-
Medical journals
- Career
The influence of temperature adjustment on thromboelastography results: prospective cohort study
Authors: Čundrle Ivan jr. 1; Šrámek Vladimír 1; Pavlík Martin 1; Suk Pavel 1; Radousková Iveta 1; Zvoníček Václav 1
Authors‘ workplace: Department of Anaesthesiology and Intensive Care, ICRC Brno, Medical Faculty of Masaryk University, St. Anna’s University Hospital, Brno 1
Published in: Anest. intenziv. Med., 22, 2011, č. 5, s. 253-259
Category: Anaesthesiology - Original Paper
Overview
Introduction:
Thromboelastography (TEG) and standard coagulation tests are carried out at a laboratory temperature of 37 °C, thus omitting the effects of real blood temperature. The aim of this study was comparing the results of kaolin – heparinase TEG and Rapid TEG during mild therapeutic hypothermia i.e. when the blood was analysed at the actual temperature (isothermia) and at 37°C (normothermia). The second aim was to evaluate the clinical relevance of the results and on their basis to determine the necessity to analyse TEG at the actual temperature.Materials and Methods:
Thirty patients following CPR (cardiopulmonary resuscitation), where therapeutic hypothermia (32–34°C) was indicated for 24 hours, were included in the study. The patients were observed for 36 hours, and the TEG measurements were taken in 12-hour intervals. Standard coagulation tests, blood count, the dose of anticoagulants/antiaggregants, bleeding complications and changes in treatment according to TEG results were monitored. Data is shown as median (IQR). The Wilcoxon match pair test was used for the statistical analysis, p < 0.05 was considered significant.Results:
In hypothermic kaolin-heparinase TEG, all parameters describing clot formation (R, K, Angle, TMA and CI) were significantly different from those measured at 37 °C (p < 0.01). Clot strength parameters (MA, G) did not differ. In Rapid TEG, the differences between hypothermia and normotermia were less pronounced. There were mostly minor signs of bleeding only and clinical judgement was not influenced by the difference in TEG measurements.Conclusions:
Statistically significant differences in TEG results were more pronounced in kaolin-heparinase TEG than in Rapid TEG. These differences were related just to clot formation. The laboratory changes had no impact on clinical judgement.Keywords:
thromboelastography – cardiopulmonary resuscitactionIntroduction
The main aim of this study was comparing the results of standard kaolin-heparinase thromboelastography (KH-TEG) and Rapid TEG during mild therapeutic hypothermia in patients after cardiac arrest, when the blood was analysed at the actual temperature (hypothermia) and at 37 °C (normothermia). The second aim was to evaluate the clinical relevance of the results.
Hypothermia itself conveys a greater risk of infection [1], it alters the pharmacokinetics and pharmacodynamics of drugs, and it can induce ventricular arrhythmias in predisposed patients [1]. It also significantly interferes with coagulation [2–3] by enzyme inhibition, thrombocyte dysfunction, and changes in the fibrinolytic process [4]. During hypothermia below 35 °C, pooling of thrombocytes in the spleen and liver is initiated [5]. At 33 °C, primary adhesion and aggregation of thrombocytes begins to be disturbed [6].
Currently, mild hypothermia (32–34°C) has become the therapeutic standard of patient care after CPR [7]. These patients are at an increased risk of bleeding complications due to CPR, trauma and frequent administration of anticoagulation and antiaggregation drugs after percutaneous coronary interventions (PCI) [8]. On the other hand, inadequate anticoagulation and antiaggregation can also increase the morbidity and mortality of these patients – e.g. by an increased risk of coronary artery stent reocclusion [9]. Therefore, the monitoring of coagulation in these patients seems very important.
TEG is an accurate and relatively fast tool for the bedside monitoring of coagulation disorders. It represents a global coagulation test of the plasmatic coagulation factors, and the interaction between thrombocytes, fibrin fibres and fibrinolysis [10]. TEG and standard coagulation tests are carried out at a laboratory temperature of 37 °C, thus omitting the effects of real blood temperature. This affects the interpretation of the actual coagulation status and this was confirmed in in vitro [11] and in vivo studies of TEG [12], as well as in standard coagulation tests [13–14]. E.g. Wolberg [13] and Fukuda [14] proved that a pathological aPTT at hypothermic temperature is not detected at 37 °C. TEG allows simple adjustment of the temperature under which the analysis is performed, thus offering a study of the temperature effect on coagulation.
TEG during therapeutic hypothermia after CPR has not been studied so far. We hypothesised that performing standard TEG at actual blood temperature during therapeutic hypothermia will be significantly different from an analysis at 37 °C. We also wanted to test a new TEG variant – Rapid TEG – in this respect, and to evaluate the clinical impact of temperature adjusted TEG during hypothermia.
Materials and Methods
The study was performed from January 2009 until July 2010 at the Department of Anaesthesiology and Intensive Care, St. Anna’s University Hospital Brno, Czech Republic. All TEG measurements were done on Haemoscope (Haemoscope Corp., Sokie, IL) [10] by the first author with the support of a trained research nurse. The study was approved by the local Ethics Committee of St. Anna’s University Hospital Brno and a waiver to the informed patient consent was obtained. Nevertheless informed consent was obtained from every patient who survived and left our ICU without cognitive dysfunction.
Patients following CPR for cardiac reasons in whom therapeutic hypothermia (32–34 °C) was indicated for 24 hours, and who were expected to survive at least 48 hours in the ICU, were included. No specific exclusion criteria were set.
Therapeutic hypothermia was performed according to the local ICU protocol, independent of the study. Direct continual temperature measurement was performed using a Swan-Ganz catheter, PiCCO or a thermo-sensor in the nasopharynx. All the patients were under deep sedation during the study using a combination of propofol, sufentanil and midazolam, and were observed for 36 hours. Each patient had an arterial and a central venous catheter. Every TEG measurement in each patient during the whole study was taken using the same invasive access – the arterial catheter in the majority of cases. This was strictly adhered to because of the known arterio-venous coagulation differences [15].
TEG was analysed in 12-hour intervals: before hypothermia was started (T0), at 12 hours of hypothermia (T1), at 24 hours of hypothermia (T2), and after 12 hours of rewarming (T3). A freshly drawn blood sample using a 22G needle and 5ml syringe from the invasive access as mentioned above was divided into four aliquots, and analysed immediately and simultaneously on two thromboelastographs. Two samples were activated with kaolin for two minutes and then analysed with additional heparinase. Heparinase type 1 and 2 was used to eliminate the effects of unfractioned heparin, low molecular weight heparin, and heparin-like substances which were reported to be released during hypothermia [16]. One sample was run at the actual temperature of the patient and the other one at 37 °C. The other two samples were analysed using Rapid TEG – a new method of TEG analysis which shortens analysis time significantly by using kaolin to activate the “internal pathway”, and recombinant tissue factor to activate also the “external pathway” of the coagulation cascade. These samples were analysed at the actual body temperature of the patient and at 37 °C as well.
Table 1 shows the analysed TEG parameters and a brief description of their characteristics.
1. TEG parameters and brief description of their characteristics 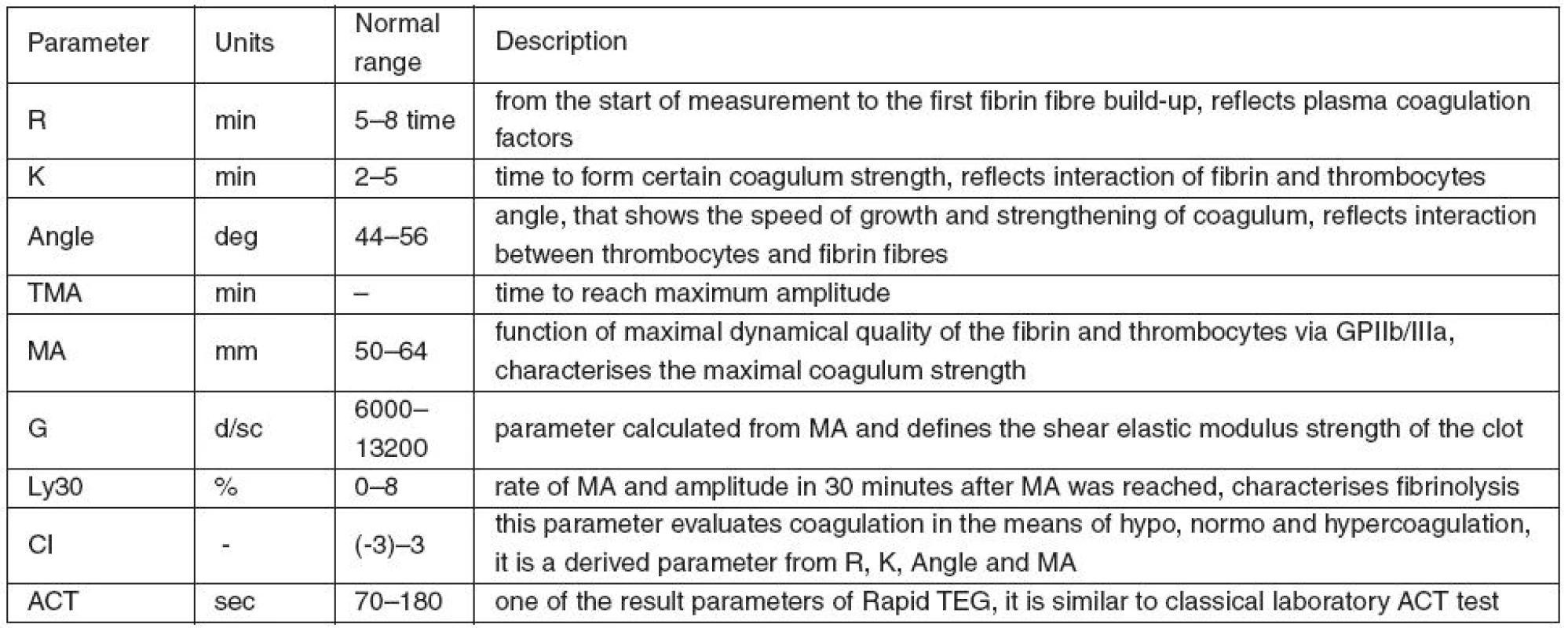
Standard coagulation tests (INR and aPTT), blood count, manifest bleeding and therapeutic interventions aimed at reduction of possible adverse effects of hypothermia were also monitored during the study. Treatment with anticoagulants and antiaggregants was independent of the study protocol.
Minor signs of bleeding were defined as discrete bleeding during suction from the tracheal tube, minor bleeding from the oral cavity or the wound, and minor haematuria. Major signs of bleeding were defined as haemoptysis, massive bleeding from the tracheal tube, major bleeding from the oral cavity or the wound, massive haematuria and large skin haematomas.
Data is presented as median (lower quartile, upper quartile). After a test for normality, the Wilcoxon match pair test was used for the statistical analysis of the differences between the normothermic (analysed at 37 °C) and isothermic (analysed at actual temperature) pair samples at each time-point. P < 0.05 was considered significant.
Results
We obtained 400 samples from 30 patients following CPR (22 men; 8 women). Three patients died during the study. Median age was 65.5 (52–74) years and median length of CPR was 15 (10–20) minutes. The primary cause of the cardiac arrest was malignant arrhythmia, which in 12 patients complicated acute myocardial infarction (AMI).
Temperatures achieved were 35.9 °C (35.4–36.0 °C) at T0; 33°C (32.6–33.9 °C) at T1; 33.1°C (32.8–33.9°C) at T2 and 36.4°C (36.0–36.9°C) at T3.
During hypothermia (T1, T2), all parameters connected to clot formation (R, K, Angle and TMA) and CI in KH TEG were significantly different from those measured at 37 °C. Clot strength parameters (MA, G) did not differ.
Table 2 summarizes the results of KH TEG.
2. Results of Kaolin heparinase TEG at actual temperature (isothermia) and 37 °C (normothermia) 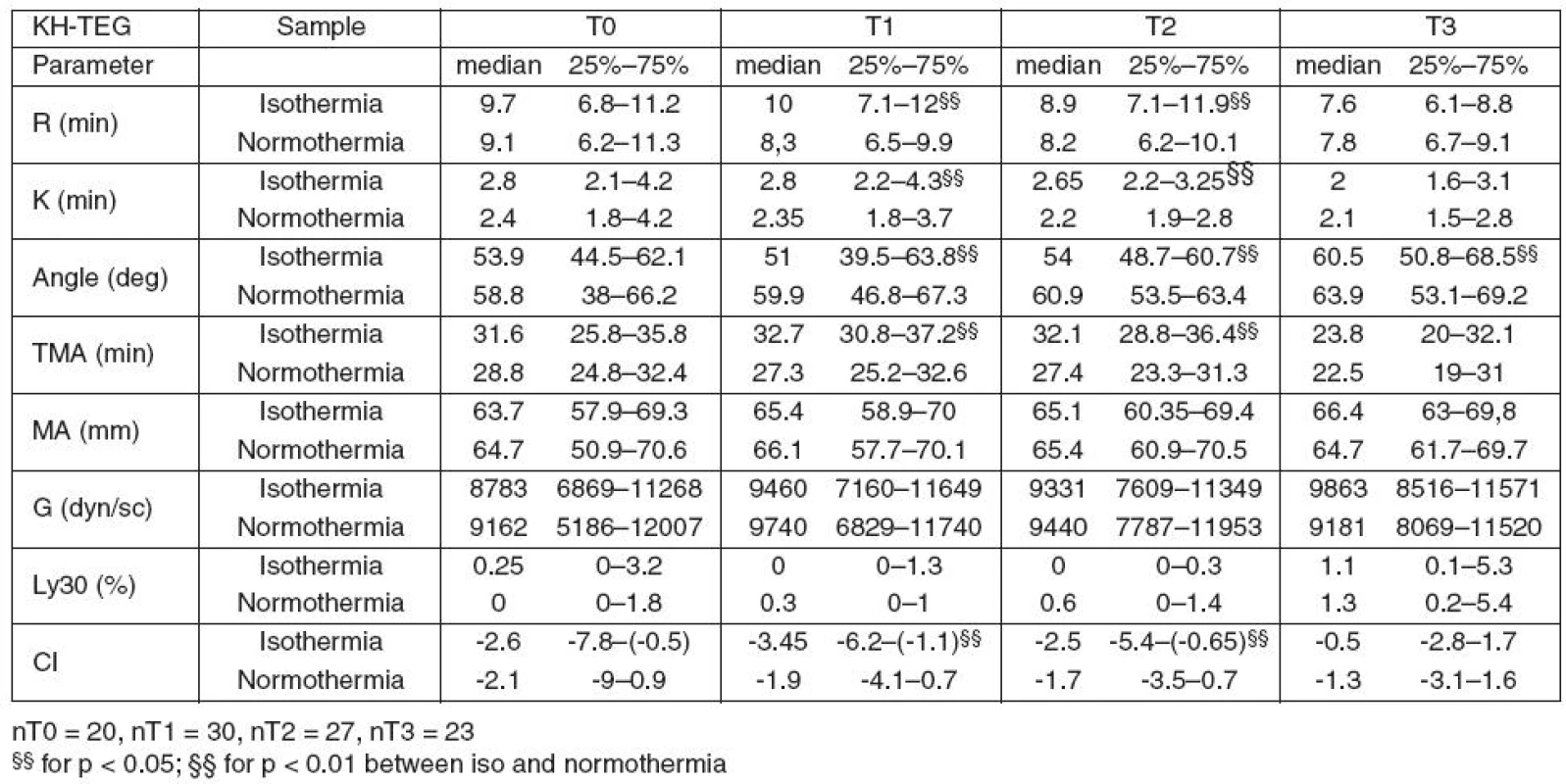
In hypothermic Rapid TEG, only the plasmatic coagulation parameters (R, ACT and TMA) were different from the normothermic values at T1 and partially also at T2 (R and ACT).
Table 3 summarizes the results of Rapid TEG.
3. Results of Rapid TEG at actual temperature (isothermia) and 37°C (normothermia) 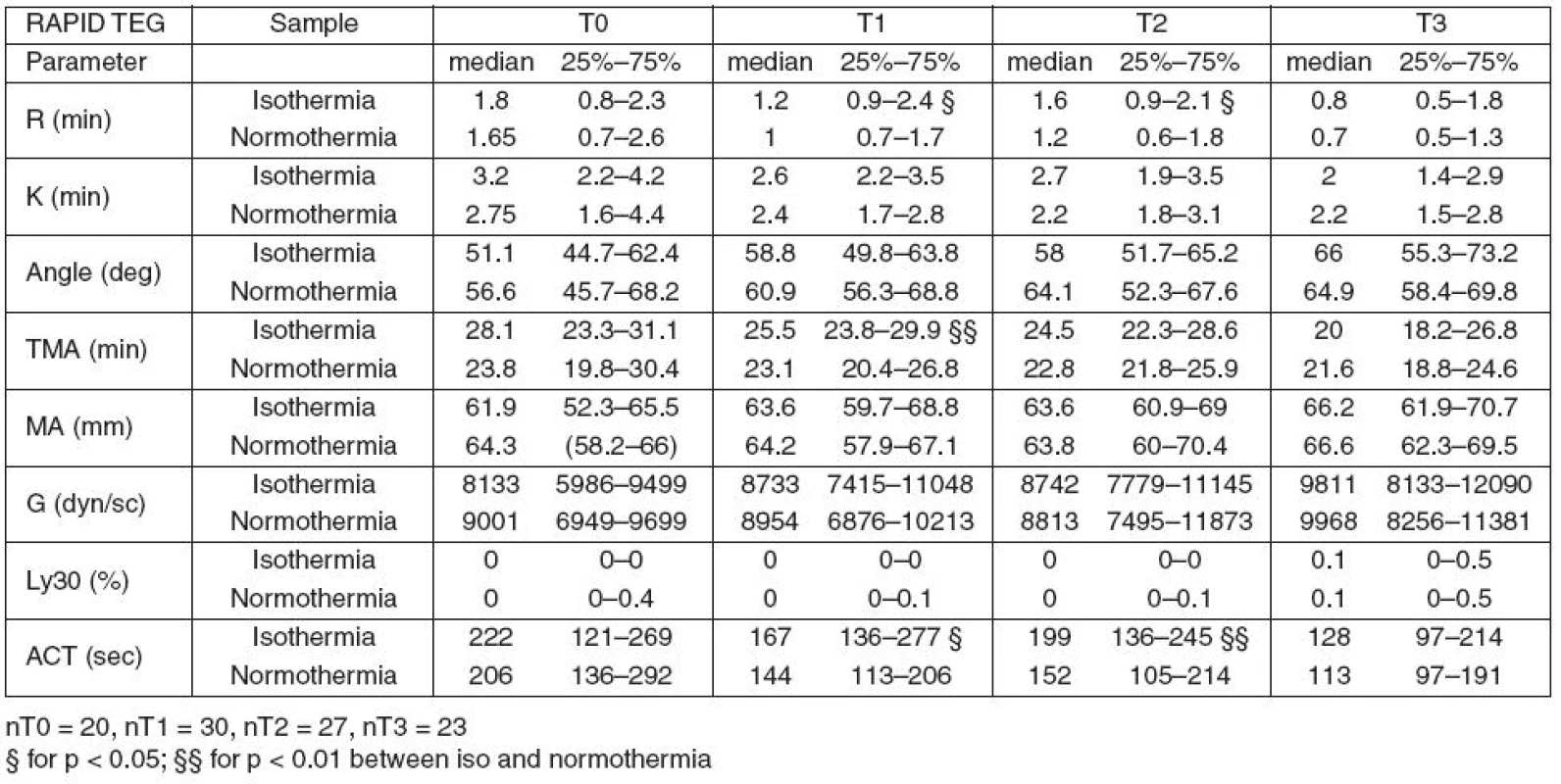
Parameters MA and G (clot strength and quality) and parameter LY30 (fibrinolysis) measured by KH TEG and Rapid TEG showed no effect of temperature.
Table 4 shows types of therapy with anticoagulant and/or antiaggregant drugs.
4. Therapy with anticoagulants and/or antiaggregants drugs during the study 
There were minor signs of bleeding in five patients during time-points T0 and T1 in the group with the combination of unfractioned heparin and clopidogrel and acetylsalicylic acid. Major signs of bleeding (skin haematoma or haemoptysis) were present in two patients whose standard coagulation tests and TEG on ICU admission were normal, despite the fact that one was on chronic warfarin medication. Only one patient received a single unit of blood during the whole study due to low haemoglobin level on admission. No therapeutic interventions were done on the basis of the TEG results.
Table 5 summarises the changes of the standard coagulation tests and the blood count during the study.
5. Changes in blood count and classical coagulation tests during the study 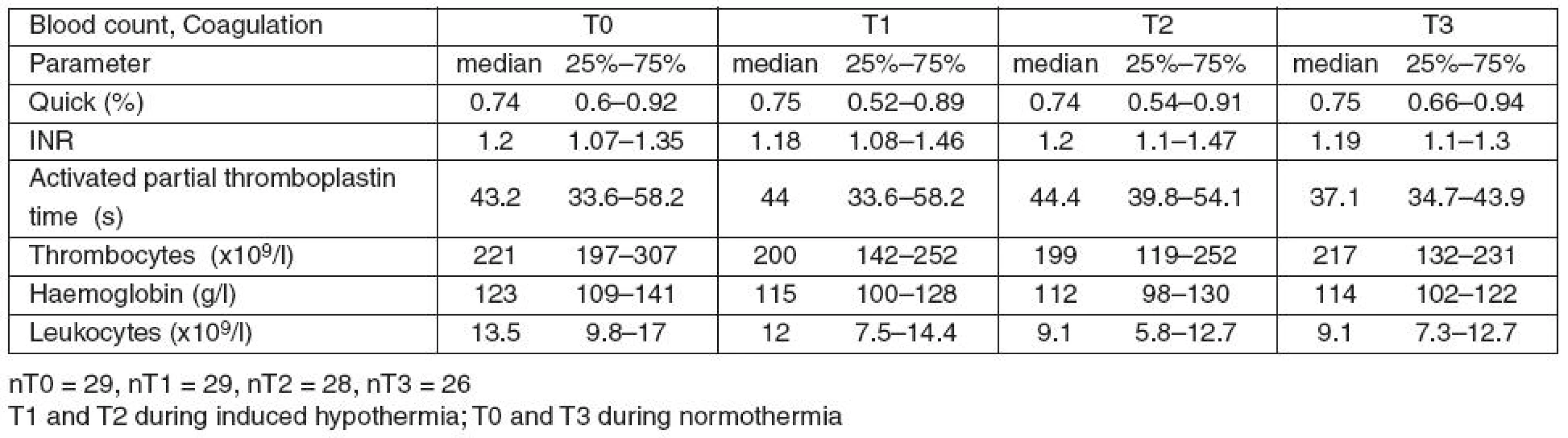
Discussion
Major findings
In standard TEG, clot formation parameters measured during hypothermia were significantly different from the parameters measured at 37 °C. Rapid TEG was less able to trace the hypothermia influence on coagulation. Coagulum strength, quality, and fibrinolysis were not influenced by hypothermia. Despite these statistically significant differences the clinical judgement was not influenced.
KH-TEG
Our findings of diminished clot formation during hypothermia in KH-TEG are in consensus with an experimental study in rabbits by Shimokawa [17], clinical studies in liver transplant patients by Douning [12], and with studies in patients under general anaesthesia by Kettner [18] who also found prolonged parameters R and K, and Angle α decrease during hypothermia. Similarly, parameter MA was free of any significant change. The limitations of these studies, in our opinion, include liver failure in the Douning study and a relatively short period of hypothermia in both of them. All of these studies (ours included) differ from the study by Watts [19] on hypothermic trauma patients, who found a decrease in MA parameter on top of diminished clot formation. Nevertheless, in trauma patients, multiple factors influence coagulation and either the pooling of thrombocytes in the spleen [20], or their shape changes [21] can be responsible for the difference. Interestingly, there is a discrepancy between in vitro and in vivo studies concerning MA parameter during hypothermia. There is either no change or a decline in MA [18–19] in vivo, in contrast to in vitro studies. For example Ramaker [11] who tested different conditions including hypothermia in vitro found significantly impaired K and Angle α, whereas maximum amplitude MA significantly increased.
Rapid TEG
We also tested a new method of TEG analysis – Rapid TEG – which has not been tested in hypothermia so far. Our findings suggest that Rapid TEG due to its maximal activation of internal and external pathways by kaolin and tissue factor lost the ability to trace delicate changes of coagulation status caused by hypothermia. No parameters differed during hypothermia with exception of significantly prolonged ACT (median 30–50 s). As parameter R (a parameter also testing plasmatic coagulation like ACT) was only insignificantly prolonged this observation needs further testing.
The risk of bleeding in patients following CPR
We chose to study patients after CPR because of the high risk of bleeding complications. Multiple factors are involved; e.g. CPR trauma, anticoagulation and antiaggregation therapy, and further factors causing or worsening coagulopathy (e.g. hypoperfusion [22], acidosis [23] and therapeutic hypothermia that are part of standard care after CPR [7]). Therefore, we believe that the monitoring of coagulopathy, and the distinction of its causes, are important in this group of patients and can influence the clinical decisions of pharmacotherapy; e.g. LMWH.
Clinical relevance of mild hypothermia impact on coagulation
The ability to perform measurements at different temperatures at the bedside, which is not the case in standard laboratory tests, is a great advantage of TEG. Though a highly statistically significant impact of hypothermia on clot formation was found, we judge its clinical relevance to be insignificant and see no need to perform TEG analysis in non-trauma hypothermic patients at the actual temperature. Although plasmatic coagulation changes start at temperatures below 34 °C, the thrombocytes are altered at temperatures below 28°C as elegantly shown in vitro in Rundgren’s study [24].
A comment on warfarin on admission, ICU anticoagulants/antiaggregants treatment and dropouts
We had practically no exclusion criteria for the study except for moribund patients. Previous treatment with anticoagulant drugs (two patients) was also not considered inappropriate for study inclusion as relative changes during hypothermia were studied. Interestingly, six patients received no anticoagulation/antiaggregation during the first 36 hours in the ICU. Four of them had ASA in their chronic medication and it was restarted after 48 hours. Indeed, the rather conservative approach of our ICU – i.e. antiaggregants given only in cases of proven or highly suspicious AMI – is clearly visible (see Table 4). Anticoagulants and antiaggregants were given independently of the study protocol and an eventual impact of hypothermia on the pharmacokinetics and pharmacodynamics of the drugs given cannot be excluded. Missing measurements at some of the time-points were caused by various factors – i.e. the death of three patients during the study, lack of time to perform TEG before therapeutic hypothermia was commenced and technical difficulties in some cases. On the whole we think that the number of measurements is representative.
Conclusion
Hypothermia interferes with clot formation. Standard TEG performed at 37 °C does not track these effects and there are major statistically significant differences in clot formation. Rapid TEG was less influenced by temperature with the exception of ACT. Nevertheless, from a clinical point of view, the effect of mild hypothermia on TEG analysis is insignificant. We conclude that there seems no need to analyse TEG at the actual temperature in patients with mild hypothermia following CPR.
Key messages
- Standard TEG performed at 37 °C does not record changes in coagulation during hypothermia.
- When measured at actual temperature, KH-TEG shows significant differences in all clot formation parameters. Rapid-TEG is less influenced by hypothermia.
- Clot strength and quality parameters were not influenced by the temperature adjustment during hypothermia in KH-TEG or Rapid-TEG.
- The clinical significance of these laboratory changes is negligible.
- To obtain relevant ACT values Rapid-TEG should be analysed at the actual temperature during hypothermia.
List of abbreviations
ACT – activated clotting time
AMI – Acute Myocardial Infarction
aPTT – activated partial thromboplastin time
ASA – acetylsalicylic acid
BC – blood count
CI – coagulation index
CPR – cardiopulmonary resuscitation
Hb – haemoglobin
ICU – intensive care unit
INR – international normalized ratio
IQR – inter quartile range
KH – kaolin-heparinase
Leu – leukocytes
LMWH – low molecular weight heparin
MA – maximal amplitude
PCI – percutaneous coronary intervention
TEG – thromboelastography
TMA – time to maximal amplitude
Tr – thrombocytes
UFH – unfractioned heparin
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IC designed the study, collected data, performed the statistical analysis, and wrote the manuscript. VZ participated on the study design, statistical analysis, and gave the final approval for publishing of the manuscript. VS participated on the interpretation of the data, writing and revising the manuscript, and also gave the final approval for publishing of the manuscript. IR participated on the study design, collecting of data, writing the manuscript, and statistical analysis. PS and MP participated on study design, statistical analysis and interpretation of the data.
Acknowledgements and Funding
The whole study and everything connected to it was funded with the support of Grant IGA MZCR NS 10097-3.
Došlo dne 10. 6. 2011.
Přijato dne 20. 9. 2011.
Adresa pro korespondenci:
MUDr. Václav Zvoníček, PhD.
KARIM LF MU a FN u sv. Anny
Pekařská 53
656 91 Brno
e-mail: vaclav.zvonicek@fnusa.cz
Sources
1. Sessler, D. I. Complications and Treatment of Mild Hypothermia. Anesthesiology, 2001, 95, p. 531–543.
2. Rohrer, M. J., Natale, A. M. Effect of hypothermia on coagulation cascade. Critical Care Med., 1992, 20, p. 1402–1405.
3. Reed, R. L., Johnson, T. D., Hudson, J. D., Fischer, R. P. The disparity between hypothermic coagulopathy and clotting studies. J. Trauma, 1992, 33, p. 465–470.
4. Britt, L., Dascombe, W., Rodriguez, A. New horizons in management of hypothermia and frostbite injury. Surg. Clin. North. Am., 1991, 71, p. 345–370.
5. Lier, H. Hypothermie und die tödliche Triade. Notfall Rettungsmed, 2008, 11, p. 377–380.
6. Lier, H., Krep, H., Schöchl, H. Gerinnungsmanagement bei der Polytraumaversorgung. Anaesthesist, 2009, 58, p. 1010–1026.
7. Nolan, J. P., Morley, P. T., Vanden Hoek, T. L., Hickey, R. W., Kloeck, W. G., Billi, J., Böttiger, B. W., Morley, P. T., Nolan, J. P., Okada, K., Reyes, C., Shuster, M., Steen, P. A., Weil, M. H., Wenzel, V., Hickey, R. W., Carli, P., Vanden Hoek, T. L., Atkins, D., International Liaison Committee on Resuscitation Therapeutic Hypothermia After Cardiac Arrest. Circulation, 2003, 108, p. 118–121.
8. Karrillon, G. J., Morice, M. C., Benveniste, E., Bunouf, P., Aubry, P., Cattan, S., Chevalier, B., Commeau, P., Cribier, A., Eiferman, C., Grollier, G., Guerin, Y., Henry, M., Lefevre, T., Livarek, B., Louvard, Y., Marco, J., Makowski, S., Monassier, J. P., Pernes, J. M., Rioux, P., Spaulding, C., Zemour, G. Intracoronary Stent Implantation Without Ultrasound Guidance and With Replacement of Conventional Anticoagulation by Antiplatelet Therapy. Circulation, 1996, 94, p. 1519–1527.
9. Gurbel, P. A., Tantry, U. S. Aspirin and Clopidogrel Resistance: Consideration and Management. J. Interven. Cardiol., 2006, 9, p. 439–448.
10. Luddington, R. J. Thrombelastography/thromboelastometry. Clin. Lab. Haematol., 2005, 27, 2, p. 81–90.
11. Ramaker, A. J., Meyer, P., van der Meer, J., Struys, M. M., Lisman, T., van Oeveren, W., Hendriks, H. G. Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: results of point of care testing with the thromboelastography analyser. Blood Coagulation & Fibrinolysis, 2009, 20, 6, p. 436–439.
12. Douning, L. K., Ramsay, M. A., Swygert, T. H., Hicks, K. N., Hein, H. A., Gunning, T. C., Suit, C. T. Temperature Corrected Thromboelastography in hypothermic patiens. Anesth. Analg., 1995, 81, p. 608–611.
13. Wolberg, A. S., Meng, Z. H., Monroe, M. D., Hoffman, M. A systematic evaluation of the effect of temperature on coagulation enzyme aktivity and palatelet function. J. Trauma, 2004, 56, p. 1221–1228.
14. Fukuda, A., Ishida, H., Kubota, M., Kojima, Y., Mizobata, Y., Matsuoka, T., Yokota, J. Clinical data obtained through coagulation testing suggests that hypothermia exerts influence on patient’s blood coagulation retraction. Rinsho Byori, 2000, 48, p. 1102–1108.
15. Naimi, S., Goldstein, R., Proger, S. Studies of Coagulation and Fibrinolysis of the Arterial and Venous Blood in Normal Subjects and Patients with Atherosclerosis. Circulation, 1963, 27, p. 904–918.
16. Paul, J., Cornillon, B., Baguet, J., Dureau, G., Belleville, J. In vivo release of heparin-like factor in dogs during profound hypothermia. J. Thorac. Cardiovasc. Surg., 1981, 82, p. 45–48.
17. Shimokawa, M., Katsuyasu, K., Kawaguchi, M., Sakamoto, T., Kakimoto, M., Furuya, H. The influence of Induced hypothermia for hemostatic function on temperature--adjusted measurements in rabbits. Anesth. Analg., 2003, 96, p. 1209–1213.
18. Kettner, S. C., Sitzwohl, C., Zimpfer, M., Kozek, S. A., Holzer, A., Spiss, C. K., Illievich, U. M. The effect of graded Hypothermia (36°C–32°C) on hemostasis in anesthetized patiens without surgical trauma. Anesth. Analg., 2003, 96, p. 1772–1776.
19. Watts, D. D., Trask, A., Soeken, K., Perdue, P., Dols, S., Kaufmann, C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J. Trauma, 1998, 44, 5, p. 846–854.
20. Villalobos, T., Adelson, E., Barilia, T. Hematolytic changes in hypothermic dogs. Proc. Soc. Exp. Biol. Med., 1955, 89, p. 192–196.
21. White, T., Krivit, W. An ultrastructural basis for the shape changes induced in platelets by chilling blood. Blood, 1967, 30, p. 635–675.
22. Brohi, K., Cohen, M. J., Ganter, M. T., Matthay, M. A., Mackersie, R. C., Pittet, J. F. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann. Surg., 2007, 245, p. 812–818.
23. Lier, H., Krep, H., Schroeder, S., Stuber, F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia and hypothermia on functional hemostasis in trauma. J. Trauma, 2008, 65, p. 951–960.
24. Rundgren, M., Engström, M. A thromboelastometric evaluation of the effects of hypothermia on the coagulation system. Anesth. Analg., 2008, 107, 5, p. 1465–1468.
Labels
Anaesthesiology, Resuscitation and Inten Intensive Care Medicine
Article was published inAnaesthesiology and Intensive Care Medicine

2011 Issue 5-
All articles in this issue
- Ultrasound guided medial cervical plexus blockade
- Fibrinolysis in cardiac surgery in the post-aprotinin era
- The mechanism of hypotension with intravenous paracetamol in the critically ill
- Does measuring the intracranial pressure influence survival rates in patients with brain injury?
- The influence of temperature adjustment on thromboelastography results: prospective cohort study
- Anaesthesiology and Intensive Care Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Ultrasound guided medial cervical plexus blockade
- Does measuring the intracranial pressure influence survival rates in patients with brain injury?
- The mechanism of hypotension with intravenous paracetamol in the critically ill
- Fibrinolysis in cardiac surgery in the post-aprotinin era
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

