-
Medical journals
- Career
The Anti-apoptotic Mechanism of Metformin Against Apoptosis Induced by Ionizing Radiation in Human Peripheral Blood Mononuclear Cells
Authors: S. Kolivand 1; E. Motevaseli 2,3; M. Cheki 4; A. Mahmoudzadeh 5; A. Shirazi 6; V. Fait 7
Authors‘ workplace: Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences and Health Services Tehran, Iran 1; Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences and Health Services Tehran, Iran 2; Food Microbio logy Research Center, Tehran University of Medical Sciences and Health Services, Tehran, Iran 3; Department of Radiologic Technology, Faculty of Paramedicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 4; Department of Biosciences and Biotechnology, Malek Ashtar University of Technology, Tehran, Iran 5; Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences and Health Services, Tehran, Iran 6; Department of Surgical Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 7
Published in: Klin Onkol 2017; 30(5): 372-379
Category: Original Articles
doi: https://doi.org/10.14735/amko2017372Overview
Background:
In a previous article, we showed that metformin (MET) can reduce ionizing radiation (IR) induced apoptosis in human peripheral blood mononuclear cells. However, the anti-apoptotic mechanism of MET against IR remains unclear. The present study attempts to investigate the mechanism of action of MET in limiting X-ray induced apoptosis in human peripheral blood mononuclear cells.Material and Methods:
Mononuclear cells were treated with MET for 2 hours and irradiated with 6 MV X-rays. The gene expression levels of BAX, CASP3 and BCL2 were determined 24 hours post irradiation using real time quantitative polymerase chain reaction (qPCR) technique. Furthermore, the protein levels of BAX, CASP3 and BCL2 were analyzed by Western blotting assay.Results:
Radiation exposure increased the expressions of BAX and CASP3 genes, and decreased the expression of BCL2 gene in mononuclear cells. Conversely, an increase in BCL2 gene expression along with a decrease in BAX and CASP3 genes expression was observed in MET plus irradiated mononuclear cells. It was found that radiation increased BAX/BCL2 ratio, while MET pretreatment reduced these ratios. Also, treatment with MET without irradiation did not change the expressions of BAX, CASP3 and BCL2 genes. On the other hand, downregulated expression of BCL2 protein and upregulated expressions of BAX and CASP3 proteins were found in 2 Gy irradiated mononuclear cells, while pretreatment with MET significantly reversed this tendency.Conclusion:
These results suggest that MET can protect mononuclear cells against apoptosis induced by IR through induction of cellular anti-apoptotic signaling.Key words:
ionizing radiation – metformin – apoptosis – genes – proteins – blood cellsIntroduction
Interaction between ionizing radiation (IR) and cells leads to production of free radicals that arise in the process of energy absorption and breakdown of chemical bonds in molecules. Free radicals play a major role in radiation effects on biological tissues and organisms. Since cellular damage induced by IR is attributed primarily to the harmful effects of free radicals, molecules with direct free radical scavenging properties are particularly promising as radioprotectants. Initial efforts were centralized on synthetic thiol compounds. These compounds are effective at reducing IR-induced lethality. Amifostine as the most effective compound of this type is the only radioprotector that has been clinically approved by the Food and Drug Administration (FDA) for reducing side effects in cancer patients undergoing radiotherapy. Because of some undesirable side effects of amifostine, such as hypotension, nausea, vomiting, sneezing, hot flashes, mild somnolence and hypocalcemia, researchers diverted their attention toward other compounds as radioprotectors [1–3]. Over the years, a number of compounds have been tested for their radioprotective efficacy with generally limited success. Thus, there is still an urgent need to identify novel, nontoxic compounds to protect humans from the damaging effects of IR.
Metformin (MET) is a widely used drug in the treatment of diabetes mellitus type 2 [4]. MET can neutralize reactive oxygen species (ROS) as a direct and indirect free radical scavenger through donation of H atom from its CH3 or NH groups, and upregulation of the antioxidant thioredoxin, and/or suppression of NAD (P) H oxidase activity [5–9]. Several studies have confirmed that MET possesses anti-inflammatory and antiapoptotic properties [10–13]. MET can act as a radiosensitizer under in vitro conditions in a variety of cancer cell lines. For example, MET potentiated the effects of irradiation on human hepatic, lung, pancreatic, esophageal, prostate, and breast cancer cells [14]. Recently, we have shown that MET plays a role in reduction of radiation induced apoptosis and genotoxicity in cultured human blood lymphocytes (HBLs) [15]. Although, the radioprotective effect of MET on cultured HBLs is clear, but its mechanism is still unknown. Thus, the present study attempts to investigate the mechanism of action of MET in limiting X-ray induced apoptosis in human peripheral blood mononuclear cells (PBMCs).
Materials and Methods
Isolation of mononuclear cells and treatment procedure
This research was approved by the ethics committee of the Tehran University of Medical Sciences and Health Services. Fifteen ml of blood samples were collected in ethylenediaminetetraacetic acid (EDTA) coated tubes from the median cubital vein of each of the three healthy male volunteers. The donors were 25–30 year old non-smokers with no history of radiotherapy, no alcohol or medication consumption and no disease at the time of blood collection. Written consent was obtained from each blood donor. Mononuclear cells were isolated by Ficoll-Hypaque (Baharafshan Co., Iran) using the protocol suggested by the manufacturer. The blood was diluted 1 : 1 with phosphate buffered saline (PBS) and layered onto Ficoll-Hypaque solution with the ratio of blood and PBS – Ficoll-Hypaque maintained at 2 : 1. The blood was centrifuged at 3000 rpm for 30 minutes at room temperature. The mononuclear cells layer was removed, washed twice with PBS and centrifuged at 2000 rpm for 10 minutes each. After separation, the mononuclear cells were cultivated overnight at concentration of 3 × 106 cells/ml in Roswell Park Memorial Institute (RPMI) Medium supplemented with 10% fetal bovine serum (Gibco Co., USA), 10 mg/ml streptomycin (Sigma-Aldrich Co., USA), 10,000 U penicillin (Sigma-Aldrich Co., USA) and 2 mmol/mL L-glutamine (Gibco Co., USA) in a humidified atmosphere with 5% CO2 at 37 °C. The number of viable cells was assessed by staining the cells with trypan blue (Gibco Co., USA) and counting the cells by hemocytometer. Trypan blue exclusion showed their viability to be > 96%.
The results of our previous article showed that the maximum protective effect of metformin against apoptosis induced by IR in HBLs occurred at the dose of 2 Gy radiation and 50 μM of metformin [15]. Hence, 2 Gy radiation and 50 μM of metformin were chosen for this study. In our experiment, cells were divided into four groups and in each group, three samples were processed with triplicate:
- Group I – Control – mononuclear cells were treated with MET solvent (PBS + RPMI Medium).
- Group II – 50 μM MET – mononuclear cells were treated with 50 μM of MET (Exir Pharmaceutical Co., Iran).
- Group III – Radiation only – mononuclear cells were treated with MET solvent (PBS + RPMI medium) for 2 hours before exposure to 2 Gy X-radiation.
- Group IV – 50 μM MET + Radiation – mononuclear cells were treated with 50 μM of MET for 2 hours before exposure to 2 Gy X-radiation.
Irradiation
Mononuclear cells were irradiated with 6 MV X-rays from a medical linear accelerator (Elekta, Stockholm, Sweden) at a dose rate of 2 Gy/min, 100 cm distance from the source (SSD), over an area of 20 × 20 cm2, at room temperature.
Real time quantitative polymerase chain reaction
Real time quantitative polymerase chain reaction (RT-qPCR) was used to measure the expression of BAX, CASP3 and BCL2 genes. The total RNA was extracted 24 hours after irradiation using RNA extraction kit (SinaClon BioScience Co., Iran) according to manufacturer’s instructions. RNA purity was quantified by spectrophotometry at 260/280 nm ratio and the integrity was confirmed by electrophoresis on a denaturing agarose gel. For each RNA sample, absence of contaminating DNA was examined by a PCR without preceding RT-qPCR and no amplification product was observed. Further, complementary DNA (cDNA) synthesis kit (Yekta Tajhiz Azma Co., Iran) was used to prepare cDNA directly from cultured cells, according to the manufacturer’s instructions. The primers and cDNA sample qualities were analyzed using PCR and agarose gel electrophoresis. RT-qPCR was performed in a reaction volume of 25 μL according to the manufacturer’s instructions. Briefly, 12.5 μL of master mix, 1 μL of primer assay, 9 μL PCR grade H2O and 2.5 μL of template cDNA were added to each sample. After a brief centrifugation, the PCR plate was subjected to 40 cycles of the following conditions – (i) PCR activation at 95 °C for 15 minutes, (ii) denaturation at 95 °C for 15 seconds and (iii) annealing/extension at 60 °C for 30 seconds. All samples and controls were run in duplicate on a Roter-Gene 6000 instrument (Corbett Life Science, Australia) using RealQ Plus 2x Master Mix Green (Ampliqon Co., Denmark). The quantitative RT-PCR data was analyzed by a comparative threshold (Ct) method, and the fold inductions of samples were compared with the untreated samples. GAPDH was used as an internal reference gene to normalize the expression of the genes. The Ct cycle was used to determine the expression level in control and treated mononuclear cells. The gene expression level was then calculated as described by Yuan et al. [16]. The results were expressed through the ratio of reference gene to target gene by using the following formula – ΔCt = Ct (BAX, CASP3, BCL2 genes) – Ct (GAPDH). To determine the relative expression levels, the following formula was used – ΔΔCt = ΔCt (treated) – ΔCt (control). Thus, the expression levels were expressed as n-fold differences relative to the calibrator. The value was used to plot the expression of genes using the expression of 2–ΔΔCt. The primers sequences that were used for BAX, CASP3 and BCL2 in real time PCR experiments, are illustrated in Tab. 1.
1. The primer sequences that were used in real time PCR. 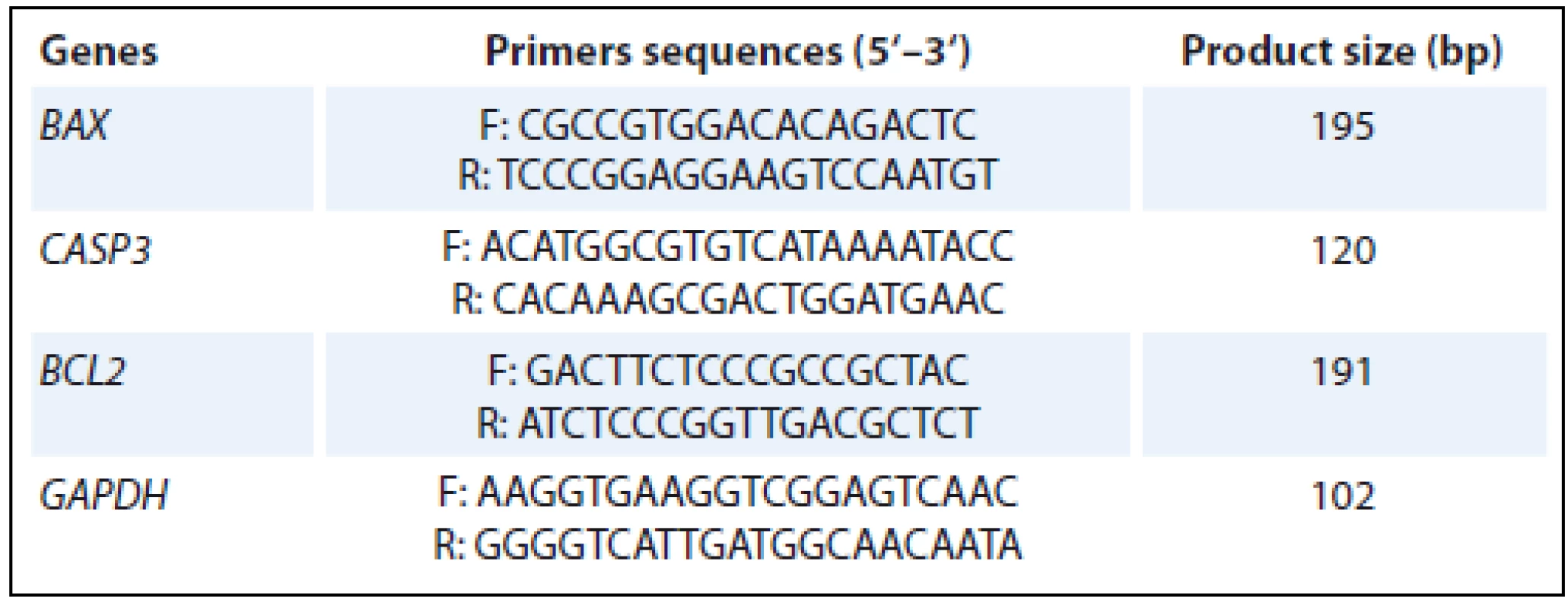
PCR – polymerase chain reaction Western blotting assay
After 24 hours from irradiation, the mononuclear cells were washed twice in PBS and suspended in radioimmunoprecipitation assay buffer (RIPA buffer), and placed on ice for 30 minutes. The supernatant was collected after centrifugation at 12,000 g for 10 minutes at 4 °C and the protein concentration in supernatant was determined using the Lowry method. Equal amounts of protein (50 µG) were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose acetate membrane. Nitrocellulose membrane were blocked with 5% (w/v) nonfat milk in TBST (1.5 M NaCl, 20 mM Tris-HCl, 0.05% [v/v] Tween-20) for overnight and then incubated with primary antibodies (mouse monoclonal antibodies anti-BAX, anti-Bcl-2, anti-CASP3, and anti-β-Actin; Sigma-Aldrich Co., USA) for 6 hours. The membranes were washed with TBST thrice for 10-minute interval and then incubated with horseradish peroxidase conjugated secondary antibody (goat anti-Mouse IgG-HRP polyclonal antibody; Sigma-Aldrich Co., USA) for 2 hours. Then, the membranes were washed with TBST thrice for 10-minute interval and the bands were detected using a 3,3‘-diaminobenzidine solution. The relative amount of protein expressed was determined by using β-Actin as an internal control. The reative intensity of bands was analyzed by Image J software (v. 1.47, Rasband W S, National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data values are presented as means ± standard error of mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA), as well as post hoc Turkey tests. Value p < 0.05 was considered to represent a statistically significant difference.
Results
As shown in Graph 1, irradiation with 2 Gy caused a marked increase in the expression of BAX as compared with the control group (5.23 ± 0.39-fold; p < 0.001). However, treatment with 50 μM of MET 2 hours before irradiation showed a significant decrease in the expression of BAX when compared to radiation only group (2.69 ± 0.27-fold vs. 5.23 ± 0.39-fold, resp. p < 0.001) (Graph 1). Furthermore, treatment with 50 μM of MET had no effect on BAX expression when compared to control group (1.07 ± 0.22-fold; p = 0.997) (Graph 1). On the other hand, in the irradiation only group, the expression of BCL2 was significantly decreased as compared to control group (0.34 ± 0.04-fold; p < 0.05) (Graph 2). However, treatment with 50 μM of MET 2 hours before irradiation caused a significant increase in BCL2 expression when compared to radiation only group (1.65 ± 0.15-fold vs. 0.34 ± 0.04-fold, resp. p < 0.05) (Graph 2). The statistically significant differences in the expression of BCL2 was not seen in 50 μM of MET alone group in comparison with control group (1.00 ± 0.21-fold; p = 1.00) (Graph 2). As shown in Graph 3, irradiation with 2 Gy caused a marked increase in the expression of CASP3 as compared with the control group (3.09 ± 0.29-fold; p < 0.001). However, treatment with 50 μM of MET 2 hours before irradiation showed a significant decrease in the expression of CASP3 when compared to radiation onlygroup (1.01 ± 0.15-fold vs. 3.09 ± 0.29-fold, resp. p < 0.001) (Graph 3). Moreover, the treatment with 50 μM of MET did not lead to a significant difference in CASP3 expression when compared to the control group (p = 0.995) (Graph 3).
Graph 1. Effect of MET on the fold change of <i>BAX</i> expression at 24 hours after 2 Gy X-ray radiation (relative to control). Values are expressed as mean ± SEM of three experiments in each group with triplicate (*p < 0.001 compared to the control group, #p < 0.001 compared to the 2 Gy alone group). 
Graph 2. Effect of MET on the fold change of <i>BCL2</i> expression at 24 hours after 2 Gy X-ray radiation (relative to control). Values are expressed as mean ± SEM of three experiments in each group with triplicate (*p < 0.05 compared to the control group, #p < 0.05 compared to the 2 Gy alone group). 
Graph 3. Effect of MET on the fold change of <i>CASP3</i> expression at 24 hours after 2 Gy X-ray radiation (relative to control). Values are expressed as mean ± SEM of three experiments in each group with triplicate (*p < 0.001 compared to the control group, #p < 0.001 compared to the 2 Gy alone group). 
The ratio of pro-apoptotic BAX to anti-apoptotic BCL2 gene expression levels has been suggested as a diagnostic biomarker for radiation exposure assessment. Graph 4 illustrates the results of the BAX to BCL2 ratio for treatment with MET, radiation (2 Gy), and the combined treatment. Treatment with 50 μM of MET had no effect on the BAX/BCL2 gene expression ratio when compared to control group (1.23 ± 0.40-fold; p = 0.996) (Graph 4). A 2 Gy dose of radiation resulted in 15.60 ± 1.47-fold increase in the BAX/BCL2 ratio in comparison with control group (p < 0.001) (Graph 4). When 50 μM of MET was combined with radiation, there was a significant decrease in the BAX/BCL2 gene expression ratio in comparison with 2 Gy alone group (1.70 ± 0.30-fold vs. 15.60 ± 1.47-fold, resp. p < 0.001) (Graph 4).
Graph 4. Effect of MET on the fold change of <i>BAX/BCL2</i> ratio at 24 hours after 2 Gy X-ray radiation (relative to control). Values are expressed as mean ± SEM of three experiments in each group with triplicate (*p < 0.001 compared to the control group, #p < 0.001 compared to the 2 Gy alone group). 
The protein level in 2 Gy irradiated mononuclear cells with or without MET pretreatment were examined (Graph 5). Down-regulated expression of BCL2 protein and up-regulated expressions of BAX and CASP3 proteins were found in 2 Gy irradiated mononuclear cells, while pretreatment with MET significantly reversed this tendency (p < 0.001). In addition, the ratio of BAX to BCL2 protein expression levels were calculated for treatment with MET, radiation (2 Gy), and the combined treatment (Graph 5). BAX/BCL2 ratio increased after 2 Gy radiation, while these ratio was decreased by MET pretreatment.
Graph 5. Western blotting analysis of BAX, BCL2 and CASP3. A – shows immunoblot images of BAX, BCL2 and CASP3. β-ACTIN was used as loading control. Lane A – represents the control, lane B – 50 μM MET, lane C – 2 Gy; lane D – 50 μM MET + 2 Gy. B–E – effect of MET on the expression of BAX, BCL2 and CASP3 in normal, 2 Gy and MET pretreated mononuclear cells. Values are expressed as mean ± SEM of three experiments in each group with triplicate (*p < 0.001 compared to the control group, #p < 0.001 compared to the 2 Gy alone group). 
Discussion
Lymphocytes as a component of human immune system are very sensitive to IR [17]. It is reported that the major cause of lymphocytopenia in the early phase after radiotherapy might be lymphocyte apoptosis. In cancer patients undergoing radiotherapy, lymphocytopenia as a negative prognostic marker has been accepted to be associated with a poor prognosis in terms of both response to therapy and survival time [18–22]. Hence, lymphocytopenia control by radioprotectors may result in an improved response to cancer therapy and finally longer survival time. We previously determined that pretreatment with 50 μM of MET 2 hours before a 2 Gy of 6 MV X-rays diminish apoptosis in cultured HBLs [15]. Apoptosis occurs via two different death signaling pathways – the extrinsic death receptor-dependent pathway and the intrinsic mitochondria-dependent pathway [23–25]. For the intrinsic mitochondria-dependent pathway, the release of cytochrome c from the inner mitochondrial membrane space into the cytosol will cause Caspase-3 activation and apoptosome formation [26]. The Bcl-2 family members have important role in modulation of cytochrome c release in the context of apoptotic stimuli [27]. Within the Bcl-2 family, BAX is a pro-apoptotic protein and Bcl-2 is an anti-apoptotic protein. BAX or Bcl-2 may control mitochondrial permeability and facilitate the passage of cytochrome c [2]. Thus, the BAX/Bcl-2 ratio determines the fate of many cells [29]. An imbalance of BAX and Bcl-2 proteins may lead to the loss of mitochondrial membrane potential and the release of cytochrome c, which triggers Caspase-3 activation and results in apoptosis [30]. Our study showed that 2 Gy of X-radiation may produce the upregulation of BAX and CASP3, and downregulation of BCL2 and subsequently increase in BAX/BCL2 ratio in irradiated mononuclear cells. Moreover, our findings revealed that 50 μM of MET pretreatment reduced the expression of BAX and CASP3, and increased the BCL2 expression in irradiated mononuclear cells. In addition, the significant decrease was observed in the BAX/BCL2 ratio following irradiation in the 50 μM of MET pretreatment group. On the other hand, the results of this study show an increase in BCL2 protein expression along with a decrease in BAX and CASP3 proteins expression in MET plus irradiated mononuclear cells. Ullah et al. [31] showed that MET prevented neuronal apoptosis by decreasing the expression of proapoptotic BAX protein, and increasing the expression of anti-apoptotic Bcl-2 protein in primary cortical neurons. They also observed that MET attenuated the elevation of cytosolic free [Ca2+]c, the activation of Caspases-3 and -9 and the cleavage of poly (ADP-ribose) polymerase 1 (PARP-1), and releasing of cytochrome c from mitochondria. Furthermore, pretreatment of nucleus pulposus cells with MET attenuated tert-butyl hydroperoxide-induced increases in protein content of BAX and cleaved Caspase-3, and decreases in Bcl-2 [32]. Zhou et al. [33] observed that MET diminished neuronal apoptosis in glutamate treated cerebellar granule neurons by reducing cytochrome c re-lease, Caspase-3 activation and phosphorylation of MAP kinases. Another study demonstrated that MET inhibited menadione-induced apoptosis, Caspases-9, -6 and -3 activation and PARP cleavage in primary rat hepatocytes via induction of heme oxygenase-1 and Bcl-xl, and inhibition of c-Jun N-terminal kinase (JNK) activation [34]. It is reported that gentamicin increases PARP and Caspase-3 in auditory cell line, while pretreatment with MET reduced all of these changes [13]. Asensio-Lopez et al. [35] showed that pretreatment with MET significantly attenuated doxorubicin-induced apoptosis and Caspases-8, -9 and -3 activation in adult mouse cardiomyocytes. In mouse Schwann cells, MET reduced apoptosis, the activation of Caspase-3 and JNK induced by methylglyoxal [36]. Recent investigations have showed that MET prevented oxidative stress-induced death in several cell types through a mechanism dependent on the mitochondrial permeability transition pore (MPTP) opening and cytochrome c release [37–39]. These studies established that significant decrease in the BAX/BCL2 ratio by MET can prevent the translocation of BAX from cytoplasm to mitochondria, inhibit opening of MPTP in the mitochondrial membrane, reduce release of cytochrome c to the cytosol and inactivate Caspase cascade and finally reduce irradiation-induced apoptosis.
The majority of radioprotectors under active investigation are designed to scavenge IR-induced intracellular free radicals and inhibit apoptosis, averting initial cascades of radiochemical events in cells following IR exposure [40,41]. Edaravone as a ROS scavenger protects HBLs against apoptosis induced by γ-irradiation through downregulation of BAX, upregulation of BCL2, and consequent reduction of the BAX – BCL2 ratio [42]. Begum et al. [43] showed the anti-apoptotic effect of apigenin against IR in HBLs by increasing BCL2 and decreasing BAX, p53, p21 and NF-κB expressions. In addition, black tea and tetramethylpyrazine as two radioprotective agents reduced apoptosis, Caspases activation and BAX expression, and also increased BCL2 expression in irradiated HBLs [44,45]. Studies have suggested that ROS are associated with the BAX activation in apoptosis induced by some stimuli [46]. MET is effective in scavenging ROS and modulating the intracellular production of superoxide radicals [47]. Regarding the close relationship between ROS and apoptosis, the anti-apoptotic effect of MET is supposed to have resulted from the action of MET as direct free radical scavengers against ROS generated by radiation.
Conclusion
To the best of our knowledge, this is the first study to present the molecular mechanism of MET that it may be beneficial in reduction of X-ray induced apoptosis in PBMCs. This study reveals that MET may reduce irradiation induced apoptosis in mononuclear cells presumably by induction of cellular anti-apoptotic signaling. However, further in vivo studies are needed to clarify anti-apoptotic mechanism of MET.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Mohsen Cheki, PhD.
Department of Radiologic Technology Faculty of Paramedicine,
Ahvaz Jundishapur University of Medical Sciences Golestan Avenue Ahvaz,
Iran
email: mohsencheky@gmail.com
Submitted: 2. 8. 2017
Accepted: 7. 9. 2017
Sources
1. Cheki M, Mihandoost E, Shirazi A et al. Prophylactic role of some plants and phytochemicals against radio-genotoxicity in human lymphocytes. J Cancer Res Ther 2016; 12 (4): 1234–1242. doi: 10.4103/0973-1482.172131.
2. Cheki M, Shahbazi Gahrouei D, Moslehi M. Determination of organ absorbed doses in patients following bone scan with using of MIRD method. Iran South Med J 2013; 16 (5): 296–303.
3. Shahbazi-Gahrouei D, Cheki M, Moslehi M. Estimation of organ absorbed doses in patients from 99mTc-diphosphonate using the data of MIRDose software. J Med Signals Sens 2012; 2 (4): 231–234.
4. Viollet B, Guigas B, Sanz Garcia N et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012; 122 (6): 253–270. doi: 10.1042/CS20110386.
5. Halicka HD, Zhao H, Li J et al. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging (Albany NY) 2011; 3 (10): 1028–1038. doi: 10.18632/aging.100397.
6. Hou X, Song J, Li XN et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 2010; 396 (2): 199–205. doi: 10.1016/j.bbrc.2010.04. 017.
7. Piwkowska A, Rogacka D, Jankowski M et al. Metformin induces suppression of NAD (P) H oxidase activity in podocytes. Biochem Biophys Res Commun 2010; 393 (2): 268–273. doi: 10.1016/j.bbrc.2010.01.119.
8. Brunmair B, Staniek K, Gras F et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic action? Diabetes 2004; 53 (4): 1052–1059.
9. Drose S, Hanley PJ, Brandt U. Ambivalent effects of diazoxide on mitochondrial ROS production at respiratory chain complexes I and III. Biochim Biophys Acta 2009; 1790 (6): 558–565. doi: 10.1016/j.bbagen.2009.01.011.
10. Lee SY, Lee SH, Yang EJ et al. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS One 2015; 10 (9): 1358–1358. doi: 10.1371/journal.pone.0135858.
11. Liu Y, Yang F, Ma W et al. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytes in vitro via mTOR-signaling pathway. Pharm Biol 2016; 54 (7): 1173–1178. doi: 10.3109/13880209.2015.1057652.
12. Yeh CH, Chen TP, Wang YC et al. AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators Inflamm 2010. doi: 10.1155/2010/130636.
13. Chang J, Jung HH, Yang JY et al. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res 2011; 282 (1–2): 92–96.
14. Koritzinsky M. Metformin: A novel biological modifier of tumor response to radiation therapy. Int J Radiat Oncol Biol Phys 2015; 93 (2): 454–464. doi: 10.1016/j.ijrobp.2015.06.003.
15. Cheki M, Shirazi A, Mahmoudzadeh A et al. The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat Res 2016; 809 : 24–32. doi: 10.1016/j.mrgentox.2016.09.001.
16. Yuan JS, Reed A, Chen F et al. Statistical analysis of real-time PCR data. BMC Bioinformatics 2006; 7 : 81–85. doi: 10.1186/1471-2105-7-85.
17. Kulkarni S, Ghosh SP, Hauer-Jensen M et al. Hematological targets of radiation damage. Curr Drug Targets 2010; 11 (11): 1375–1385.
18. Razzaghdoust A, Mozdarani H, Mofid B et al. Reduction in radiation-induced lymphocytopenia by famotidine in patients undergoing radiotherapy for prostate cancer. Prostate 2014; 74 (1): 41–47. doi: 10.1002/pros.22725.
19. De Giorgi U, Mego M, Scarpi E et al. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer 2012; 12 (4): 264–269. doi: 10.1016/j.clbc.2012.04.004.
20. Balmanoukian A, Ye X, Herman J et al. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest 2012; 30 (8): 571–576. doi: 10.3109/07357907.2012.700987.
21. Grossman SA, Ye X, Lesser G et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011; 17 (16): 5473–5480. doi: 10.1158/1078-0432.CCR-11-0774.
22. Lissoni P, Meregalli S, Bonetto E et al. Radiotherapy-induced lymphocytopenia: Changes in total lymphocyte count and in lymphocyte subpopulations under pelvic irradiation in gynecologic neoplasms. J Biol Regul Homeost Agents 2005; 19 (3–4): 153–158.
23. Xu P, Cai X, Zhang W et al. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2 (Ca (2+)) /Caspase-3/PARP-1 pathway. Apoptosis 2016; 21 (10): 1125–1143. doi: 10.1007/s10495-016-1270-1.
24. Shen Y, Luo Q, Xu H et al. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclopeptide, for preventing murine experimental colitis. Biochem Pharmacol 2011; 82 (3): 260–268. doi: 10.1016/j.bcp.2011.04.013.
25. Xia L, Luo QL, Lin HD et al. The effect of different treatment time of millimeter wave on chondrocyte apoptosiss, caspase-3, caspase-8, and MMP-13 express-ion in rabbit surgically induced model of knee osteoarthritis. Rheumatol Int 2012; 32 (9): 2847–2856. doi: 10.1007/s00296-011-2080-y.
26. Mohan S, Abdelwahab SI, Kamalidehghan B et al. Involvement of NF-κB and BCL2/BAX signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012; 19 (11): 1007–1015. doi: 10.1016/j.phymed.2012.05.012.
27. Kluck RM, Bossy-Wetzel E, Green DR et al. The release of cytochrome C from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997; 275 (5303): 1132–1136.
28. Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist 2005; 11 (1): 10–15. doi: 10.1177/10738 58404269267.
29. Bivik CA, Larsson PK, Kagedal KM et al. UVA/B-Induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J Invest Dermatol 2006; 126 (5): 1119–1127. doi: 10.1038/sj.jid.5700124.
30. Green DR. At the gates of death. Cancer Cell 2006; 9 (5): 328–330. doi: 10.1016/j.ccr.2006.05.004.
31. Ullah I, Ullah N, Naseer MI et al. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neuroscience 2012; 13 : 11. doi: 10.1186/1471-2202-13-11.
32. Chen D, Xia D, Pan Z et al. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis 2016; 7 (10): e2441. doi: 10.1038/cddis.2016.334.
33. Zhou C, Sun R, Zhuang S et al. Metformin prevents cerebellar granule neurons against glutamate-induced neurotoxicity. Brain Res Bull 2016; 121 : 241–245. doi: 10.1016/j.brainresbull.2016.02.009.
34. de la Rosa LC, Vrenken TE, Buist-Homan M et al. Metformin protects primary rat hepatocytes against oxidative stress-induced apoptosis. Pharm Res Perspect 2015; 3 (2): e00125. doi: 10.1002/prp2.125.
35. Asensio-Lopez MC, Lax A, Pascual-Figal DA et al. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic Biol Med 2011; 51 (10): 1861–1871. doi: 10.1016/j.freeradbiomed.2011.08.015.
36. Ota K, Nakamura J, Li W et al. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem Biophys Res Commun 2007; 357 (1): 270–275. doi: 10.1016/j.bbrc.2007.03.140.
37. Guigas B, Detaille D, Chauvin C et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J 2004; 382 (Pt 3): 877–884. doi: 10.1042/BJ20040885.
38. El-Mir MY, Detaille D, R-Villanueva G et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci 2008; 34 (1): 77–87. doi: 10.1007/s12031-007-9002-1.
39. Morales AI, Detaille D, Prieto M et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int 2010; 77 (10): 861–869. doi: 10.1038/ki.2010.11.
40. Park SJ, Ahn G, Lee NH et al. Phloroglucinol (PG) purified from Ecklonia cava attenuates radiation-induced apoptosis in blood lymphocytes and splenocytes. Food Chem Toxicol 2011; 49 (9): 2236–2242. doi: 10.1016/j.fct.2011.06.021.
41. Park E, Lee NH, Joo HG et al. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem Biophys Res Commun 2008; 372 (4): 792–797. doi: 10.1016/j.bbrc.2008.05.140.
42. Chen L, Liu Y, Dong L et al. Edaravone protects human peripheral blood lymphocytes from γ-irradiation-induced apoptosis and DNA damage. Cell Stress Chaperones 2015; 20 (2): 289–295. doi: 10.1007/s12192-014-0542-3.
43. Begum N, Prasad NR. Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damages in human peripheral blood lymphocytes. Biomed Prev Nut 2012; 2 (1): 16–24. doi: 10.1016/j.bionut.2011.11. 003.
44. Ghosh D, Dey SK, Saha C. Antagonistic effects of black tea against gamma radiation-induced oxidative damage to normal lymphocytes in comparison with cancerous K562 cells. Radiat Environ Biophys 2014; 53 (4): 695–704. doi: 10.1007/s00411-014-0551-8.
45. Wang XY, Ma ZC, Wang YG et al. Tetramethylpyrazine protects lymphocytes from radiation-induced apoptosis through nuclear factor-κB. Chin J Nat Med 2014; 12 (10): 730–737. doi: 10.1016/S1875-5364 (14) 60112-6.
46. Villunger A, Michalak EM, Coultas L et al. p53-and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302 (5647): 1036–1038. doi: 10.1126/science.1090072.
47. Bonnefont-Rousselot D, Raji B, Walrand S et al. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003; 52 (5): 586–589. doi: 10.1053/meta.2003.50093.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2017 Issue 5-
All articles in this issue
- Potential of Using Vitamin D as an Adjuvant Treatment of Malignant Melanoma
- Controversy in the Postoperative Treatment of Low-grade Gliomas
- The Role of Chemotherapy in the Treatment of Low-grade Gliomas
- Evaluation of Anti-cancer Therapies with Reimbursement Limited to Comprehensive Cancer Centres Using the European Society for Medical Oncology Magnitude of Clinical Benefit Scale
- Treatment Refusal in Pediatric Oncology
- Isocitrate Dehydrogenase Mutations are Better Prognostic Marker than O6-methylguanine-DNA Methyltransferase Promoter Methylation in Glioblastomas – a Retrospective, Single-centre Molecular Genetics Study of Gliomas
- The Anti-apoptotic Mechanism of Metformin Against Apoptosis Induced by Ionizing Radiation in Human Peripheral Blood Mononuclear Cells
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Controversy in the Postoperative Treatment of Low-grade Gliomas
- The Role of Chemotherapy in the Treatment of Low-grade Gliomas
- Treatment Refusal in Pediatric Oncology
- Isocitrate Dehydrogenase Mutations are Better Prognostic Marker than O6-methylguanine-DNA Methyltransferase Promoter Methylation in Glioblastomas – a Retrospective, Single-centre Molecular Genetics Study of Gliomas
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

