-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaOrthotopic liver transplantation with reduced and rotated graft in adult situs inversus recipient: a case report and a review of reported cases
Ortotopická transplantace jater redukovaným a rotovaným štěpem dospělému příjemci se situs inversus: kazuistika a přehled publikovaných případů
Úvod:
Situs inversus je vzácná vrozená anomálie charakterizovaná zrcadlovým umístěním orgánů v dutině břišní a ve většině případů i orgánů hrudníku. Transplantace jater u těchto pacientů je velmi náročná z důvodu časté přítomnosti přidružených cévních abnormalit. Dosud bylo publikováno jen několik příspěvků týkajících se úspěšné transplantace jater dospělému příjemci se situs inversus. Tyto kazuistiky navrhovaly různé polohy štěpu. Zkušenost s danou problematikou je chudá.
Kazuistika:
V tomto článku prezentujeme kazuistické sdělení o úspěšné transplantaci redukovaného kadaverozního štěpu levé poloviny jater dospělému příjemci rotovaného o 90° ve směru hodinových ručiček. Bylo nutné provést komplexní rekonstrukci arteriálního zásobení. Kazuistika je doplněna přehledem dosud publikovaných případů transplantace jater dospělému příjemci spolu s použitými technikami.
Výsledky:
Použití této techniky nebylo spojeno s žádnými cévními ani prostorovými komplikacemi. 27 měsíců po transplantaci má štěp výbornou funkci.
Závěr:
Je prezentována první transplantace levou polovinou redukovaného kadaverozního štěpu jater od dárce se situs solitus příjemci se situs inversus. Použitá metoda 90° rotace štěpu ve směru hodinových ručiček poskytuje výborné prostorové uspořádání. Preferovány jsou relativně menší štěpy, protože poskytují více možností napolohování štěpu. Cévní štěpy by měly být vždy k dispozici.
Klíčová slova:
situs inversus – transplantace jater – redukovaný štěp – poloha štěpu
Authors: J. Kristek 1,2; M. Kocík 1; J. Chlupac 1,2; J. Froněk 1,2
Authors place of work: Transplant Surgery Department, Institute for Clinical and Experimental Medicine, Prague 1; Second Faculty of Medicine, Charles University, Prague 2
Published in the journal: Rozhl. Chir., 2018, roč. 97, č. 12, s. 568-575.
Category: Kazuistiky
Summary
Introduction:
Situs inversus is a rare congenital anomaly characterized by a mirror-image orientation of abdominal and mostly also thoracic organs. Liver transplantation in these patients is a demanding procedure due to the difficulties pertaining to positioning of the graft and the presence of frequently associated vascular abnormalities. Several reports have been published regarding successful liver transplantation in adult situs inversus recipients with different proposed positions of the graft. Relevant experience remains limited.
Case report:
In this paper we present a case of successful transplantation of a reduced-size cadaverous left hemi-liver graft to an adult situs inversus recipient in a 90-degree clockwise rotation. A complex arterial reconstruction was established. A review of published liver transplantations in adult situs inversus recipients along with the techniques employed is provided.
Results:
No vascular or spatial problems were encountered using this technique. The graft function is perfect at 27 months from the transplant procedure. The first liver transplantation with a reduced-size left hemi-liver graft from a situs solitus cadaveric donor to the situs inversus adult recipient is presented.
Conclusion:
The devised method of 90-degree clockwise rotation provides perfect spatial adjustment. Relatively smaller grafts are to be preferred as they allow maximum flexibility. Vascular conduits should be readily available.
Key words:
situs inversus – liver transplant – reduced graft – position
INTRODUCTION
The orthotopic liver transplantation (LT) has become a standardized and refined treatment option for various types of liver diseases, both acute and chronic. As an increasing number of patients require and undergo transplantation, more anatomical anomalies of the liver and adjacent vasculature are encountered [1]. These abnormalities may potentially pose technical difficulties and endanger the outcome of both the graft and the patient. One such anomaly is situs viscerum inversus totalis (SI). This rare congenital condition represents the mirror-image orientation of both visceral and vascular abdominal and thoracic structures relative to the midline [2]. The anatomically correct position is called situs viscerum solitus (SS) [3]. Situs inversus presents a major technical challenge to a transplant surgeon for several reasons. Firstly, with the liver being a chiral organ and the donor liver graft most likely coming from a normally arranged donor, there arises a spatial problem with placing the organ in the anatomically non-complementary site of situs inversus recipient’s hepatic fossa. Such a setting constitutes a risk of hepatic venous outflow kinking and consequential thrombosis [4]. Secondly, situs inversus is often related with other, more complex anomalies which may make the situation even more problematic [5], e.g. biliary atresia and other features characteristic for a complex of anomalies called the polysplenia syndrome (interrupted inferior vena cava with azygous drainage, confluent hepatic veins to the right atrium, preduodenal portal vein, hypoplastic or atretic portal vein, aberrant hepatic arterial anatomy, etc. [2,5,6]. In view of the fact that biliary atresia is the most frequent indication for LT in children and its high co-incidence with SI [7,8], one can easily understand why most of LTs in SI recipients have been performed in children [5,8,9,10]. LT procedures in adult situs inversus recipients are extremely rare [8,9].
Although abdominal situs inversus was once considered a contraindication to LT on account of the complexity of the potentially present vascular malformations [2,6,11], refinements of techniques and greater experience with LT have led to reconsideration of this paradigm. Although successful LTs in patients with SI have been described, and it is not deemed a contraindication any more [7,12], the experience with this procedure in SI recipients remains scarce and the related literature is limited. In this paper, we present a successful case of reduced liver graft transplantation to a recipient with situs viscerum inversus totalis and a review of the literature concerning the topic.
CASE REPORT
A 58-year-old Caucasian woman with hepatitis C (HCV) cirrhosis (Child-Turcotte-Pugh A) and situs viscerum inversus totalis presented with a solitary liver tumor consistent with the diagnosis of hepatocellular carcinoma (HCC). The tumor was located in liver segment VII and had a diameter of 25 mm. She had a known history of unsuccessful hepatitis C (HCV) treatment with PEG-interferon alpha-2B and ribavirin; the antiviral therapy was discontinued due to a side effect (depression). Following the diagnosis of HCC she underwent another course of antiviral therapy with sofosbuvir+simeprevir regimen. No signs of generalized malignancy disease or any medical contraindications to LT were revealed. Due to the presence of portal hypertension and liver cirrhosis of a very high degree (45 kPa on shear wave elastography), liver resection was contraindicated. With sustained virologic response at 12 weeks, the patient fulfilled the Milan criteria and was listed for liver transplantation.
In preoperative CT angiography of the recipient, the presence of complete thoracic and abdominal visceral as well as vascular mirror-image orientation was confirmed. The arterial blood supply to the liver originated typically from the celiac axis with no evidence of accessory or replaced hepatic artery. Besides the complete situs viscerum inversus including a left-sided vena cava inferior and dextrocardia (Fig. 1), she had no associated vascular and visceral anomalies. In other words, there were no signs of preduodenal portal vein, intestinal malrotation or intermittent inferior vena cava (IVC). One accessory spleen of 10 mm in diameter was revealed.
Fig. 1. Postoperative chest X-ray showing dextrocardia (thus complete situs inversus)
Note the hemoclips at the resection plane of the liver under the left portion of the diaphragm.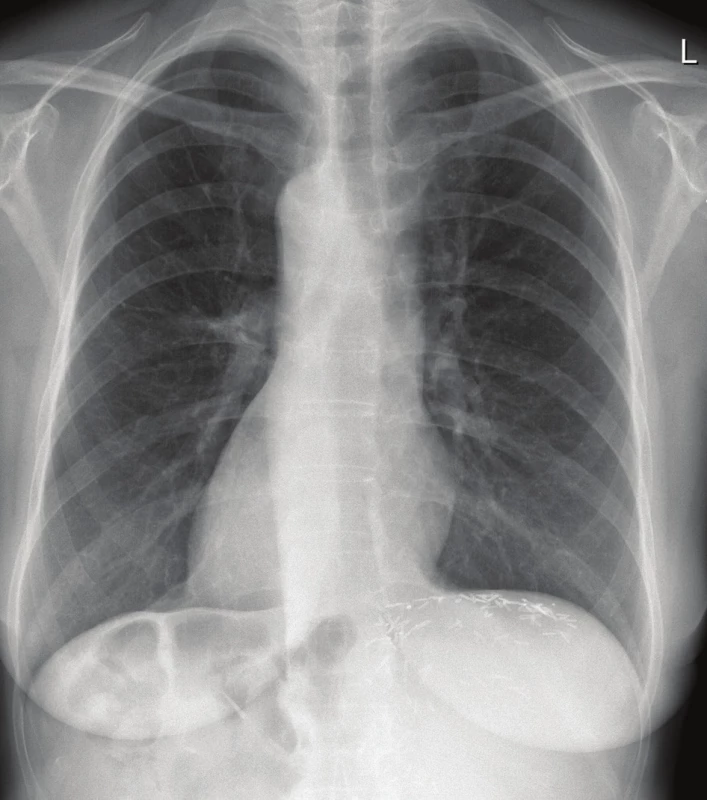
At the time of transplantation, her Body Mass Index (BMI) was 20.6 kg/m2 (height, 162 cm; weight, 54 kg) and her Model for End-Stage Liver Disease score totaled 10. After 6 days of being on the waiting list, an acceptable ABO compatible (A RhD+) 38-year-old male cadaveric SS donor was accepted with BMI of 23 kg/m2 (height, 178 cm; weight, 73 kg). Due to the donor-recipient body size discrepancy (donor-to-recipient weight ratio 1.35 : 1) and the presence of SS graft for SI recipient and thus impending complications concerning positioning of the graft, the donor liver was reduced to anatomic left hemi-liver graft (segments II+III+IV). Graft-to-recipient weight ratio was 1% (reduced graft weight 540 g, volume 607 mL). The total donor liver volume before reduction was 1777 mL. In preoperative planning, there were anomalies of hepatic arterial blood supply revealed in cross-sectional contrast imaging studies of the donor. A replaced thick-caliber right hepatic artery (HA) arising from the superior mesenteric artery (SMA), a thin proper hepatic artery supplying segment IV, and a thick-caliber replaced left hepatic artery arising from the left gastric artery were found. The right HA supplied the biliary duct. There were no anomalies regarding hepatic veins and the portal vein (PV). The procurement was done at our institution. The liver was reduced using the in situ technique (Fig. 2). The preservation solution used was HTK Solution.
Fig. 2. In situ-reduced left hemi-liver graft during the procurement period 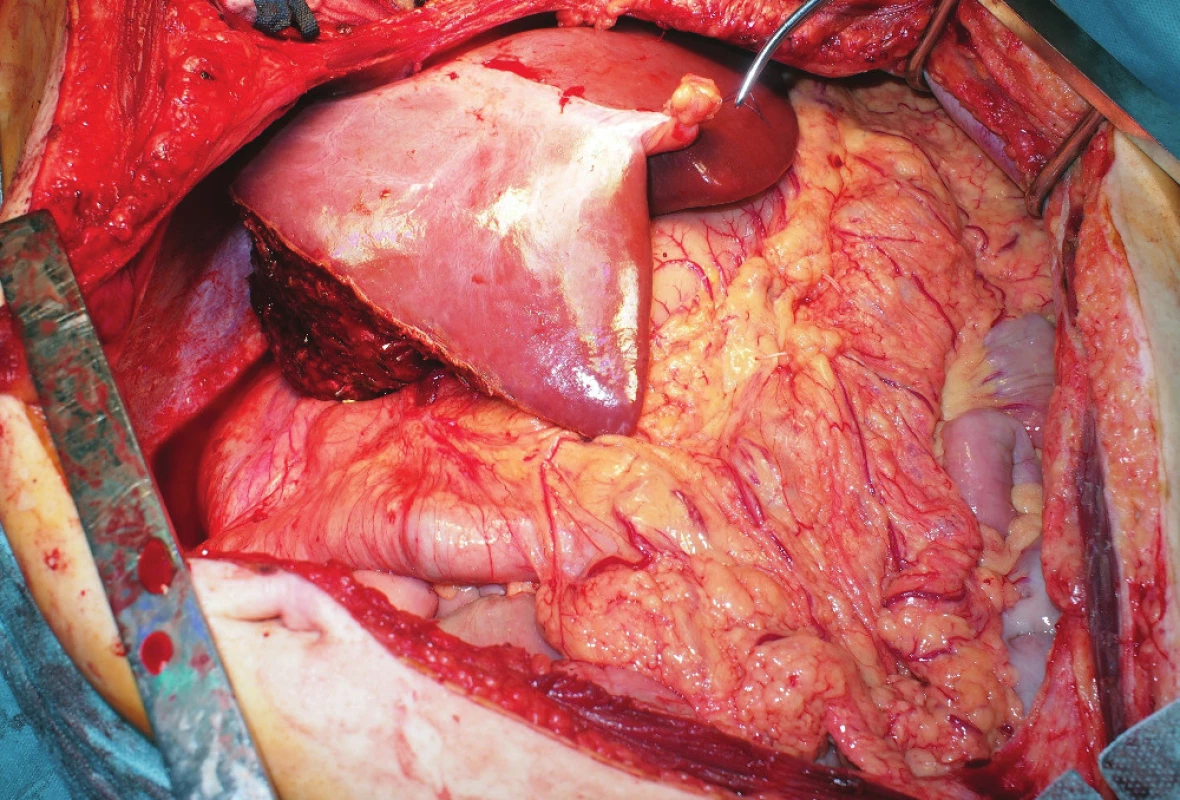
The transplantation itself was begun with a left-sided subcostal incision. Abdominal exploration confirmed the preoperative anatomical findings. The liver was cirrhotic (Fig. 3); no ascites was present. The hepatic hilum structures were carefully dissected. The HA and bile duct were ligated and divided. The PV was temporarily preserved. The liver was dissected free from its attachments. The PV and hepatic veins were divided; the recipient’s IVC was preserved and the hepatectomy completed (Fig. 4). The graft was tentatively placed in the hepatic fossa rotated 90-degree clockwise. I.e., the resection plane was located under the diaphragm, the left lateral segment pointed to the left hypogastrium, and the hepatic hilum was directed medially. The alignment of the hila in this orientation was perfect (Fig. 5). The cavocavostomy was accomplished using modified piggyback end-to-side technique. The suprahepatic end of the graft IVC was oversewn. The infrahepatic IVC constituted the outflow of the graft, and was sutured to the recipient’s left-sided IVC end-to-side. The portal veins were easily approximated without tension and anastomosis was carried out in a standard end-to-end fashion. Reperfusion was homogenous. No venovenous bypass was used during the anhepatic phase. The IVC was side-occluded by Satinsky clamp. Major hemodynamic instability with ventricular tachycardia followed and was present for 10 min. During the back-table period the arterial graft reconstruction was carried out. The celiac axis with Carrel patch had been anastomosed to the SMA, thus all three hepatic arteries of the graft were about to be supplied by the SMA. Due to the fact that there was a significant discrepancy of diameter between the recipient HA and the donor SMA (approximately 1 : 3), we were forced to construct subdiaphragmatically the aortohepaticostomy, i.e. in fact aortomesentericostomy (Fig. 6). Having cross-clamped the aorta and performed the requisite endarterectomy, the construction of the anastomosis was started with a running Prolene 5-0 suture. However, having finished one side of the anastomosis, we found out that the stitch cut through the aorta. Having started over, we performed the anastomosis with a 4-0 Prolene suture. We observed no perianastomotic bleeding following the aortic declamping. The anastomosis was secured using BioGlue®. The biliary anastomosis was constructed without any problems in the end-to-end fashion with a stent. The abdomen was packed with pads and closed. Neither venovenous bypass nor any adjunctive fixation technique of the graft was used in course of the procedure. The transplantation took 3 hours and 37 minutes. The planned second look operation was done on postoperative day 2. It revealed a source of bleeding at a resection plane and a biliary leak from the biliary anastomosis, both of which were readily taken care of. In further course, there were no significant disturbances to the patient’s recovery apart from a temporary renal function impairment that necessitated transitory volume and diuretic therapy; no dialysis was necessary. The patient’s liver function recovered quickly and did not show any signs suggestive of the small-for-size syndrome. The patient was discharged on postoperative day 12 with maintenance immunosuppressive regimen comprising of Tacrolimus, Mycophenolate Mofetil (MMF) and Prednisone. No induction immunosuppressive therapy was used.
Fig. 3. The situs inversus cirrhotic liver of the recipient before explantation 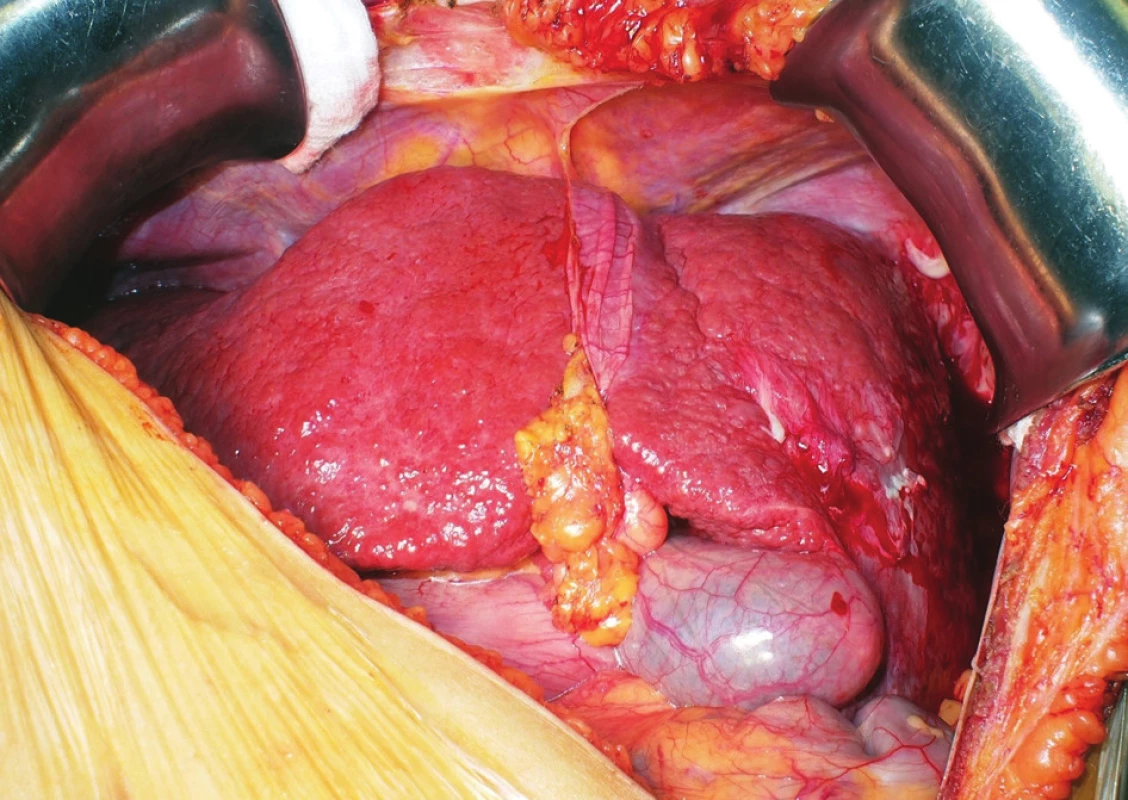
Fig. 4. Obvious spatial discrepancy between the reduced situs solitus graft and the situs inversus hepatic fossa during the anhepatic phase 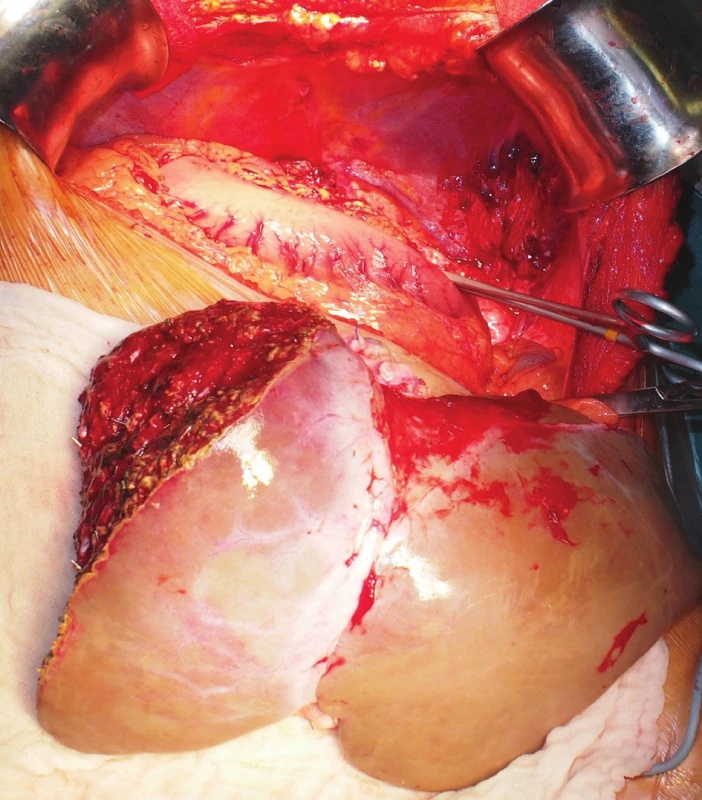
Fig. 5. Probatory placement of the rotated reduced graft in the hepatic fossa
The resection plane is located under the diaphragm; the left lateral segment points to the left hypogastrium. The picture was taken before reperfusion of the graft.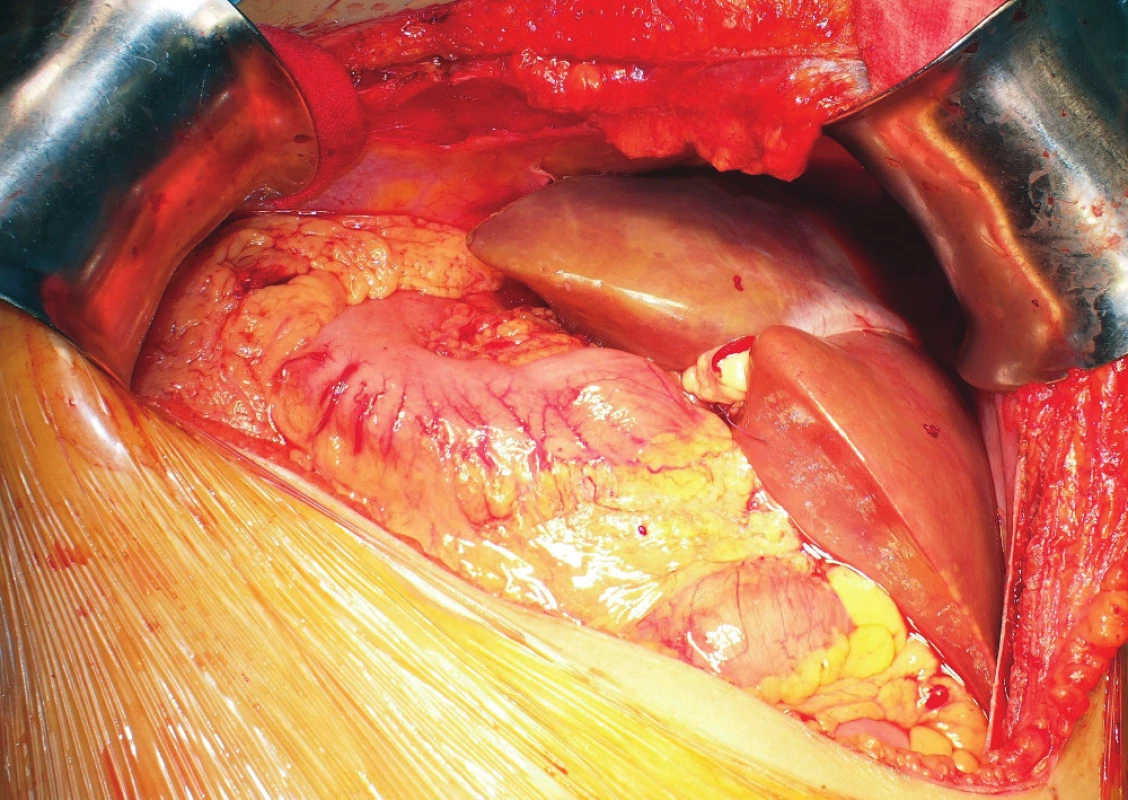
Fig. 6. A detailed view of the established portal vein anastomosis and aortohepatic bypass
The picture was taken after reperfusion of the graft. The biliary duct anastomosis is not clearly visible in this image.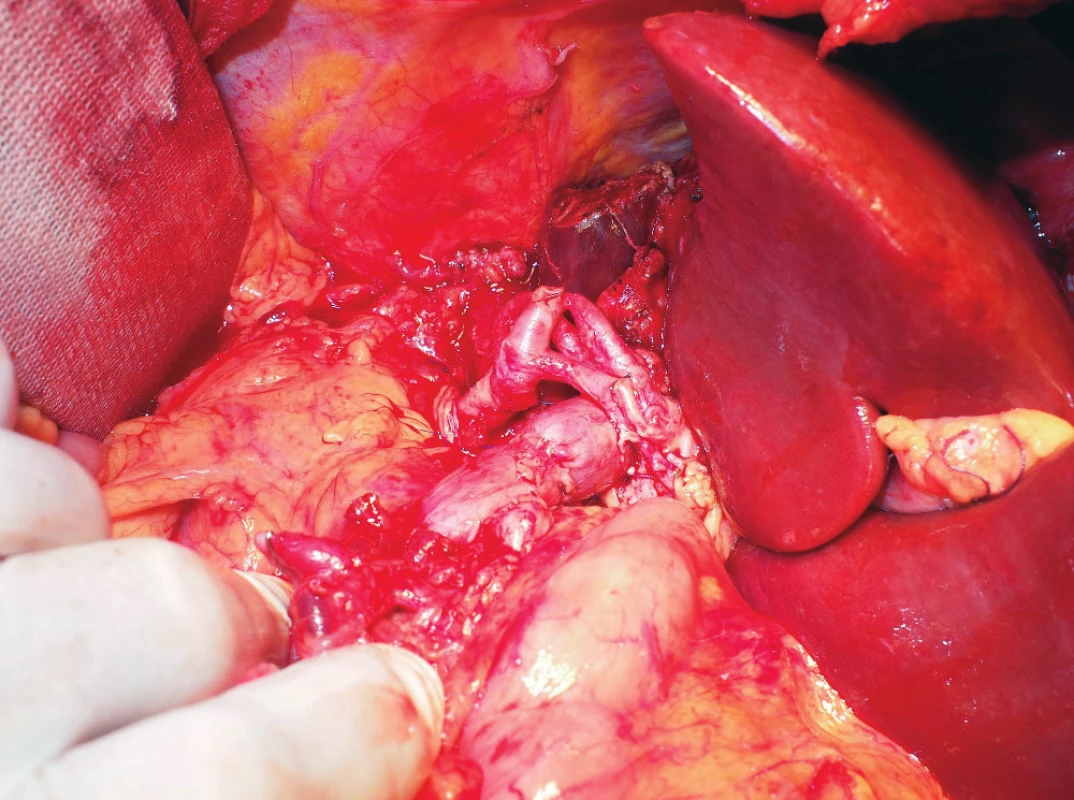
The explanted liver histology confirmed moderately differentiated (grade 2) hepatocellular carcinoma (pT2N0MX) with the proportions of 25 x 20 x 20 mm, with microvascular invasion, and with no apparent spreading through the tumor capsule. No signs of metastatic involvement of lymph nodes were present.
At 5 months follow-up pancytopenia was revealed. Myelotoxic medication including MMF was discontinued and a few courses of granulocyte-colony stimulating factor were applied. The liver enzymes were transitorily elevated. Despite thorough investigation, including a liver biopsy, the causal factor of the liver enzymes elevation was not identified. Control CT angiography confirmed perfectly strong arterial blood supply to the graft. Shortly, the liver tests normalized. In further course, chronic kidney disease grade III (CKD-EPI 34 mL.min-1) developed, probably secondary to calcineurin inhibitor renal toxicity.
At the time of publication, i.e., 27 months after the LT, the patient is in an excellent condition with normal liver function tests, negative HCV viremia and no signs of HCC recurrence (Tab. 1).
Tab. 1. Important data regarding the case report 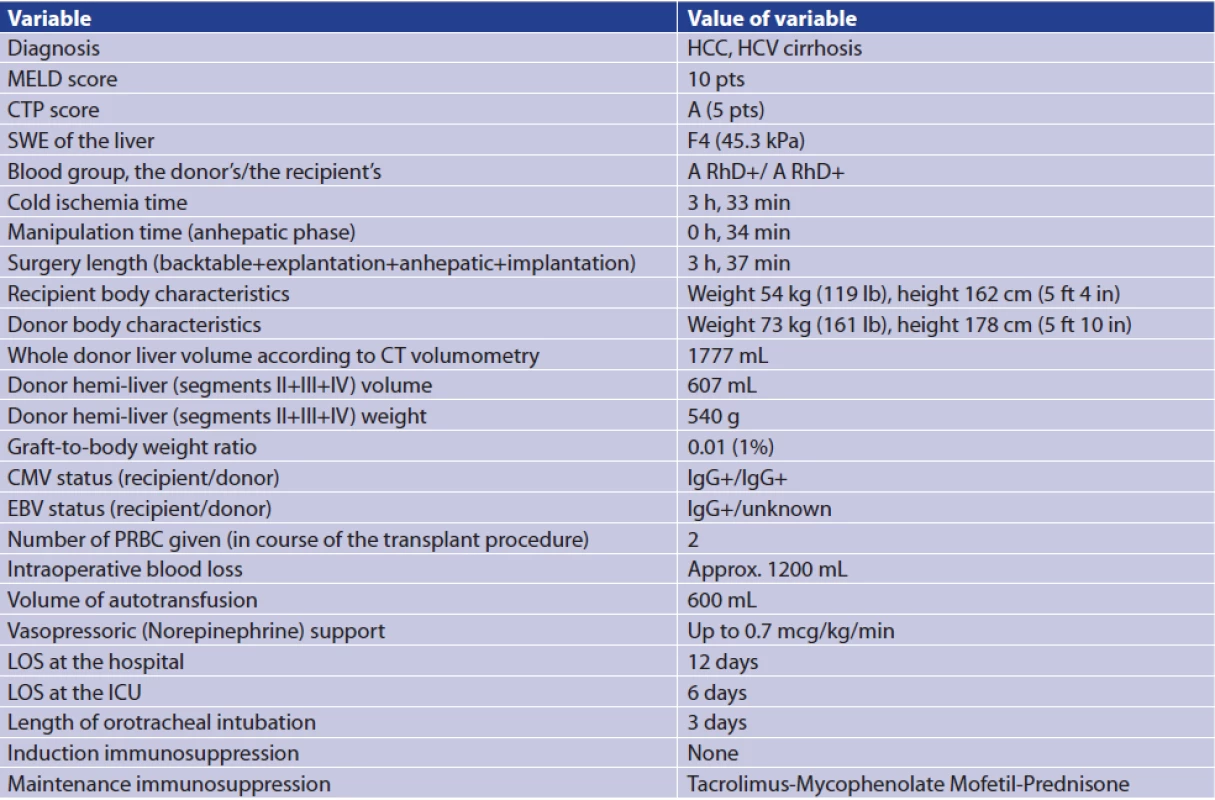
Notes: CM − cytomegalovirus; CT − computed tomography; CTP − Child-Turcotte-Pugh; EBV − Epstein-Barr virus; h − hour(s); HCC − hepatocellular carcinoma; HCV − hepatitis C virus; ICU − Intensive Care Unit; LOS − length of stay; mcg, microgram(s); MELD − Model for End-Stage Liver Disease; min − minute(s); PRBC − packed red blood cells; pts − points; SWE − shear wave elastography. DISCUSSION
According to a review of Farmer and Busuttil [9], there have been overall 90 transplantations in recipients with SI and/or polysplenia syndrome described in English written literature until 2015. Most of the recipients were children with biliary atresia and associated SI. In a mere 12 of these 90 cases the recipients were adults with situs inversus. In other words, LT procedures in adult SI recipients are extremely rare [8,9]. Since the Farmer’s and Busuttil’s publication, there have been 5 more case reports of LT in SI adult recipients published [2,6,8,13,14]. In 5 out of these 17 cases, the adult SI recipients received a reduced-size graft. However, all of these grafts came from living-related donors (Tab. 2). To the best of our knowledge, this is the first case that describes a liver transplantation with a reduced-size graft from a cadaveric SS donor to an adult SI recipient.
Tab. 2. All published experience with liver transplant in situs inversus adult recipients Team Reference number Year Age Sex Technique, adjunct maneuvers Diagnosis Donor type 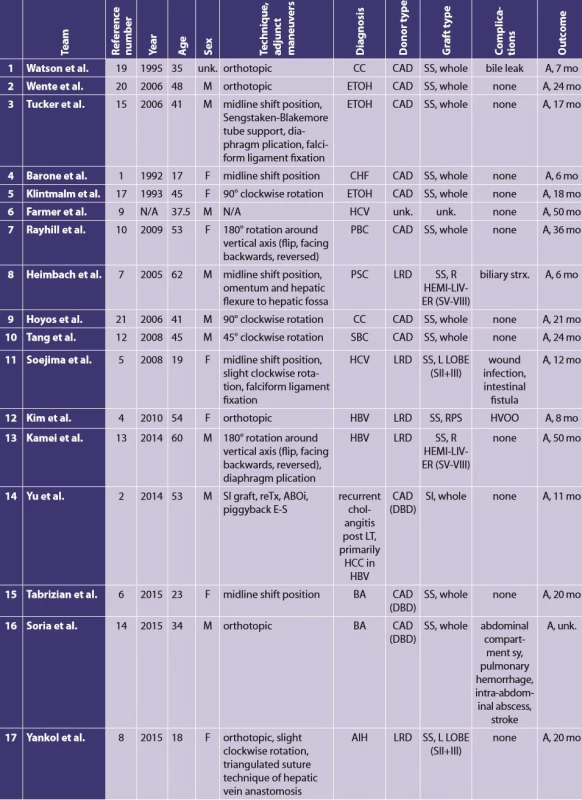
Notes: A − alive; ABOi − ABO incompatible; AIH − autoimmune hepatitis; BA − biliary atresia; CAD − cadaveric donor; CC − cryptogenic cirrhosis; CHF − congenital hepatic fi brosis; DBD − donor after brain death; E-S − end-to-side; ETOH − ethylism; F − female; HA − hepatic artery; HBV − hepatitis B virus; HCC − hepatocellular carcinoma; HV − hepatic vein; HVOO − hepatic venous outfl ow obstruction; L LOBE − left lobe; LRD − living related donor; LT − liver transplant; M − male; mo − month(s); n − normal; N/A − not available; PBC − primary biliary cirrhosis; PV − portal vein; R − right; reTx − retransplantation; RHV − right hepatic vein; R LOBE − right lobe; RPS − right posterior sector; SBC − secondary biliary cirrhosis; SI − situs inversus; SS − situs solitus; SII+III − liver segments II and III; SV-VIII − liver segments V-VIII; sy − syndrome; unk. − unknown In the presented case, the patient suffered from HCC in a cirrhotic liver. Due to the presence of portal hypertension and a very high degree of liver cirrhosis, liver resection was contraindicated. The patient met the Milan criteria for LT. The patient was on the list for not more than a few days when a potentially suitable donor was identified. However, there was a size discrepancy present between the donor and the recipient. In regard to the recipient’s relatively small body proportions (weight 54 kg, height 162 cm) and her diagnosis of HCC, it could be tricky to wait for an optimal whole graft not requiring a reduction. Of course, another possibility may have been to treat the patient with locoregional therapy while she was waiting for the adequate full-size graft. However, we preferred to accept this relatively large graft for this recipient and to reduce it to a left hemi-liver graft.
Since patients with SI make up a heterogeneous group with respect to often accompanying abdominal and/or cardiopulmonary malformations, there is no standard surgical technique of LT in this setting. For this reason, one of the most important technical aspects of LT in patients with SI remains resourcefulness of the surgeon [5]. The performing surgeon must be ready to apply any of a multitude of possible reconstructive techniques during the procedure. All in all, smaller grafts permit flexibility and donor-to-recipient weight ratio of <1 has been described as optimal for whole-organ donors. Also segmental grafts (either a deceased-donor, or living-related) are frequently used and allow easier placement in the abdominal cavity [5]. Thus, careful donor selection including precise size matching is of paramount importance [5]. According to the literature, pediatric patients are expected to exhibit less graft displacement and hepatic venous torsion on account of their relatively smaller abdominal cavity, even in cases where split and reduced grafts are used [5]. On the contrary, in adults following a hepatectomy, a large empty space can be formed in the left upper quadrant which predisposes the graft to lateral displacement with supero-lateral rotation and torsion of the hepatic venous pedicle [5,15]. A particularly crucial point to be noted is that vascular conduits should always be available [6], which is a significant advantage of cadaverous grafts and at the same time, a considerable limitation to living donor grafts. In our case, although we had to construct an arterial bypass there was no need of using vascular conduits. Due to the discrepancy between the caliber of the recipient’s and graft’s HA (the risk of thrombosis) we had to establish an aortohepaticostomy. A perfect patency of the anastomosis without signs of stenosis was confirmed with CT angiography 5 months later. There are several other aspects worth mentioning regarding the preoperative planning. Due to the high incidence of associated cardiopulmonary abnormalities, evaluation of the functional reserve should be considered. An extensive preoperative recipient anatomy work-up including contrast-enhanced cross-sectional imaging is a matter of course, as well as the previously mentioned meticulous size-matching [16]. The hemodynamic instability in course of the anhepatic phase we observed in this case may have been preventable by establishment of a temporary venovenous bypass.
The abovementioned will probably not provoke any discussion, but it is the placement and orientation of the SS liver graft in an SI recipient which is a matter of debate. To date, several possible techniques have been described in the literature. By and large, the location of the recipient’s suprahepatic IVC or hepatic veins (left - or right-sided in relation to the vertebral column) will most likely determine the position of the graft. In other words, positioning of the graft will most probably differ in the recipients with dextrocardia from those with levocardia. Most of the patients with situs inversus have dextrocardia and left-sided IVC (situs inversus totalis), which means – in the case of using the midline position (“orthotopic”) method – that the right lobe of the SS whole graft will occupy the midline, where the space is scarce due to the presence of the vertebral column and the stomach. Surprisingly and somewhat paradoxically, in most of the cases reported, the graft was placed in this way. The problem with this position is that the anatomical right lobe overlies the recipient’s stomach and could possibly cause IVC compression and, most importantly, rotation of the graft with aforementioned consequences. The second most common technique reported used a segmental allograft, either from a living related donor or a cadaveric one. Other alternatives have been infrequent. Klintmalm et al. ingeniously invented a technique of placing the graft rotated 90-degrees clockwise around its anteroposterior axis [17]. Using this method, the larger right lobe fits better the hepatic fossa; the left lateral segment points to the left lower quadrant and the hepatic hilum is in favorable syntopy with the structures of the hepatoduodenal ligament. A modified hepatic outflow needs to be set up between the graft’s infrahepatic and recipient’s IVC in the end-to-side fashion. In contrast with the midline position, using the Klintmalm’s 90-degree rotation method, the risk of the laterodisplacement of the graft and consequent kinking of the venous outflow is minimized. Also the recipient’s stomach is not compromised by the donor right lobe. In our case, we made use of this method and it proved very helpful. We did not face any problems concerning this position. The hepatic hila were perfectly aligned and cavocavostomy was also constructed conveniently. Another technique described by Todo et al. mentions an auxiliary LT in a patient with complex vascular anomalous anatomy [18]. A fascinating solution to this problem was devised by Rayhill et al. placing the graft orthotopically, but reversely, i.e. 180-degree rotated around the axis of the IVC (facing backwards) [10]. The hepatic hilum faced anteriorly, well aligned with the recipient SI hepatoduodenal structures. Hepatic outflow was established using reversed cavaplasty (end-to-side anastomosis between suprahepatic IVCs).
The potentially most threatening peril of the midline position in SI LT recipients is the lateral displacement or a rotation of the graft and torsion of the venous pedicle with consequent thrombosis and impending failure of the graft. According to the literature, in an attempt to solve this anatomically unfavorable condition of empty hepatic fossa, several maneuvers have been proposed and used: 1) Plication of the diaphragm [15]; 2) Temporary placement of the inflated Sengstaken-Blakemore tube in the hepatic fossa [15]; 3) Fixation of the falciform ligament to the diaphragm [15]; 4) Filling the fossa up with omentum [7,15]; and 5) Mobilizing the hepatic flexure and positioning it in the relatively empty hepatic fossa [7,15]. We feel obliged to mention that we have no experience with using any of these methods apart from the method number 3.
Postoperative management of these patients does not differ significantly from that required for other patients undergoing LT. However, medical teams must be aware of the anatomical variety present. This is of importance in postoperative radiographic studies and, first of all, in invasive procedures such as liver biopsy.
Conclusion
In this paper, we present the first liver transplantation with reduced-size left hemi-liver graft from a situs solitus cadaveric donor to the situs inversus adult recipient. The graft was placed orthotopically 90-degree clockwise rotated and a complex reconstruction of the arterial blood supply was performed. There were no difficulties regarding positioning of the graft, nor were any vascular abnormalities in the postoperative course encountered. Generally speaking, the experience with this type of operation is rare. In our opinion, smaller grafts are to be preferred as they allow maximum flexibility. Vascular conduits should be readily available. The situs inversus is not a contraindication to liver transplantation. However, thorough preoperative planning is a matter of course because recipients with this anomaly are often afflicted with other cardiac and non-cardiac malformations.
List of abbreviations
BMI − body mass index
CT − computed tomography
HA − hepatic artery
HCC − hepatocellular carcinoma
HCV − hepatitis C virus
IVC − inferior vena cava
LT − liver transplantation
MMF − mycophenolate mofetil
PV − portal vein
SI − situs viscerum inversus
SMA − superior mesenteric artery
SS − situs viscerum solitus
Conflict of interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.
Assoc. Prof. Jiri Fronek, M.D., Ph.D., FRCS
Transplant Surgery Department
Institute for Clinical and Experimental Medicine
Vídeňská 1958/9
140 21 Prague
e-mail: jiri.fronek@ikem.cz
Zdroje
- Barone GW, Henry ML, Elkhammas EA, et al. Orthotopic liver transplantation with abdominal situs inversus and dextrocardia. Am Surg. 1992;58 : 651−3.
- Yu S, Guo H, Zhang W, et al. Orthotopic liver transplantation in situs inversus adult from an ABO-incompatible donor with situs inversus. BMC Gastroenterol 2014;14 : 46.
- Tawfik AM, Batouty NM, Zaky MM, et al. Polysplenia syndrome: a review of the relationship with viscero-atrial situs and the spectrum of extra-cardiac anomalies. Surg Radiol Anat 2013;35 : 647−53.
- Kim BW, Bae BK, Xu W, et al. Living donor liver transplantation for an adult patient with situs inversus totalis. World J Gastroenterol 2010;16 : 2311−3.
- Soejima Y, Meguro M, Taketomi A, et al. Left lobe living donor liver transplantation in an adult patient with situs inversus: technical considerations. Transpl Int 2008;21 : 384−9.
- Tabrizian P, Joseph TT, Radkani P, et al. Liver transplantation in an adult recipient with situs inversus totalis: Case report and review of the literature. Transplant Proc 2016;48 : 3163−6.
- Heimbach JK, Menon KV, Ishitani MB, et al. Living donor liver transplantation using a right lobe graft in an adult with situs inversus. Liver Transpl 2005;11 : 111−3.
- Yankol Y, Mecit N, Kanmaz T, et al. Living donor liver transplantation in an adult patient with situs inversus totalis. Ulus Cerrahi Derg. 2015;31 : 232−4.
- Farmer DG, Busuttil RW. Transplantation: situs inversus and polysplenia syndrome. In: Busuttil RW, Klintmalm GB. Transplantation of the liver. Third edition. ed. Philadelphia, PA: Elsevier/Saunders 2015.
- Rayhill SC, Scott D, Orloff S, et al. Orthotopic, but reversed implantation of the liver allograft in situs inversus totalis-a simple new approach to a difficult problem. Am J Transplant 2009;9 : 1602−6.
- Lilly JR, Starzl TE. Liver transplantation in children with biliary atresia and vascular anomalies. J Pediatr Surg 1974;9 : 707−14.
- Tang DN, Wei JM, Liu YN, et al. Liver transplantation in an adult patient with situs inversus: a case report and overview of the literature. Transplant Proc 2008;40 : 1792−5.
- Kamei H, Onishi Y, Ogawa K, et al. Living donor liver transplantation using a right liver graft with additional vein reconstructions for patient with situs inversus. Am J Transplant 2014;14 : 1453−8.
- Fernandez Soria N, Garcia Novoa MA, Rivas Polo JI, et al. Orthotopic liver transplantation in an adult with biliary atresia, situs inversus, and inferior cava vein absence: A case report. Transplant Proc 2015;47 : 2407−9.
- Tucker O, Prachalias A, Kane P, et al. Graft positioning at liver transplantation in situs inversus. Liver Transpl 2006;12 : 1720−2.
- Farmer DG, Shaked A, Olthoff KM, et al. Evaluation, operative management, and outcome after liver transplantation in children with biliary atresia and situs inversus. Ann Surg 1995;222 : 47−50.
- Klintmalm GB, Bell MS, Husberg BS, et al. Liver transplant in complete situs inversus: a case report. Surgery 1993;114 : 102−6.
- Todo S, Hall R, Tzakis A, Starzl TE. Liver transplantation in patients with situs inversus. Clin Transplant 1990;4 : 5−8.
- Watson CJ, Rasmussen A, Jamieson NV, et al. Liver transplantation in patients with situs inversus. Br J Surg 1995;82 : 242−5.
- Wente MN, Thorn M, Radeleff B, et al. A routine liver transplantation in a patient with situs inversus: a case report and an overview of the literature. Clin Transplant 2006;20 : 151−5.
- Hoyos S, Guzman C, Correa G, et al. Orthotopic liver transplantation in an adult with situs inversus: an easy way to fit the liver. Ann Hepatol 2006;5 : 53−5.
- Raynor SC, Wood RP, Spanta AD, et al. Liver transplantation in a patient with abdominal situs inversus. Transplantation 1988;45 : 661−3.
Štítky
Chirurgie všeobecná Ortopedie Urgentní medicína
Článek Zemřel primář Josef Kalný
Článek vyšel v časopiseRozhledy v chirurgii
Nejčtenější tento týden
2018 Číslo 12- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Hojení análních fisur urychlí čípky a gel
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Vzdělávání chirurgů a kompetence
- Confocal laser endomicroscopy in the diagnostics of gastrointestinal lesions − literary review and personal experience
- Surgical site infections after degenerative lumbar spine surgery
- Splenectomy in patients older than 65 years – a single-center experience
- Targeted axillary dissection and sentinel lymph node biopsy in breast cancer patients after neoadjuvant chemotherapy – a retrospective study
- Current treatment procedures for civilian gunshot wounds
- Crohn’s disease of the appendix – a case report
- Zemřel primář Josef Kalný
- Orthotopic liver transplantation with reduced and rotated graft in adult situs inversus recipient: a case report and a review of reported cases
- Vzpomínka na primáře Vladimíra Černého
- Zápis z jednání schůze Redakční rady časopisu Rozhledy v chirurgii, konané dne 7. 11. 2018
- Rozhledy v chirurgii
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Current treatment procedures for civilian gunshot wounds
- Surgical site infections after degenerative lumbar spine surgery
- Targeted axillary dissection and sentinel lymph node biopsy in breast cancer patients after neoadjuvant chemotherapy – a retrospective study
- Crohn’s disease of the appendix – a case report
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



