows, sheep) are incidental hosts [2]. An infrequent but possible transmission pathway involves ingestion of non‑pasteurized milk of infected animals (mostly goat and sheep) or products made from such milk [2,3]. The clinical picture of the disease was first described in 1930s, when an Austrian doctor Schneider described seasonality of the occurrence of aseptic meningitis [4]. Incubation period of the disease is 2 – 28 days. Just as in other arboviral diseases, course of the disease is mostly asymptomatic. Symptomatic phase, however, follows typical biphasic course in two thirds of patients. The first, so ‑ called febrile phase of the disease, characterized by flu‑like symptoms, is a manifestation of viraemia. A period of 2 – 10 days of temporary improvement follows. The second phase of the disease then commences, characterized by development of neurological symptoms [5,6]. There are several forms of the disease, depending on the type of neural involvement – meningitis (approx. 50% of patients), encephalitis (40%) and meningoencephalomyelitis (10%); the most serious bulbar form affects the brain stem. It is typical for tick ‑ borne encephalitis that pareses can develop as late as 5 – 10 days after the febrile stage [7,8]. Development of so called post‑encephalitic syndrome, a collection of reversible non‑specific neuropsychiatric complaints resulting from nervous system inflammation (cephalea, fatigue, sleep disturbances, emotional lability etc.) represent another possible complication that may last for years [9]. TBE diagnostics is based on clinical examination, history of tick bite, inflammatory changes in the cerebrospinal fluid (CFS) (tens to hundreds of predominantly polynuclear cells) and the presence of specific antibodies in the serum [10]. TBE therapy is symptomatic, there is no causal medication available so far. Application of a specific immunoglobulin has also been previously tested. However, the effect of this treatment was questionable; clinical condition of six children worsened after the application [6,7,11]. TBE is, however, preventable by vaccination. An effective vaccine has been available since 1970s. Currently, two vaccines are available on the Czech market, both also in a pediatric form [12]. Vaccine manufacturers claim higher than 98% efficacy of the vaccines [13]. Preventive vaccination should be recommended mainly to older persons living in endemic areas as well as to persons with higher occupational risk, such as those employed in agriculture and forestry (125 cases of occupational TBE infections have been recorded in the Czech Republic between 1994 and 2009) [14].
Patients and methods
Patients diagnosed with TBE and hospitalized at the Department of Infectious Diseases at the University Hospital (UH) in Brno between 2009 and 2012, were included. Data were collected between May 2009 and June 2012. According to clinical symptoms, course of the disease was classified as meningitis (fever, cephalea, pleocytosis in the cerebrospinal fluid, nausea, photophobia), meningoencephalitis (concurrent tremor, ataxia, qualitative or quantitative disturbance of consciousness) and meningoencephalomyelitis (development of flaccid pareses). Patients were monitored both during hospitalization and in the course of three follow‑ups scheduled throughout the following year. Patient participation was voluntary. The follow‑ups were scheduled at 1, 6 and 12 months after discharge from the hospital. Presence of the following subjective complaints was evaluated: headache, fatigue, vertigo, and sleep, memory and concentration disturbances. Paresis, basic neurological examination including EEG, duration of persistence of TBE ‑ specific IgM antibodies in the serum and detection of concomitant Lyme disease (defined by the presence of erythema migrans or fulfilling diagnostic criteria for neuroborreliosis) were also assessed [15]. The parameters were recorded in a recording sheet. Collected data were evaluated using the STATISTIKA CZ software, version 10, and Microsoft Excel. Correlation between complications (development of pareses, impaired consciousness, death) and concomitant presence of Lyme disease was tested by the Pearson χ2 test at a significance level of 0.05. Given the sample size, distribution of quantitative variables (age, duration of hospitalization) was considered parametric.
Results
The study sample included 107 patients. Their mean age was 45.6 years (median 47.0 and standard deviation (SD) 16.1). The youngest was 18 (patients 18 years old and older only are hospitalized at the Department of Infectious Diseases, UH Brno) and the oldest was 88 years of age. 41.1% of the monitored patients were females, 58.9% were males. 28% of patients did not notice a tick bite prior to the illness. Possibility of alimentary acquisition of the disease (via a home ‑ made sheep’s milk cheese) was considered in one female patient. The most frequently reported incubation period was 3 – 8 days (27.1%). 21% of patients did not observe biphasic course. Meningitis symptoms appeared in 50 patients, meningoencephalitis in 45 patients, and meningoencephalomyelitis in 12 patients (46.7%, 42.1% and 11.2%, respectively). Mean duration of hospitalization was 14.3 days (median 14.0, SD 16.1, range 7 – 49 days). Patients with impaired consciousness were hospitalized for the mean of 15.7 days (median 15.0, SD, 3.2, range 9 – 22 days) and patients with paretic complications for the mean of 16.3 days (median 16.0, SD 4.8, range 9 – 30 days). A combination of impaired consciousness and paresis was identified in 6.5% of patients. Tab. 1 lists the different types of paretic complications (three patients had a combination of two types of paresis) and Tab. 2 their duration (two patients died during hospitalization). Clinical condition of two patients (44 - and 51‑year ‑ old ‑ men with no previous co ‑ morbidities) was complicated as they developed the bulbar form of the disease, necessitating vital function support (mechanical ventilation, temporary external ventricular stimulation), and paretic complications (triparesis and quadruparesis). Both cases were lethal (cause of death was bronchopneumonia). Concomitant Lyme disease was diagnosed in 12 patients. Two patients had typical erythema migrans (both had meningoencephalitis), 10 patients had neuroborreliosis (nine meningitis, one meningoencephalitis). Patient with meningoencephalitis had a combination of paresis (cranial nerves) and qualitative consciousness disturbance. One patient was admitted again three weeks after discharge because of gradual development of lower limb paraparesis. Neuroborreliosis was diagnosed (during the first examination, the patient had positive serology in the blood only, second examination revealed inthratecal production of specific antibodies). There also were another 11 patients with suspected neuroborreliosis (CFS pleocytosis and neurological symptoms with positive serology in the blood but no intrathecal production of anti‑borrelial antibodies). Follow‑up monitoring after discharge had a form of voluntary ambulatory examinations over one year. One month after discharge, 33/ 105 patients (30.8%) had abnormal EEG; EEG results were not documented in three patients. Tab. 3 presents selected symptoms of post‑encephalitic syndrome six and twelve months after discharge. For various reasons, only 97 of 107 patients completed the 6 – month follow‑up and 90 patients completed 12 – month follow‑up after discharge from the hospital. Specific IgM class of anti‑TBE antibodies were found in 36 patients (41.4%) one year after discharge.
1. Forms of paretic affection. 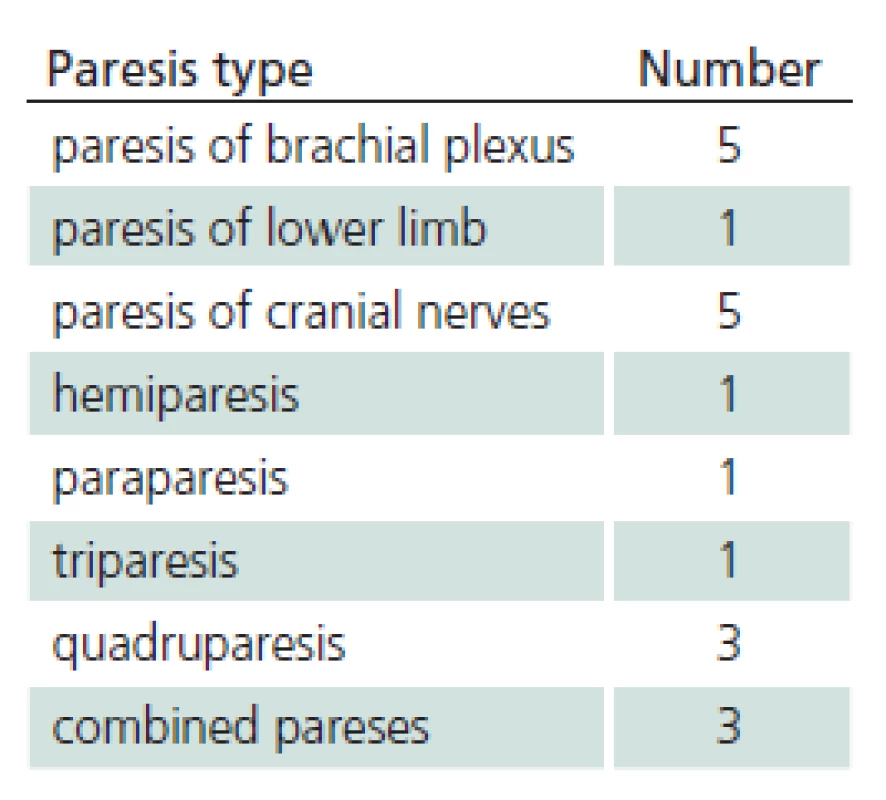
2. Duration of paretic affection. 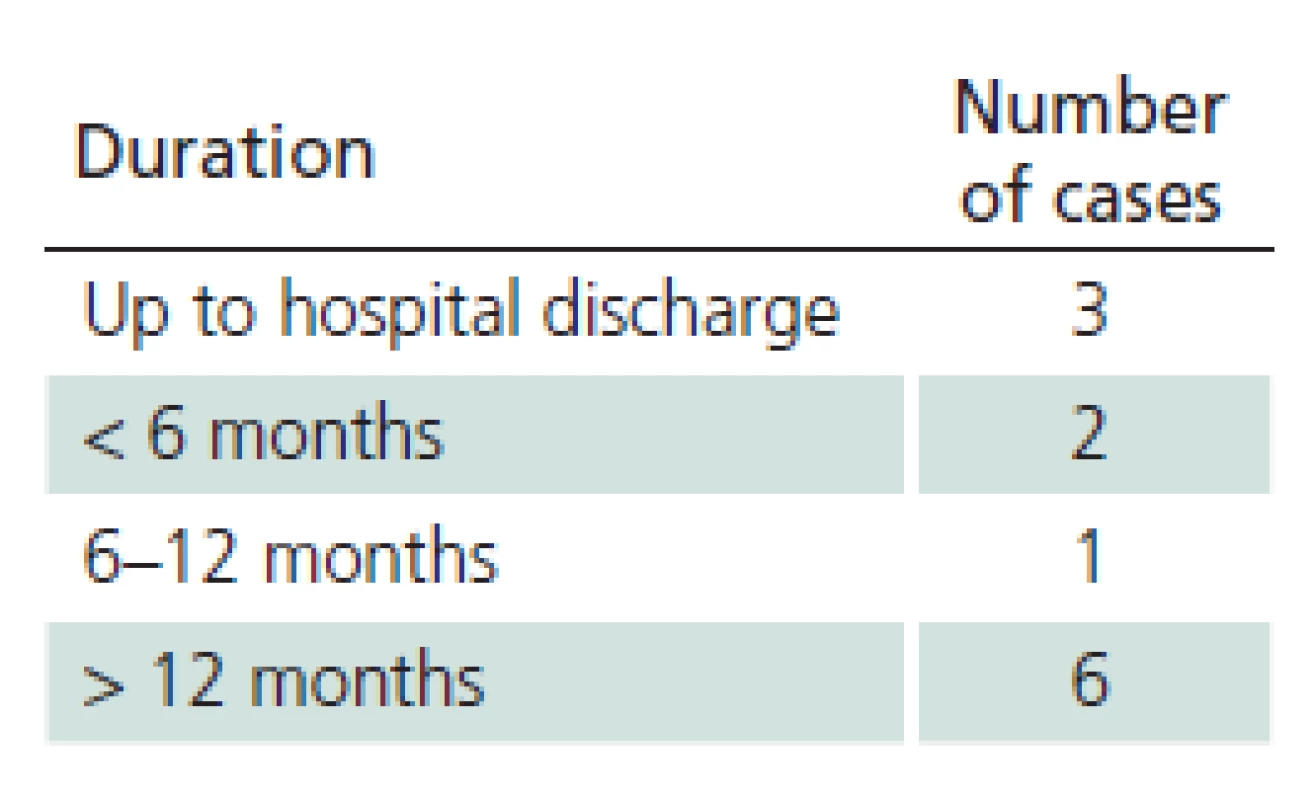
3. Post-encephalitic syndrome – monitored manifestations. 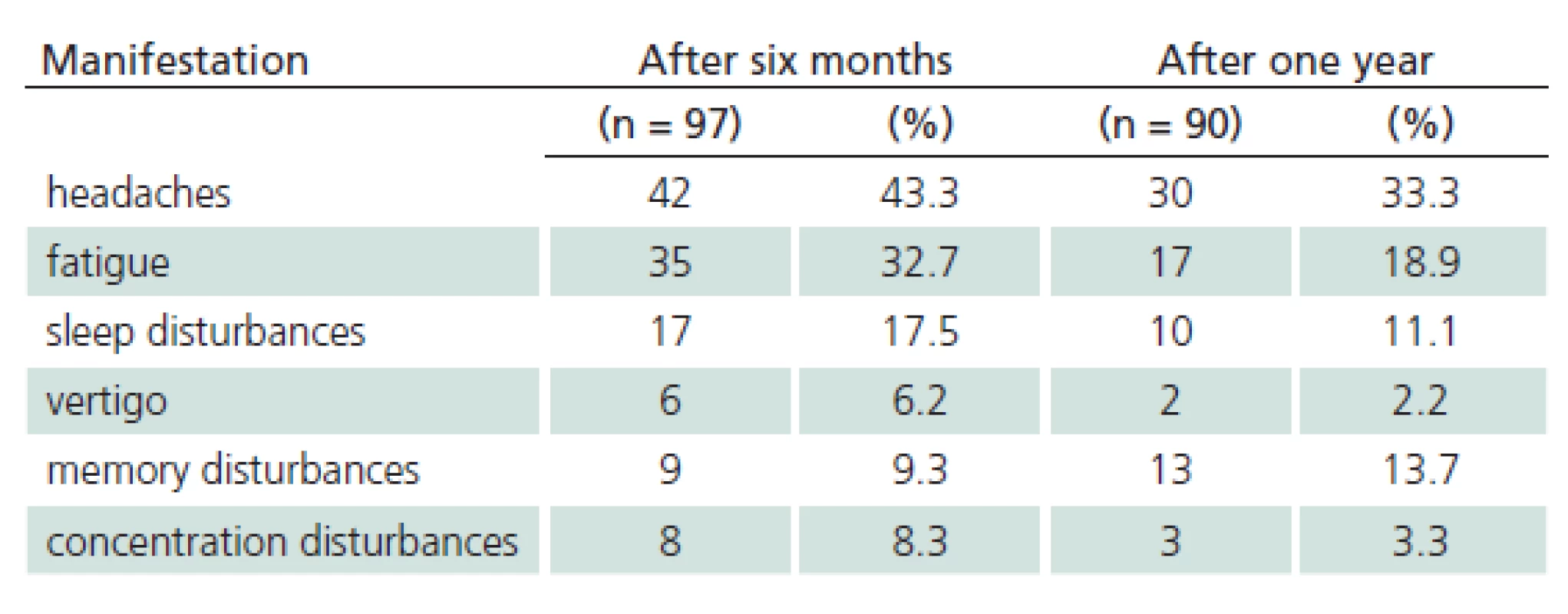
Discussion
The majority of our patients presented with meningitis (46.7%). This correlates with the results of similar prospective studies by Günther (55%) and Kaiser (49%) [16,17]. The typical biphasic course of the disease was observed in 2/ 3 of our patients only, and up to 1/ 3 of patients did not notice a tick bite. Paresis was observed in all meningoencephalomyelitis patients (12) and in two patients with meningoencephalitis (two patients with a paresis of a cranial nerve (one with concomitant neuroborreliosis), one patient with hemiparesis – 4.5% of patients with meningoencephalitis). Similar to our results (5.6%), Mickiene and Kaiser reported permanent residual paresis in 2.6 – 7% of respondents. In affected patients, and these are predominantly middle ‑ aged and older, neurological deficit is likely to be permanent. Lethality in our patient sample was 1.9%, corresponding to the available data for the European subtype of the virus [5,18]. Both deceased patients were middle ‑ aged. Approximately one tenth of patients (11.2%) were diagnosed with definitive Lyme disease. We did not identify any statistically significant correlation between selected TBE complications and concomitant Lyme disease (p = 0.87 for pareses, p = 0.25 for impaired consciousness). Also, no patient with concomitant borreliosis died. A group of Finnish authors reported a fatal case of TBE with concomitant borreliosis [19]. Each our patient with definite Lyme disease or possible borreliosis was treated with antibiotics [15,20]. We also monitored post‑encephalitic syndrome. In their prospective studies focusing on the course and complications of TBE, Smiskova and Günther reported on prolonged symptoms of post‑encephalitic syndrome in approximately one ‑ third of their patients; this is in line with our findings (39.6%) [8,16,17]. Post‑encephalitic syndrome is observed more often in TBE than other viral neuroinfections [16].
Conclusion
Uncomplicated clinical course of TBE requires several weeks of convalescence. Paresis frequently has permanent sequelae. Development of post‑encephalitic syndrome also significantly affects quality of life in the horizon of many months or years after infection. Our results suggest that, after the acute illness, approximately one third of patients has long‑term complaints, and their subsequent quality of life is significantly altered. The risk of complications increases with age. There is no causal therapy. It is, however, a vaccine ‑ preventable disease. Despite this, less than 20% of the Czech Republic population have been vaccinated.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Martina Pychova, MD
Department of Infectious Diseases, Faculty of Medicine
Masaryk University and University Hospital Brno
Jihlavska 20
625 00 Brno
e-mail: martina.pychova@fnbrno.cz
Accepted for review: 15. 8. 2013
Accepted for print: 31. 10. 2013
Sources
1. Kunze U. Tick ‑ borne encephalitis (TBE): An underestimated risk…still: Report of the 14th Annual Meeting of the International Scientific Working Group on Tick ‑ Borne Encephalitis (ISW ‑ TBE). Ticks Tick Borne Dis 2012; 3(3): 197 – 201.
2. Dobler G, Gniel D, Petermann R, Pfeffer M. Epidemiology and distribution of tick ‑ borne encephalitis. Wien Med Wochenschr 2012; 162(11 – 12): 230 – 238.
3. Cisak E, Wójcik ‑ Fatla A, Zając V, Sroka J, Buczek A,Dutkiewicz J. Prevalence of tick ‑ borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in eastern Poland. Ann Agric Environ Med AAEM 2010; 17(2): 283 – 286.
4. Dumpis U, Crook D, Oksi J. Tick ‑ borne encephalitis. Clin Infect Dis 1999; 28(4): 882 – 890.
5. Bogovic P, Lotric ‑ Furlan S, Strle F. What tick ‑ borne encephalitis may look like: clinical signs and symptoms. Travel Med Infect Dis 2010; 8(4): 246 – 250.
6. Lindquist L, Vapalahti O. Tick ‑ borne encephalitis. Lancet 2008; 371(9627): 1861 – 1871.
7. Mansfield KL, Johnson N, Phipps LP, Stephenson JR,Fooks AR, Solomon T. Tick ‑ borne encephalitis virus – a review of an emerging zoonosis. J Gen Virol 2009; 90(8): 1781 – 1794.
8. Smíšková D, Polívková S, Blechová Z, Marešová V. Klíšťová meningoencefalitida, klinický průběh a komplikace. Vakcinologie 2010; 4(3): 106 – 109.
9. Pícha D. Infekce nervové soustavy. In: Bednařík J, Ambler Z, Růžička E (eds). Klinická neurologie část speciální. 1st ed. Praha: Triton 2010.
10. Holzmann H. Diagnosis of tick ‑ borne encephalitis. Vaccine 2003; 21 (Suppl 1): S36 – S40.
11. Waldvogel K, Bossart W, Huisman T, Boltshauser E,Nadal D. Severe tick ‑ borne encephalitis following passive immunization. Eur J Pediatr 1996; 155(9): 775 – 779.
12. Zent O, Bröker M. Tick ‑ borne encephalitis vaccines: past and present. Expert Rev Vaccines 2005; 4(5): 747 – 755.
13. Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick ‑ borne encephalitis. Vaccine 2007; 25(43): 7559 – 7567.
14. Fenclová Z, Urban P, Pelclová D, Navrátil T. Neurologická profesionální onemocnění v České republice v letech 1994 – 2009. Cesk Slov Neurol N 2012; 75/ 108(1): 70 – 74.
15. Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I et al. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol Off J Eur Fed Neurol Soc 2010; 17(1): 8 – 16.
16. Günther G, Haglund M, Lindquist L, Forsgren M, Sköldenberg B. Tick ‑ borne encephalitis in Sweden in relation to aseptic meningo ‑ encephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol 1997; 244(4): 230 – 238.
17. Kaiser R. The clinical and epidemiological profile of tick ‑ borne encephalitis in southern Germany 1994 – 98: a prospective study of 656 patients. Brain J Neurol 1999; 122(11): 2067 – 2078.
18. Mickiene A, Laiskonis A, Günther G, Vene S,Lundkvist A, Lindquist L. Tick-borne encephalitis in an area of high endemicity in lithuania: disease severity and long‑term prognosis. Clin Infect Dis Off Publ Infect Dis Soc Am 2002; 35(6): 650 – 658.
19. Oksi J, Viljanen MK, Kalimo H, Peltonen R, Marttía R,Salomaa P et al. Fatal encephalitis caused by concomitant infection with tick ‑ borne encephalitis virus and Borrelia burgdorferi. Clin Infect Dis Off Publ Infect Dis Soc Am 1993; 16(3): 392 – 396.
20. Dlouhý P, Honegr K, Krbková L, Pícha D, Roháčová H, Štruncová V. Lymeská borrelióza – doporučený postup v diagnostice, léčbě a prevenci. Klin Mikrobiol Infekční Lékařství 2011; 17(4): 144 – 153.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2014 Issue 3-
All articles in this issue
- Functional Movement Disorders
- An Overview of Less Common Primary Headaches
- Post‑stroke Spasticity as a Manifestation of Maladaptive Plasticity and its Modulation by Botulinum Toxin Treatment
- Less Common Indications for Deep Brain Stimulation
- Fluorescence Guided Resection of High‑grade Gliomas
- Neurobiological Hypotheses in Panic Disorder
- Sequelae of Methanol Poisoning for Cognition
- Options for Continual Cerebral Blood Flow Monitoring to Detect Vasospasms in Patients after Severe Subarachnoid Haemorrhage
- Pupillary Response to Chromatic Stimuli
- Mobile Total Disc Replacement Prosthesis Mobi‑ C, our Experience – Results of the Study with Five Years Follow‑up
- Sacral Nerve Neuromodulation in the Treatment of Faecal Incontinence
- Combined Paramedian Supracerebellar-transtentorial and Miniinvasive Suboccipital Approach to the Entire Length of the Mediobasal Temporal Region Glioma
- Selective Denervation of the Carpus to Manage Arthritis Involvement of a Wrist
- Flexion Cervical Myelopathy (Hirayama Disease) – Reality or Myth? Two Case Reports
- Glioblastoma Multiforme with Simultaneous Leptomeningeal and Intramedulary Metastases – a Case Study
- Blood Blister‑like Aneurysm of the Internal Carotid Artery – a Case Report and Review of Literature
- Tick‑ borne Encephalitis: Course and Complications – Our Observations from 2009 to 2012
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Functional Movement Disorders
- An Overview of Less Common Primary Headaches
- Neurobiological Hypotheses in Panic Disorder
- Sacral Nerve Neuromodulation in the Treatment of Faecal Incontinence
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

