-
Medical journals
- Career
SNHG7 and FAIM2 are up-regulated and co-expressed in colorectal adenocarcinoma tissues
Authors: Farinaz Ziaee 1; Mohammadreza Hajjari 1; Reza Seyed Kazeminezhad 1; Mehrdad Behmanesh 2
Authors‘ workplace: Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran 1; Department of Genetics, Faculty of Biological Science, Tarbiat Modares University (TMU), Tehran, Iran 2
Published in: Klin Onkol 2020; 33(6): 445-449
Category:
Overview
Background: The global incidence of colorectal cancer (CRC) is expected to be increased by 60% until a few years. Despite the advances in surgical and chemotherapy techniques, a significant proportion of patients with CRC have poor responses to treatments. These are the reasons that prove the importance of identifying molecular biomarkers as potential therapeutic targets. Long non-coding RNAs (lnc RNAs) participate in the initiation, development, progression, and metastasis of cancers such as CRC. Hence, this class of noncoding RNAs is known as biomarker for cancer diagnosis and prognosis. Materials and methods: In this experimental study, the extraction of total RNA from tissues, synthesis of complementary DNA as well as quantitative real-time polymerase chain reaction (qRT - PCR) were performed. Comparative cycle threshold method was applied to quantify the expression level of lncRNA-SNHG7 and FAIM2. The relative amount of lncRNA-SNHG7 and FAIM2 was calculated using the equation 2 -DDCT. Results: In this study, by qRT PCR, we concluded that the expression level of SNHG7, as a recently identified lncRNA and FAIM2 were increased in colorectal cancer tissues compared with normal adjacent tissues. Conclusion: Our study indicates the potential importance of SNHG7 and FAIM2 expression for more studies in future.
Keywords:
lncRNA-SNHG7 – FAIM2 – colorectal cancer
Introduction
The global burden of colorectal cancer (CRC) is expected to be increased, so that it is predicted that many new cases will be affected and many patients will die until the next decade. This increase in the number of patients and deaths is partly due to a lack of early diagnosis of this cancer [1,2]. Early high sensitive detection of the colon cancer can help to manage these patients. It would also improve the prognosis of this type of cancer and improve the recurrence risk [3,4]. Long noncoding RNAs (lncRNAs) are a class of non-coding RNA molecules with more than 200 nucleotides in length. They are located in nuclear or cytoplasmic compartments of the cell. They are usually transcribed by RNA polymerase II and conventionally cannot be translated into any proteins [5,6].
Evidence has shown that lncRNAs can participate in different cellular and molecular processes [7,8], including transcriptional regulation, cellular proliferation/differentiation and apoptosis, chromosome imprinting and remodeling, etc. [9].
It has been reported that several lncRNAs are involved in the pathogenesis of different diseases, including cancers. They can work as a tumor suppressor or onco lncRNAs in the initiation and progression of different cancers [10]. Thus, their dysregulation has been reported to be associated with the occurrence, development, progression, and metastasis in cancers [11,12] such as CRC. This class of noncoding RNAs is therefore known as biomarkers for cancer diagnosis and prognosis [9,13].
Long non-coding small nucleolar RNA host gene 7 (lncRNA SNHG7) is a member of recently identified lncRNAs which is located on 9q34.3 chromosome. Its potential role in several cancers including gastric cancer [14], breast cancer [15], hepatocellular carcinoma [10], glioblastoma, lymphoma, ovarian cancer [6,16], lung cancer [17,18], prostate cancer [19], and renal cell carcinoma [20] has been reported. Therefore, it can be considered as a promising candidate biomarkers for diagnosing colorectal cancer. Hence, previous studies have suggested that SNHG7 may be a potential molecule for the diagnosis and treatment of different cancer types.
While the dysregulated expression of SNHG7 has been reported in several cancer cell lines and tumors, there is a little information about the role of SNHG7 in colorectal cancer. Thus, we investigated the expression level of SNHG7 in colorectal adenocarcinoma tissues compared with adjacent normal tissues. In the current study, we demonstrated that lncRNA-SNHG7 was aberrantly up-regulated in colorectal adenocarcinoma tissues. Furthermore, we found a correlation between the expression levels of this lncRNA with the expression of FAIM2 as an anti-apoptotic gene. So, our study confirms a potential role of SNHG7 in colorectal cancer and highlights its role in the initiation/progression of colorectal cancer.
Materials and methods
Patients and tissues collection
This study was ethically approved by Shahid Chamran University of Ahvaz. The colorectal tumoral and non-tumoral tissue samples were obtained from Iran Tumor Bank (Tehran, Iran). Totally, 25 paired tissue samples were examined for gene expression (25 colorectal cancer tissues and 25 normal adjacent tissues).
The diagnosis of all patients with CRC was histopathologically confirmed by the Tumor Bank. Distant metastasis and histological differentiation were classified according to the standard criteria. The clinical stage was evaluated on the basis of the Union for International Cancer Control (UICC) TNM classification system.
The clinicopathological characteristics of the patients are summarized in Tab. 1.
1. The clinicopathological factors of 25 patients. 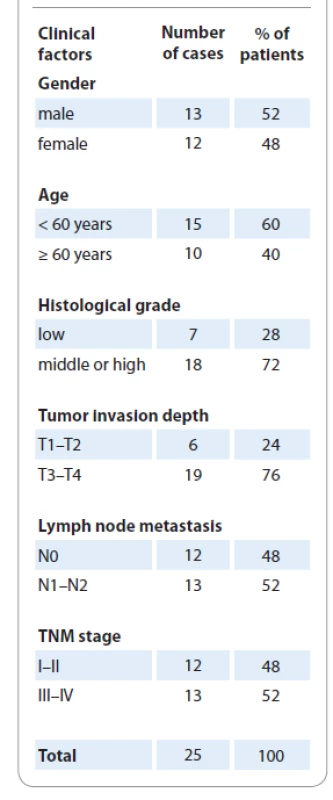
The tumor tissues and paired adjacent normal tissues were immediately frozen in liquid nitrogen and stored at −80 ºC for further experiments.
RNA preparation, cDNA synthesis, and quantitative real-time PCR
Total RNA from tissues was extracted by using RNXTM-plus reagent (CinnaGen, Iran) according to the protocol of the manufacturer. The concentration of precipitated RNAs was measured by a NanoDrop 2000 spectrophotometer RNA (Thermo Fisher Scientific, Waltham, USA) and the quality was evaluated by agarose gel electrophoresis.
Complementary DNA (cDNA) was synthesized from 1.5 μg total RNA using random hexamer and oligo (dT) primers through a synthesis kit (Takara, Shiga, Japan) in a total 10 μL reaction mixture, according to the manufacturer’s instructions.
The quantitative real-time PCR (qRT PCR) assays were performed using SYBR Green (Takara, Dalian, China) on the Applied Biosystems StepOne Real-time PCR System (Applied Biosystems, Foster City, USA) with suitable controls. All reactions were run in duplicate and the comparative cycle threshold (CT) method was applied to quantify the expression level of lncRNA-SNHG7 and FAIM2.
The results were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR primers were designed and analyzed by Oligo 7 and Gene Runner software. The primer sequences used for the studies are shown in Tab. 2. The relative amount of lncRNA-SNHG7 and FAIM2 was calculated using the equation 2 -DDCT.
2. Sequence of primers for GAPDH, SNHG7 and FAIM2 genes. 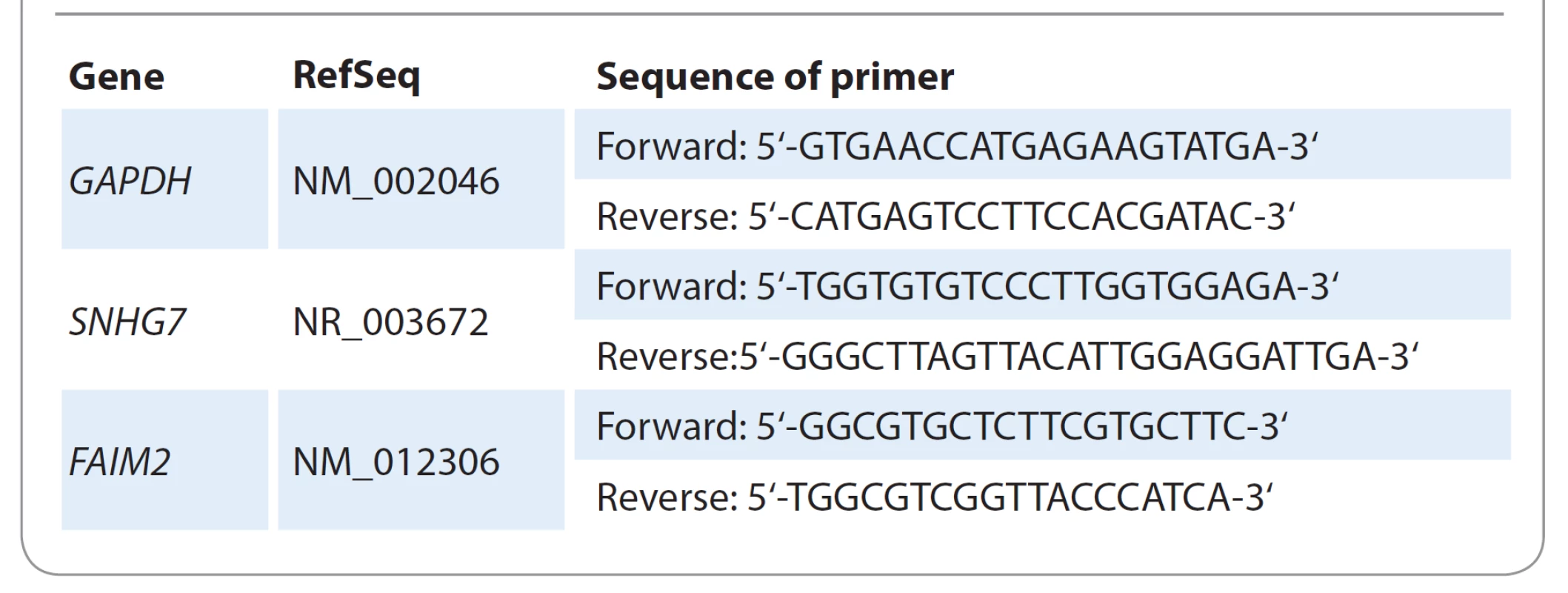
Statistical analysis
The data were used for statistical analysis by GraphPad Prism 5. The t-test was used for the statistical analysis of the data. The significance was considered at the level of P < 0.05. In addition, the relationship between gene expression changes was determined using Pearson correlation coefficient.
Results
SNHG7 was highly expressed in CRC tissues
We performed qRT-PCR in order to detect the relative expression of SNHG7 in CRC tumor tissues and normal adjacent tissues. The results showed that the expression of SNHG7 is increased in the tumor samples in comparison with normal tissues (fold change 3.491; P = 0.024) (Fig. 1A).
FAIM2 expression was increased in tumor samples compared to normal tissues
The FAIM2 gene in the tumor samplesshowed a higher expression compared with the normal tissues (fold change 4.395; P = 0.0396). The following diagram illustrates this comparison statement (Fig. 1B).
Positive correlation between the FAIM2 and SNHG7 expression
The relationship between genes expression changes was determined in GraphPad Prism 5 using Pearson correlation coefficient (P < 0.0001, correlation coefficient = 0.7027). The model shows the correlation between changes in the expression of FAIM2 and SNHG7 genes (Fig. 1C).
1. Comparison between normalized expression levels of the desired genes. A) SNHG7 was highly expressed in CRC tissues compared with adjacent normal tissues. B) The FAIM2 gene in the tumor samples showed a higher expression than the normal tissues. C) Change in the expression of FAIM2 and SNHG7 genes indicated a positive correlation between their expression levels. 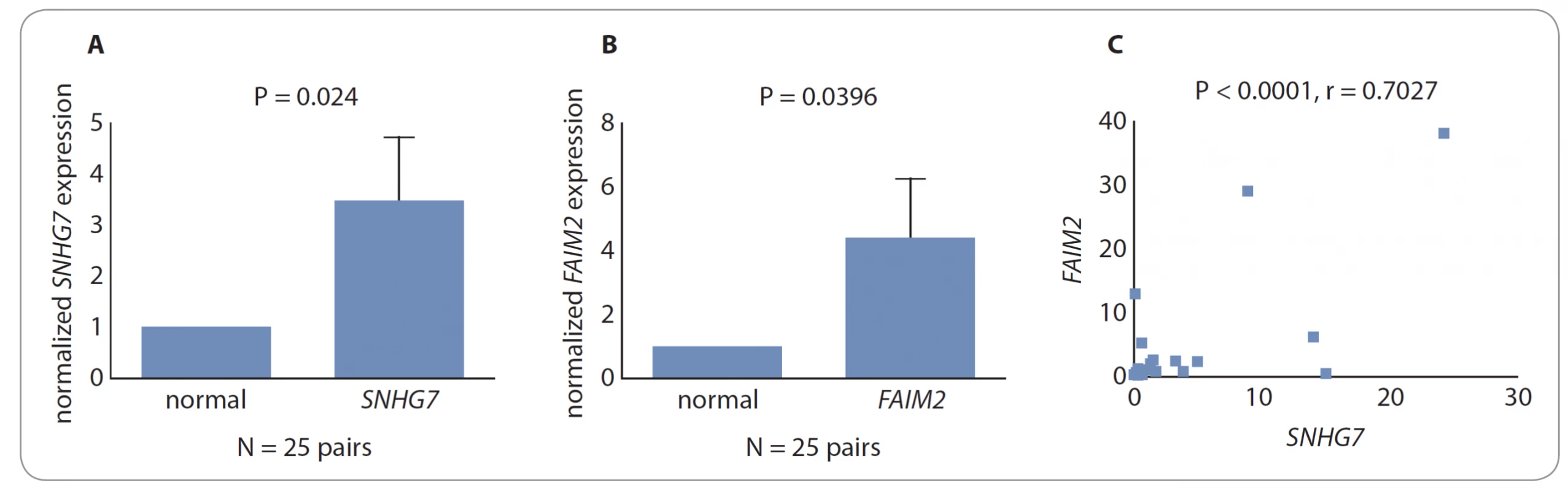
Correlation of SNHG7 and FAIM2 expression with clinicopathological features of CRC patients
In addition to previous results, the correlation of SNHG7 and FAIM2 expression with clinicopathological characteristics of colorectal cancer samples was analyzed by t-test.
In this study, the relationship between tumor stages and grades, lymph node invasion and tumor location for both genes were examined. The increase in the expression of FAIM2 and SNHG7 genes was associated with high stages of cancer (III–IV) and metastasis to lymph nodes (N1–N2), although these relationships were not significant. The increase in the expression of SNHG7 was also correlated with high tumor grades (II–III) (Fig. 2).
2. The high expression level of FAIM2 and SNHG7 genes was associated with high stages of cancer (stage III–IV) and metastasis to lymph nodes (N1–N2). Increasing the expression of SNHG7 also correlated with high tumor grades, although these relationships were not signifi cant. The expression rate of SNHG7 in the colon was higher than the rectum with P = 0.0296, also over expression of FAIM2 correlated with colon with P = 0.0462. 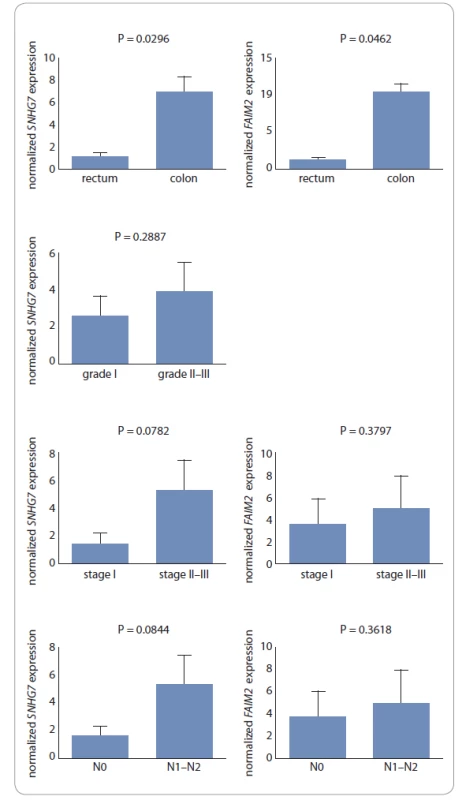
Discussion
Colorectal cancer is the third most commonly diagnosed cancer in males and the second one in females worldwide [21]. Although surgery is an effective way for removing the primary tumor, many patients are diagnosed with metastases after this primary surgery. Despite the advances in chemotherapy and surgical methods, a significant number of patients with CRC have poor responses to treatments. A suitable biomarker or biomarker panel along with colonoscopic method would help screening and improving the diagnosis protocols [22].
Long noncoding RNAs (lncRNAs) are a type of non-coding RNA molecules which are longer than 200 nucleotides and do not have any open reading frame. They participate in many biological processes in different levels and compartments [23–25].
However, researchers have found that lncRNAs exhibit critical roles in the development of many diseases with complicated mechanism, such as tumor [26]. In fact, lncRNAs participate in the occurrence and progression of metastasis of different cancers such as CRC. This class of non-coding RNAs is therefore known as potential biomarkers for cancer diagnosis and prognosis.
The Small Nucleolar Host Gene (SNHG) is a gene family with more than 10 members. Their potential role in tumor progression has been investigated in recent years. However, their functional mechanism in cancers has not been diagnosed yet. As an example, SNHG1, a potential oncogene, is over-expressed in colorectal cancer. It induces cell proliferation by sponging a microRNA named miR-145, and has a correlation with poor prognosis of CRC [27,28]. The RNA acts via Wnt/b-catenin signaling pathway, and may significantly be associated with the development and progression of this cancer [29,30].
In this study, the expression level of another member of this family called SNHG7 and its potential collaborative gene called FAIM2 were investigated in colorectal cancer. FAIM2 is a gene encoding the FAIM2 protein that plays a role in the Fas signaling pathway [17,31].
In recent years, changes in lncRNA-SNHG7 expression have been reported in hepatocellular carcinoma [10], glioblastoma [6,16], gastric cancer [14], breast cancer [15], lung cancer [17,18], prostate cancer [19], and renal cell carcinoma [20]. High levels of SNHG7 expression are involved in cell proliferation, migration, invasion, lymph node metastases as well as in TNM stage. On the other hand, increased level of SNHG7 expression inhibits apoptosis and negatively correlates with the prognosis and survival rate of patients with various cancers.
She et al [17,18] found that the changes in the expression of SNHG7 and FAIM2 can be found in lung cancer. Their results showed that the expression level of FAIM2 and SNHG7 are up-regulated in lung cancer tissue. It was found that the expression of lncRNA-SNHG7 had a positive association with FAIM2 in this type of cancer. They knock down the lncRNA-SNHG7 and FAIM2 by siRNA which inhibits proliferation, cell migration, and invasion; and it also induces apoptosis.
Studies have shown that the FAIM2 gene is involved in the Fas signaling pathway, which is an apoptotic pathway. This gene produces a membrane protein called Fas apoptotic inhibitory molecule 2 (FAIM2), which is considered as an anti-apoptotic protein and a member of the lifeguard (LFG) family [18]. In the Fas signaling pathway, binding of the Fas receptor (CD95) to FasL (Fas Ligand) on the surface of the other cells can induce apoptosis signaling [31,32].
In this study, we recognized a significant increase in the expression of lncRNA-SNHG7 in tumor tissues compared with normal adjacent tissues (P = 0.024) by checking 25 patients with CRC. The expression of FAIM2 was also found to be increased in tumor tissues compared with normal tissues (P = 0.0396). To our knowledge, this study is the first one reporting the over-expression of FAIM2 as well as its correlation with SNHG7 in colorectal cancer. During the processing of the current study and manuscript, there were published two relevant studies which also showed the over-expression of SNHG7 in colorectal cancer [33,34]. In the current study, we investigated the possible relationship between the changes of SNHG7 and FAIM2 expression in colorectal cancer for the first time. We found that the correlation coefficient was positive and equal to 0.7027, which indicates a positive relationship between the expressions of these two genes. This correlation indicates a potential correlation between the roles of these molecules in colorectal cancer. The results are consistent with the previous study [18] which showed this correlation.
Based on the results of this study as well as on the results of previous reports, it can be argued that SNHG7 and FAIM2 probably collaborate for colorectal cancer progression.
Conclusion
In summary, our data demonstrate that abnormal expression of SNHG7 and FAIM2 and their correlation may play important roles in CRC and may serve as a target for diagnosis and therapy of colorectal cancer. Our results suggest further in vitro and in vivo studies to reveal their role in oncogenesis.
Acknowledgments: The authors thank Shahid
Chamran University of Ahvaz for supporting
this study.
The authors declare they have no potential
conflicts of interest concerning drugs,
products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem
studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript
met the ICMJE recommendation for
biomedical papers.
Redakční rada potvrzuje, že rukopis práce
splnil ICMJE kritéria pro publikace zasílané do
bi omedicínských časopisů.
Mohammadreza Hajjari
Department of Biology,
Faculty of Science
Shahid Chamran University of Ahvaz
Ahvaz
Iran
e-mail: mohamad.hajari@gmail.com;
m-hajari@scu.ac.ir
Submitted/Obdrženo: 1. 5. 2020
Accepted/Přijato: 16. 7. 2020
Sources
1. Arnold M, Sierra MS, Laversanne M et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016; 66 (4): 683–691. doi: 10.1136/gutjnl-2015-310912.
2. Deen KI, Silva H, Deen R et al. Colorectal cancer in the young, many questions, few answers. World J Gastroint Oncol 2016; 8 (6): 481–488. doi: 10.4251/wjgo.v8.i6.481.
3. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clini Colon Rectal Surg 2009; 22 (4): 191–197. doi: 10.1055/s-0029-1242458.
4. Lu M, Liu Z, Li B et al. The high expression of long non-coding RNA PANDAR indicates a poor prognosis for colorectal cancer and promotes metastasis by EMT pathway. J Cancer Res Clin Oncol 2017; 143 (1): 71–81.doi: 10.1007/s00432-016-2252-y.
5. Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med 2015; 12 (1): 1.9 – doi: 10.7497/j.issn.2095-3941.2015.0006.
6. Ren J, Yang Y, Xue J et al. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem Biophys Res Com 2018; 496 (2): 712–718. doi: 10.1016/j.bbrc.2018.01.109.
7. Gu L-Q, Xing X-L, Cai H et al. Long non-coding RNA DILC suppresses cell proliferation and metastasis in colorectal cancer. Gene 2018. 666 : 18–26. doi: 10.1016/j.gene.2018.03.100.
8. Paralkar VR, Weiss MJ. Long noncoding RNAs in biology and hematopoiesis. Blood 2013; 121 (24): 4842–4846. doi: 10.1182/blood-2013-03-456111.
9. Wang F, Ni H, Sun F et al. Overexpression of lncRNA AFAP1-AS1 correlates with poor prognosis and promotes tumorigenesis in colorectal cancer. Biomed Pharmacother 2016; 81 : 152–159. doi: 10.1016/j.biopha.2016.04.009.
10. Cui H, Zhang Y, Zhang Q et al. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer medicine 2017; 6 (12): 2932–2941. doi: 10.1002/cam4.1180.
11. Han Y, Yang Y-n, Yuan H-h et al. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology 2014; 46 (5): 396–401. doi: 10.1097/PAT.0000000000000125.
12. Ding J, Lu B, Wang J et al. Long non-coding RNA Loc554202 induces apoptosis in colorectal cancer cells via the caspase cleavage cascades. J Exp Clin Cancer Res 2015; 34 (1): 100. doi: 10.1186/s13046-015-0217-7.
13. Li Z, Yu X, Shen J. ANRIL: a pivotal tumor suppressor long non-coding RNA in human cancers. Tumor Biol 2016; 37 (5): 5657–5661. doi: 10.1007/s13277-016-4808-5.
14. Wang M, Liu J, Liu Q et al. LncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric cancer cells by repressing the P15 and P16 expression. Eur Rev Med Pharmacol Sci 2017; 21 (20): 4613-4622.
15. Zhou M, Zhong L, Xu W et al. Discovery of potential prognostic long non-coding RNA biomarkers for predicting the risk of tumor recurrence of breast cancer patients. Sci Rep 2016; 6 : 31038. doi: 10.1038/srep31038.
16. Leng J, Xiong W, Wang X et al. Long non-coding RNA SNHG7 promotes proliferation and self-renewal of glioblastoma cells. Int J Clin Exp Pathol 2016; 9 (11): 11352–11360.
17. She K, Yan H, Huang J et al. miR-193b availability is antagonized by lnc RNA-SNHG 7 for FAIM 2-induced tumour progression in non-small cell lung cancer. Cell Prolif 2018; 51 (1): e12406. doi: 10.1111/cpr.12406.
18. She K, Huang J, Zhou H et al. lncRNA-SNHG7 promotes the proliferation, migration and invasion and inhibits apoptosis of lung cancer cells by enhancing the FAIM2 expression. Oncol Rep 2016; 36 (5): 2673–2680. doi: 10.3892/or.2016.5105.
19. Qi H, Wen B, Wu Q et al. Long noncoding RNA SNHG7 accelerates prostate cancer proliferation and cycle progression through cyclin D1 by sponging miR-503. Biomed Pharmacother 2018; 102 : 326–332. doi: 10.1016/j.biopha.2018.03.011.
20. He H-T, Xu M, Kuang Y et al. Biomarker and competing endogenous RNA potential of tumor-specific long noncoding RNA in chromophobe renal cell carcinoma. OncoTargets Ther 2016; 9 : 6399–6406. doi: 10.2147/OTT.S116392.
21. Arnold M, Sierra MS, Laversanne M et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66 (4): 683–691. doi: 10.1136/gutjnl-2015-310912.
22. Gupta AK, Brenner DE, Turgeon DK. Early detection of colon cancer. Mol Diagn Ther 2008; 12 (2): 77–85. doi: 10.1007/BF03256273.
23. Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 2013; 14 (11): 699–712. doi: 10.1038/nrm3679.
24. Chen J, Liu S, Hu X. Long non-coding RNAs: crucial regulators of gastrointestinal cancer cell proliferation. Cell Death Discov 2018; 4 (1): 50. doi: 10.1038/s41420-018-0051-8.
25. Rion N, Rüegg MA. lncRNA-encoded peptides: More than translational noise? Cell Res 2017; 27 (5): 604–605. doi: 10.1038/cr.2017.35.
26. Liu Y, Zhang M, Liang L et al. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol 2015; 8 (9): 11480–11484.
27. Zhu Y, Li B, Liu Z et al. Up-regulation of lncRNA SNHG1 indicates poor prognosis and promotes cell proliferation and metastasis of colorectal cancer by activation of the Wnt/b-catenin signaling pathway. Oncotarget 2017; 8 (67): 111715–111727. doi: 10.18632/oncotarget.22903.
28. Tian T, Qiu R, Qiu X. SNHG1 promotes cell proliferation by acting as a sponge of miR-145 in colorectal cancer. Oncotarget 2018; 9 (2): 2128–2139. doi: 10.18632/oncotarget.23255.
29. Qi H, Wang J, Wang F et al. Long non-coding RNA SNHG1 promotes cell proliferation and tumorigenesis in colorectal cancer via Wnt/b-catenin signaling. Pharmazie 2017; 72 (7): 395–401. doi: 10.1691/ph.2017.7463.
30. Sun X, Wang Z, Yuan W. Down-regulated long non-coding RNA SNHG1 inhibits tumor genesis of colorectal carcinoma. Cancer Biomark 2017; 20 (1): 67–73. doi: 10.3233/CBM-170112.
31. Wajant H. The Fas signaling pathway: more than a paradigm. Science 2002; 296 (5573): 1635–1636. doi: 10.1126/science.1071553.
32. Scaffidi C, Fulda S, Srinivasan A et al. Two CD95 (APO-1/Fas) signaling pathways. The EMBO J 1998; 17 (6): 1675–1687. doi: 10.1093/emboj/17.6.1675.
33. Shan Y, Ma J, Pan Y et al. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis 2018; 9 (7): 722. doi: 10.1038/s41419-018-0759-7.
34. Li Y, Zeng C, Hu J et al. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J Hematol Oncol 2018; 11 (1): 89. doi: 10.1186/s13045-018-0632-2.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

-
All articles in this issue
- Practical instructions for testing and targeted therapy in adult patients with solid tumours with NTRK gene fusion in common clinical practice
- Treatment opinion of rehabilitation in sarcopenia and cachexia for oncological patients
- Stomatitis in mTOR inhibitors treatment and other targeted cancer therapy, possibilities of infl uencing it, and the use of local corticotherapy
- First experience in the Czech Republic with perirectal hydrogel injection before radiotherapy for prostate cancer
- SNHG7 and FAIM2 are up-regulated and co-expressed in colorectal adenocarcinoma tissues
- A Ukrainian multicenter prospective study of the value of PET/ CT prognostic role in primary patients with Hodgkin’s lymphoma in a real-life cohort
- Dabrafenib monotherapy in BRAF+ non-small cell lung cancer – our experience
- Multiresistent opportunistic talaromycosis in a patient with ovarian cancer
- Low dose exposure evaluation for hypofractionated breast intensity modulated radiation therapy – taking into account the fraction-size effect with the linear quadratic model
- Chemie jménem CRISPR
- Immunostimulatory and anticancer effect of Reishi and Coriol extracts at the level of clinical studies and their implementation in practice
- Atezolizumab a bevacizumab v léčbě hepatocelulárního karcinomu
- Aktuality z odborného tisku
- Profesor Pavel Šlampa slaví 60 let
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Immunostimulatory and anticancer effect of Reishi and Coriol extracts at the level of clinical studies and their implementation in practice
- First experience in the Czech Republic with perirectal hydrogel injection before radiotherapy for prostate cancer
- Stomatitis in mTOR inhibitors treatment and other targeted cancer therapy, possibilities of infl uencing it, and the use of local corticotherapy
- Treatment opinion of rehabilitation in sarcopenia and cachexia for oncological patients
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

