-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAutologous transplantation of mesenchymal stem cells into the portal vein of the miniature pig; a preliminary experiment for NOTES approach
Autologní transplantace mezenchymálních kmenových buněk do vena portae miniprasete; úvodní experiment pro NOTES metodiku
Úvod: Existuje důkaz, že mezenchymální kmenové buňky (MSC) mohou trans-diferencovat do jaterních buněk in vitro a in vivo a mohou tak být použity jako spolehlivý zdroj pro terapii kmenovými buňkami jaterních nemocí. Kombinace MSC (s nebo bez trans-diferenciace v in vitro podmínkách) a miniinvazivních technik, jako je laparoskopie nebo NOTES, představuje šanci pro mnohé pacienty marně čekající na transplantaci jater.
Metody: Více než 30 miliónů autologních MSC na 3. pasáži bylo transplantováno přes vena portae u osm měsíců starého miniprasete. Depozice transplantovaných buněk v jaterním parenchymu byla hodnocena histologicky. Trans-diferenciační potenciál CM-DiI značených buněk byl hodnocen pomocí exprese prasečího albuminu pomocí imunofluorescence.
Výsledky: Tři týdny po transplantaci jsme detekovali značené buňky (jednotlivě, malé shluky) ve všech 10 vzorcích (2 vzorky z každého laloku). K difuzní distribuci ve vzorkách nedošlo. CM-DiI+ buňky byly pozorovány převážně v okolí portálních triád. Také jsme detekovali lokalizaci signálu pro albumin v CM-DiI značených buňkách.
Závěr: Výsledky studie ukazují, že transplantace autologních MSC (bez dodatečné jaterní diferenciace in vitro) přes vena portae vedla k úspěšné infiltraci intaktního jaterního parenchymu miniprasete s detekovatelnou in vivo trans-diferenciací. NOTES, tak jako jiné nově vyvinuté chirurgické přístupy v kombinaci s buněčnou terapií, se zdají být velmi slibné pro léčbu jaterních nemocí v blízké budoucnosti.
Klíčová slova:
buněčná terapie jater – miniprase – mezenchymální kmenové buňky – Natural Orifice Transluminal Endoscopic Surgery
Authors: S. Juhas 1; J. Martínek 1,2; O. Ryska 1,3; R. Dolezel 1,4; M. Ryska 1,4; J. Juhásová 1
Authors place of work: Institute of Animal Physiology and Genetics, Czech Academy of Science, PIGMOD, Liběchov 1; Department of Hepatogastroenterology, Institute for Clinical and Experimental Medicine, Prague 2; Royal Lancaster Infirmary, University Hospitals of Morecambe Bay, NHS Foundation Trust, Lancaster 3; Department of Surgery, 2nd Faculty of Medicine, Charles University and Central Military Hospital, Prague 4
Published in the journal: Rozhl. Chir., 2019, roč. 98, č. 9, s. 350-355.
Category: Original article
doi: https://doi.org/10.33699/PIS.2019.98.9.350–355Summary
Introduction: There is evidence that mesenchymal stem cells (MSCs) could trans-differentiate into the liver cells in vitro and in vivo and thus may be used as an unfailing source for stem cell therapy of liver disease. Combination of MSCs (with or without their differentiation in vitro) and minimally invasive procedures as laparoscopy or Natural Orifice Transluminal Endoscopic Surgery (NOTES) represents a chance for many patients waiting for liver transplantation in vain.
Methods: Over 30 millions of autologous MSCs at passage 3 were transplanted via the portal vein in an eight months old miniature pig. The deposition of transplanted cells in liver parenchyma was evaluated histologically and the trans-differential potential of CM-DiI labeled cells was assessed by expression of pig albumin using immunofluorescence.
Results: Three weeks after transplantation we detected the labeled cells (solitary, small clusters) in all 10 samples (2 samples from each lobe) but no diffuse distribution in the samples. The localization of CM-DiI+ cells was predominantly observed around the portal triads. We also detected the localization of albumin signal in CM-DiI labeled cells.
Conclusion: The study results showed that the autologous MSCs (without additional hepatic differentiation in vitro) transplantation through the portal vein led to successful infiltration of intact miniature pig liver parenchyma with detectable in vivo trans-differentiation. NOTES as well as other newly developed surgical approaches in combination with cell therapy seem to be very promising for the treatment of hepatic diseases in near future.
Keywords:
mesenchymal stem cells – liver cell therapy – miniature pig – Natural Orifice Transluminal Endoscopic Surgery
Introduction
At the present time liver transplantations are performed at many centers worldwide and the transplantation technique has been constantly studied and improved [1]. Unfortunately, the supply of liver allografts from living and non-living donors is still insufficient and high numbers of people are waiting for liver transplants [2] despite the decrease in the prevalence of hepatitis B virus (HBV). Cell or stem cell therapy should be an alternative treatment for patients with end-stage liver diseases and could save patients who are in life-threatening situations, enabling them to gain more time and increase their prospect of survival [3]. Hepatocytes transplantation represents one of the clinically used cell therapy possibilities and is metabolically less stressful than transplantation of the whole organ. In addition therapeutic genes can be transferred into the cultured hepatocytes to prevent immune rejection of allografted or xenografted hepatocytes [4]. On the other hand the primary cultures of hepatocytes in monolayers are frequently associated with cell dedifferentiation, which is accompanied by spontaneous cell death [5]. Another possible way how to regenerate injured liver tissue is the use of stem cells from different origin and their capability to trans-differentiate into the hepatocytes functionally mimicking cells. Mesenchymal stem cells (MSCs) are multipotent stem cells that can differentiate into a variety of cell types (osteoblasts, chondrocytes and adipocytes). There are also various studies that declare trans-differentiation of MSCs into albumin-positive hepatocyte-like cells in vivo and in vitro [6,7]. In the case of a diffuse disease such as liver fibrosis, local intravascular injection has been successfully used to accomplish the delivery of stem cells through the whole organ. For example, mesenteric vein as a branch of portal vein was previously used as a place of magnetically labeled MSCs delivery in rats with liver fibrosis [8]. Moreover bioimaging rats were used to detect an improvement in acute and chronic liver damage after administration of genetically stained MSCs via portal vein [9].
Various invasive and minimally-invasive techniques are now available for the introduction of stem cells into the portal system [10–12]. Natural Orifice Transluminal Endoscopic Surgery (NOTES) represents a modern and developing form of endoscopic surgery [13]. For the abdominal cavity, NOTES procedures can be done through the transgastric, transcolonic, transvaginal, or transvesical approach, predominantly performed as a hybrid technique [14]. NOTES approach promises many benefits as improved cosmetics, earlier recovery and easier access to the retroperitoneum; however, achievement of secure luminal closure and development of a multitasking platform with dedicated instruments are essential for the success of this new endoscopic surgery. Thus the miniature pig was used as a large animal model for the development of safe techniques for successful access site closure in NOTES or for comparison with other standard surgical techniques (laparotomy, laparoscopy) [15,16].
The goal of our experiment was to transplant the autologous miniature pig MSCs into the intact liver by standard laparotomy through the portal vein and to observe cells distribution with their trans-differentiation potential in vivo as a preliminary experiment for NOTES application.
Methods
The experiment was carried out according to the guidelines for the care and use of experimental animals and was approved by the Resort Professional Commission of the CAS for Approval of Projects of Experiments on Animals (Approved protocol No. 6/2010). One miniature pig (male, 8 months old) from the Libechov breeding station (IAPG CAS, v.v.i.; PIGMOD; http://www.iapg.cas.cz/en/) was used for the experimental study. The animal was premedicated with intramuscular application of TKX mixture containing Tiletamine 4 mg/kg and Zolazepam 4 mg/kg (Zoletil 100, Virbac), Ketamine 10 mg/kg (Narketan 10, Chassot) and Xylazine 2 mg/kg (Rometar 2%, Spofa) as well as Atropine 0.05 mg/kg (BB Pharma a.s., Praha). After intravenous cannulation and intubation, Isoflurane (1.5%) combined with i.v. Fentanyl (3–5 ml/h) was used for inhalation anesthesia. Then we aspirated the bone marrow blood from tuber coxae ala ossis ilii using a bioptic needle (15G/70mm). The separation of mononuclear cells from the whole bone marrow was performed by density gradient centrifugation at 400 g for 30 min at room temperature using Ficoll-PaqueTM PLUS (Stemcell Technologies; Canada). Mononuclear cells in an opalescent layer between Ficoll and blood plasma were collected, washed in a culture medium and used for propagation under in vitro conditions [17,18]. The average amount of mononuclear cells was 50x106 cells per isolation. Cell count and viability were analyzed on Vi-CELL (Series × Cell Viability Analyzers) and about 98% of viable cells were detected. The cells were seeded in tissue culture flasks (1.3x106/cm2) and cultured at 37°C in 5% CO2. The culture medium was alpha-MEM medium (GIBCO, Invitrogen) supplemented with 10% FBS (Sigma-Aldrich) and gentamycin (50 mg/ml; Sigma-Aldrich). After reaching 80% confluence (Fig. 1A) the adherent MSCs were passaged using trypsin (0.5% trypsin-EDTA solution; Sigma-Aldrich) and reseeded at a density of 15,000 cells/cm2. After the 3rd passage the MSCs were harvested (34x106) and labeled with Cell TrackerTM CM-DiI (2 μg/ml; for 20 minutes). Subsequently, midline laparotomy was performed and the labeled MSCs in 2% FBS/5000 IU heparin/PBS (50 ml) were injected into the portal vein. After three weeks we deeply anesthetized the animal with TKX mixture and transcardially perfused the pig with heparinized (4 units/ml) PBS, followed by administration of 4% paraformaldehyde (5 liters; pH 7.4). The liver was then removed and 10 samples (2x2 cm) were randomly collected from each main liver lobe (diaphragmatic surface – right lateral (RL), right medial (RM), left lateral (LL), left medial (LM), and the lobus caudatus (LC) – 2 pieces from each) (Fig. 1D). The samples were postfixed for 2 weeks in 4% paraformaldehyde at 4°C. After postfixation, the liver pieces were cryoprotected in 10%, 20% and 30% sucrose with 0.02% sodium azide and 10–40 μm thick sections cut on a cryostat. Fluorescent immunostaining of the sections with a pig albumin antibody (Abcam; ab79960) was consequently performed.
Fig. 1. Tracking of mesenchymal stem cells on the culture plastic dish just before transplantation by CM-DiI staining
Phase contrast (A) or fluorescence microscopy (B) (100x magnification). Administration of tracked MSCs into the portal vein via the catheter under standard laparotomy. Sampling (2 samples from each lobe, 10 samples in total) of the liver after whole-body perfusion of the animal.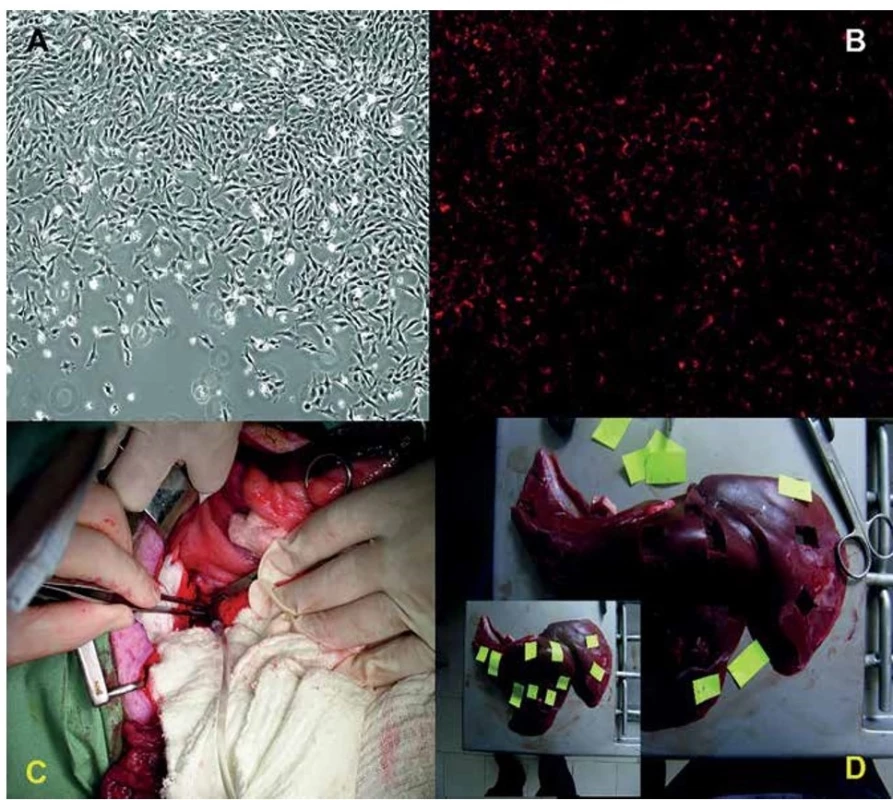
Hybrid NOTES method was performed in another animal (Libechov miniature pig, male, 8 months old) with application of saline (50ml) into the portal vein by an endoscopic injector through a double-channel endoscope (GIF 2T160; Olympus Medical Co., Tokyo, Japan). We used a large endoscopic grasper (FG-48L, 026235; Olympus Medical Co.) in the second working channel for assistance during solution application. The access site was created in the rectum on the anterior wall, 15 cm from the anal verge. Balloon dilatation (CRE balloon 20 mm; Boston Scientific, Natick, MA) was used for entry into the abdominal cavity. Laparoscopic fan liver retractor and grasper were used for liver lobes elevation as well as for spleen, stomach and intestine dislocation. A laparoscopic camera was introduced through laparoscopic trocar in the abdominal wall to record the entire procedure. Finally the entry site was closed by KING closure [16].
Results
The presence of CM-DiI labeling in the miniature pig MSCs was confirmed by fluorescence in vitro (Fig. 1B). The portal vein was visualized using the midline standard laparotomy approach and the CM-DiI labeled cells were injected as a suspension (50ml syringes) by an 18G (1.2x40mm) injection needle adapted onto a transparent PVC infusion cannula 2.9/4.1 mm (Fig. 1C). We detected the CM-DiI positive cells in the liver parenchyma of all 10 collected pieces (Fig. 2A). The CM-DiI+ cells were not equally distributed between the pieces and were predominantly observed around the portal triads. Localization of the cells was solitary or in small clumps. We observed albumin positivity in the liver parenchyma. The co-localization of albumin signal with CM-DiI labeled cells was also detected (Fig. 2B−C).
Fig. 2. Tracked mesenchymal stem cells in the liver 21 days after transplantation detected by confocal microscopy
Fluorescent detection of tracked mesenchymal stem cells (A, red, 400x magnification) in different liver lobes. Co-localization analysis of tracked MSCs (red) with pig specific albumin (green) in liver parenchyma (B, C, 630x magnification). B´, C´ – DAPI; B´´, C´´– pig albumin; B´´´, C´´´– CM-DiI.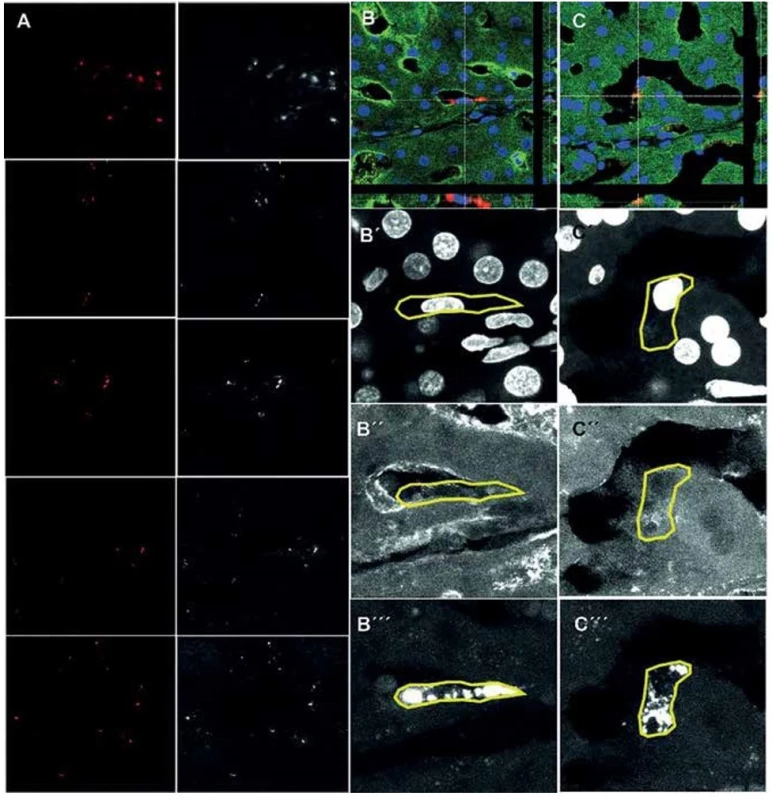
Endoscopic rectotomy for entrance into the abdominal cavity as well as hybrid NOTES procedure for injection of saline into the portal vein were performed without any serious complication (Fig. 3). Only minimal diffuse bleeding from spleen and liver parenchyma occurred during laparoscopic manipulation before the endoscopic injection. KING closure of the entry incision was done without any complication and leakage.
Fig. 3. Hybrid NOTES procedure of saline application into the portal vein
Laparoscopic view of the site of application. In the center of the view there is the tip of the endoscope with the injector (front working channel) and grasper (back working channel). Endoscopic grasper is pulling and fixing tunica adventitia/serosa of the portal vein. The endoscopic injector is moving closely to the portal vein.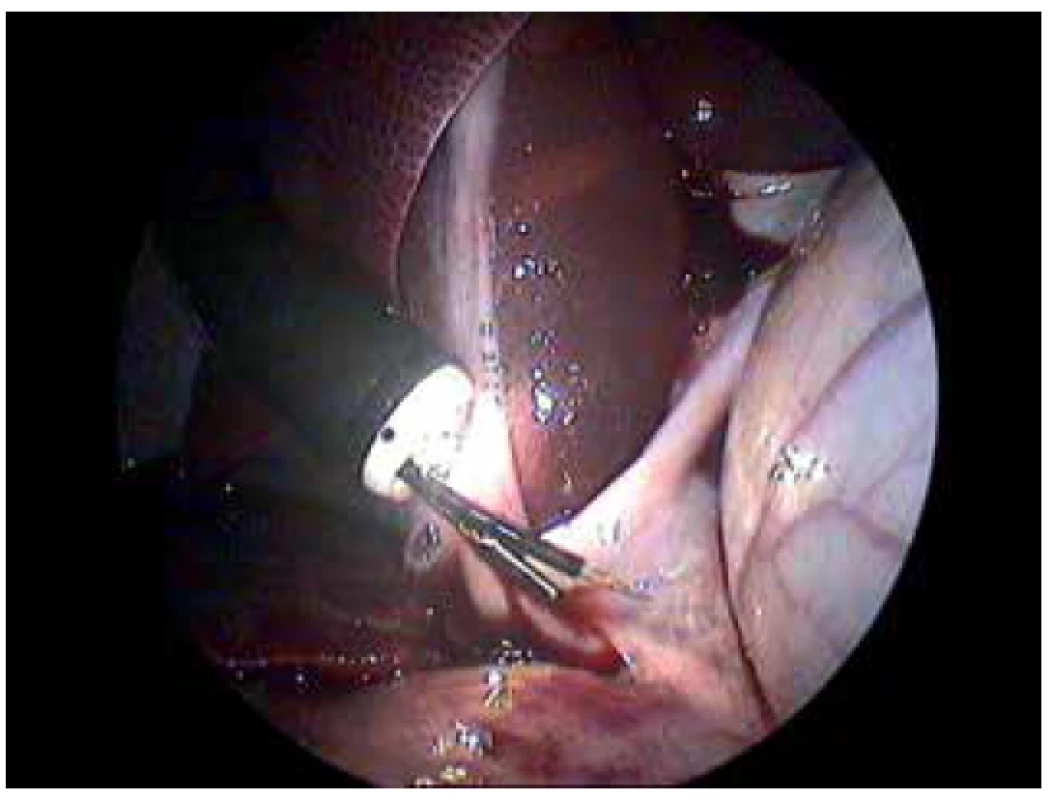
Discussion
Animal model studies are necessary for introducing of experimental cell-based therapy into the human practice. Major problems connected with cell therapy of hepatic diseases in preclinical experiments include the impossibility of introducing cells to integrate into the recipient liver, their poor trans-differentiation or differentiation and early loss of their functionality [19]. However, different cell types as iPSCs, MSCs, endothelial, embryogenic or hematopoetic have been tested as a source of hepatocytes or hepatocyte-like cells in animal models with intact or damaged liver parenchyma. Predominantly used cell sources in liver cirrhosis clinical studies included mesenchymal stem cells derived from bone marrow, umbilical cord or adipose tissue infused intravenously or via the hepatic artery. The major advantage of administration of stem cells through the portal vein is no loss of the stem cells in the periphery, outside the liver (predominantly the lungs); the stem cells reside in the portal areas and repopulate the areas faster compared to intrahepatic infusion [20]. A similar localization pattern was also detected in our study with the miniature pig as a large animal model where the cells were able to express albumin as a hepatocyte marker. Analogous to our result, human albumin positivity together with HEPAR1 was declared after transplantation of human MSCs into the splenic vein of the miniature pig with an ischemia-reperfusion injury [21]. The fundamental principle behind the stem cell therapy is that after undifferentiated cells are delivered to the injured host and migrate to the site of injury, they differentiate (under influence of local signals) into cells of the appropriate phenotype [22]. It is interesting that also a relatively intact liver parenchyma without any induced liver damage or inflammation process was colonized by transplanted MSCs with albumin positivity detection in the miniature pig. Mesenchymal stem cells represent not only a potential source of new hepatocyte-similar cells for liver disease therapy, but they also possess an additional positive function, namely the production of modulatory cytokines with inhibition of immunocytes proliferation and their migration into the liver, resulting in an attenuation of the liver injury and reduction of hepatocytes apoptosis. Moreover MSCs play an important role in terms of regression of liver fibrosis and support of endogenous hepatocytes under appropriate conditions [23].
Hybrid and pure NOTES procedures have been recently used in different application as gastroenterostomy or tumor resection [24,25]. Our successful injection of saline via the portal vein in the miniature pig represents a promising preclinical experiment for introducing hybrid NOTES procedures in hepatic cell-based therapy in the future.
Conclusion
Autologous MSCs isolated from the miniature pig, introduced via the portal vein, were able to infiltrate intact hepatic parenchyma and survive over 3 weeks. The presence of albumin signal in CMDiI+ cells indicates the presence of the trans-differentiation process into hepatocyte-like cells under normal healthy conditions. Use of the NOTES approach, which was recently successfully introduced at the Czech clinic, may provide a useful improvement in application of hepatocytes, MSCs or other stem cells (e.g. induced pluripotent stem cells) into the portal vein with consequent treatment of damaged hepatic tissue in man.
This work was supported by the grants NV16-27653A, NV16-31806A (Czech Health Research Council), the National Sustainability Program I, project number LO1609 (Czech Ministry of Education, Youth and Sports), and RVO: 67985904.
Conflict of interests
The authors declare that they have no conflict of interest in connection with this paper and that the article has not been published in any other journal.
MVDr. Štefan Juhás, PhD.
PIGMOD center
Laboratory of Cell Regeneration and Plasticity
Institute of Animal Physiology and Genetics
Czech Academy of Science v.v.i.
Rumburská 89
Liběchov, 27721
e-mail: juhas@iapg.cas.cz
Zdroje
1. Testino G, Leone S, Pellicano R. Liver transplantation: a new era. Minerva Gastroenterol Dietol. 2019;65 : 163−6. doi:10.23736/s1121-421x.19.02555-8.
2. Lan X, Zhang H, Li HY, et al. Feasibility of using marginal liver grafts in living donor liver transplantation. World J Gastroenterol. 2018;24 : 2441–56. doi:10.3748/wjg.v24.i23.2441.
3. Horisawa K, Suzuki A. Cell-based regenerative therapy for liver disease. In: Nakao K, Minato N, Uemoto S, editors. Innovative Medicine. Tokyo, Springer Japan 2015 : 327–39.
4. Barahman M, Asp P, Roy-Chowdhury N, et al. Hepatocyte transplantation: Quo Vadis? Int J Radiat Oncol Biol Phys. 2019; 103 : 922-934. doi:10.1016/j.ijrobp.2018.11.016.
5. Vinken M, Decrock E, Doktorova T, et al. Characterization of spontaneous cell death in monolayer cultures of primary hepatocytes. Arch Toxicol. 2011;85 : 1589–96. doi:10.1007/s00204-011-0703-4.
6. Zhou X, Cui L, Zhou X, et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017;21 : 881–93. doi:10.1111/jcmm.13027.
7. Stock P, Bruckner S, Ebensing S, et al. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc. 2010;5 : 617–27. doi:10.1038/nprot.2010.7.
8. Zhou B, Shan H, Li D, et al. MR tracking of magnetically labeled mesenchymal stem cells in rats with liver fibrosis. Magn Reson Imaging 2010;28 : 394–9. doi:10.1016/j.mri.2009.12.005.
9. Haga J, Enosawa S, Kobayashi E. Cell therapy for liver disease using bioimaging rats. Cell Med. 2017;9 : 3–7. doi:10.3727/215517916X693104.
10. Avritscher R, Abdelsalam ME, Javadi S, et al. Percutaneous intraportal application of adipose tissue–derived mesenchymal stem cells using a balloon occlusion catheter in a porcine model of liver fibrosis. J Vasc Interv Radiol. 2013;24 : 1871–8. doi:10.1016/j.jvir.2013.08.022.
11. Yu F, Ji S, Su L, et al. Adipose-derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis in vivo. J Formos Med Assoc. 2015;114 : 130–8. doi:10.1016/j.jfma.2012.12.002.
12. Chetty SS, Praneetha S, Govarthanan K, et al. Non-invasive tracking and regenerative capabilities of transplanted human umbilical-cord derived mesenchymal stem cells labeled with I-III-IV semiconducting nanocrystals in liver-injured living mice. ACS Appl Mater Interfaces. American Chemical Society 2019. doi:10.1021/acsami.8b19953.
13. Schwaitzberg SD, Roberts K, Romanelli JR, et al. The NOVEL trial: natural orifice versus laparoscopic cholecystectomy—a prospective, randomized evaluation. Surg Endosc. 2017;32 : 2505–16. doi:10.1007/s00464-017-5955-5.
14. Bernhardt J, Sasse S, Ludwig K, et al. Update in natural orifice translumenal endoscopic surgery (NOTES). Curr Opin Gastroenterol. 2017;33 : 346–51. doi:10.1097/MOG.0000000000000385.
15. Martínek J, Ryska O, Filípková T, et al. Natural orifice transluminal endoscopic surgery vs laparoscopic ovariectomy: Complications and inflammatory response. World J Gastroenterol. 2012;18 : 3558−64. doi:10.3748/wjg.v18.i27.3558.
16. Dolezel R, Ryska O, Kollar M, et al. A comparison of two endoscopic closures: over-the-scope clip (OTSC) versus KING closure (endoloop + clips) in a randomized long-term experimental study. Surg Endosc. 2016;30 : 4910–6. doi:10.1007/s00464-016-4831-z.
17. Juhásová J, Juhás Š, Klíma J, et al. Osteogenic differentiation of miniature pig mesenchymal stem cells in 2D and 3D environment. Physiol Res. 2011;60 : 559–71.
18. Smatlikova P, Juhas S, Juhasova J, et al. Adipogenic differentiation of bone marrow-derived mesenchymal stem cells in pig transgenic model expressing human mutant huntingtin. J Huntingtons Dis. 2019;8 : 33–51. doi:10.3233/jhd-180303.
19. Vosough M, Moslem M, Pournasr B, et al. Cell-based therapeutics for liver disorders. Br Med Bull. 2011;100 : 157–72. doi:10.1093/bmb/ldr031.
20. Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J stem cells. Korean Society for Stem Cell Research 2008;1 : 1–7.
21. Lin NC, Wu HH, Ho JH, et al. Mesenchymal stem cells prolong survival and prevent lethal complications in a porcine model of fulminant liver failure. Xenotransplantation 2019;e12542. [Epub ahead of print] doi:10.1111/xen.12542.
22. Kharaziha P, Hellström PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I–II clinical trial. Eur J Gastroenterol Hepatol. 2009;21 : 1199–205. doi:10.1097/MEG.0b013e32832a1f6c.
23. Lin H, Xu R, Zhang Z, et al. Implications of the immunoregulatory functions of mesenchymal stem cells in the treatment of human liver diseases. Cell Mol Immunol. 2011;8 : 19–22. doi:10.1038/cmi.2010.57.
24. Fernandes J, Libanio D, Giestas S, et al. Hybrid NOTES: Complete endoscopic resection of the gastric wall assisted by laparoscopy in a gastric fundus gastrointestinal stromal tumor. GE Port J Gastroenterol. 2019;26 : 215–7. doi:10.1159/000491709.
25. Liu BR, Liu D, Zhao LX, et al. Pure natural orifice transluminal endoscopic surgery (NOTES) nonstenting endoscopic gastroenterostomy: first human clinical experience. VideoGIE. 2019;4 : 206–8. doi:10.1016/j.vgie.2019.01.004.
Štítky
Chirurgie všeobecná Ortopedie Urgentní medicína
Článek vyšel v časopiseRozhledy v chirurgii
Nejčtenější tento týden
2019 Číslo 9- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Hojení análních fisur urychlí čípky a gel
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Přístroje a nástroje v chirurgii
- ERAS in colorectal surgery – neglected preadmission items
- Jubilant primář Vlastimil Bursa
- Autologous transplantation of mesenchymal stem cells into the portal vein of the miniature pig; a preliminary experiment for NOTES approach
- Use of preperitoneal wound catheter for continuous local anaesthesia after laparoscopic colorectal surgery
- Phyllodes tumours – a retrospective review of 83 clinical cases
- Foreign body ingestion in children
- Percutaneous endoscopic cecostomy in the treatment of recurrent colonic pseudo-obstruction − a case report of the first procedure in the Czech Republic
- Portal vein ligation with alcohol injection – our first experiences
- Rozhledy v chirurgii
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Foreign body ingestion in children
- Phyllodes tumours – a retrospective review of 83 clinical cases
- ERAS in colorectal surgery – neglected preadmission items
- Percutaneous endoscopic cecostomy in the treatment of recurrent colonic pseudo-obstruction − a case report of the first procedure in the Czech Republic
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



