-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Exome Sequencing of Uterine Leiomyosarcomas Identifies Frequent Mutations in , , and
Uterine leiomyosarcomas are rare, malignant smooth muscle tumors with a poor 5-year survival and high recurrence rate. They account for 1–2% of all uterine malignancies with an estimated incidence of 0.4/100,000 women per year. The symptoms and signs of this tumor type widely overlap with those of common benign uterine leiomyomas, making early diagnosis of uterine leiomyosarcomas difficult. Currently, the diagnosis of these tumors is often incidental and postoperative. Despite previous cytogenetic and molecular studies, their molecular background has remained elusive. Identification of novel molecular genetic characteristics in uterine leiomyosarcomas is clinically relevant to further improve the diagnosis and prognosis of the patients. Here, we performed exome sequencing on 19 tumors, revealing frequent mutations in TP53, ATRX, and MED12. The discovery of frequent inactivating ATRX mutations provides a potential novel therapeutic target for uterine leiomyosarcomas.
Published in the journal: . PLoS Genet 12(2): e32767. doi:10.1371/journal.pgen.1005850
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005850Summary
Uterine leiomyosarcomas are rare, malignant smooth muscle tumors with a poor 5-year survival and high recurrence rate. They account for 1–2% of all uterine malignancies with an estimated incidence of 0.4/100,000 women per year. The symptoms and signs of this tumor type widely overlap with those of common benign uterine leiomyomas, making early diagnosis of uterine leiomyosarcomas difficult. Currently, the diagnosis of these tumors is often incidental and postoperative. Despite previous cytogenetic and molecular studies, their molecular background has remained elusive. Identification of novel molecular genetic characteristics in uterine leiomyosarcomas is clinically relevant to further improve the diagnosis and prognosis of the patients. Here, we performed exome sequencing on 19 tumors, revealing frequent mutations in TP53, ATRX, and MED12. The discovery of frequent inactivating ATRX mutations provides a potential novel therapeutic target for uterine leiomyosarcomas.
Introduction
Uterine leiomyosarcoma (ULMS) is a rare, highly malignant tumor that originates from the smooth muscle layer of the uterus, the myometrium. It is the most common subtype of uterine sarcoma and accounts for 1–2% of all uterine malignancies with an estimated incidence of 0.4/100,000 women per year [1,2]. The majority of ULMSs occur in women over 50 years of age typically causing symptoms such as abnormal vaginal bleeding, palpable pelvic mass, and abdominal pain. These symptoms greatly resemble those of common benign uterine leiomyoma, making early diagnosis of ULMS difficult. Surgical resection is the primary treatment option, while the use of adjuvant therapies varies widely. ULMS show low sensitivity to both chemotherapy and radiation therapy [3,4]. In most cases, the diagnosis is made histologically after the surgery, and even then, the clinical course of ULMS is difficult to predict. Currently, the most prominent prognostic factors include stage, age, and tumor size [5–7]. The 5-year overall survival has remained <50% due to a high recurrence rate (53–71%) and metastatic capacity [6,8].
Most ULMSs are aneuploid with both complex numerical and structural chromosomal aberrations [9]. Albeit no consistent structural aberrations have been identified, abnormalities affecting chromosomal regions 1p, 10q, 13q, and 14q have been observed in multiple cases [10]. So far, only a few genes have been associated with this tumor type, including tumor protein P53 (TP53), RB1, MDM2, CDKN2A, and KIT [9,11]. These are all common cancer genes not specific for smooth muscle malignancies and the exact molecular mechanisms underlying ULMS tumorigenesis remain elusive.
During the last decade, next-generation sequencing technologies have increasingly provided genome-wide data on somatic landscapes in various cancer types enabling the discovery of novel cancer genes and mechanisms with important prognostic and therapeutic implications [12]. Here, we performed exome sequencing on 19 ULMSs to further elucidate the molecular etiology of these tumors, identifying frequent mutations in TP53, alpha thalassemia/mental retardation syndrome X-linked (ATRX), and mediator complex subunit 12 (MED12). This is the first description of high-throughput sequencing on ULMSs.
Results
Recurrently mutated genes observed in exome sequencing
We performed exome sequencing on genomic DNA of 19 formalin-fixed paraffin-embedded (FFPE) ULMSs. The average coverage of captured exonic regions reached a mean depth of 21x and 92% of the captured regions had a minimum coverage of four reads (S1 Table). After filtering the exome sequencing data, we observed a mean of 373 somatic mutations per tumor (range 240–779). The majority of mutations in each tumor specimen represented single-nucleotide variations (∼88%; range 81–95%), while deletions accounted for ∼9% (range 4–15%) and insertions ∼3% (range 1–7%) (S1 Table). Two tumors, LMS49 and LMS51, harbored more mutations than other ULMSs, but the mutation spectrum followed the common pattern.
In the exome sequencing data analysis, we focused on genes that were mutated in at least two tumors. This resulted in a list of 43 genes (S2 Table). The majority of these genes (37/43; 86%) were mutated in two tumors, while six genes, TP53, ATRX, MED12, fibrous sheath interacting protein 2 (FSIP2), ATP-binding cassette, sub-family A (ABC1), member 13 (ABCA13), and ankyrin repeat domain 26 (ANKRD26), had mutations in three or more tumors (Fig 1). The most frequently mutated gene was TP53, which was mutated in six tumors (6/19; 32%) (S1 Fig). Two mutations were nonsense mutations creating a premature stop-codon and four were missense alterations; all missense changes were predicted pathogenic by two independent in silico tools (S2 Table). All the observed TP53 mutations have been reported as somatic mutations in the COSMIC-database.
Fig. 1. Most frequently mutated genes in 19 ULMSs studied by exome-sequencing. 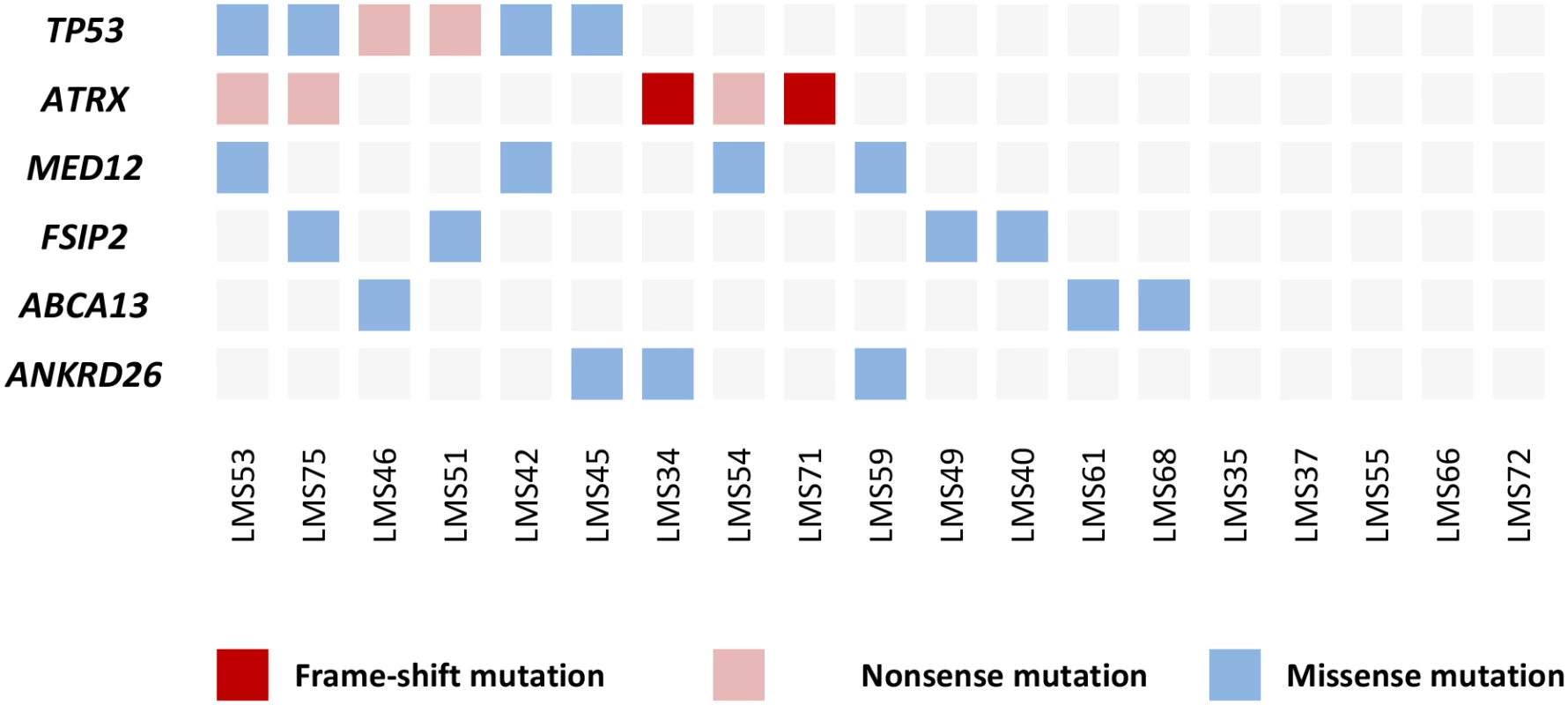
Five tumors did not have mutations in any of the six genes listed. The second most commonly mutated gene was ATRX, which was mutated in five tumors (5/19; 26%) (S1 Fig). The total number of mutations was six as one tumor (LMS71) contained two distinct mutations. All mutations were either nonsense mutations or small frameshift insertions or deletions, and were thus predicted to result in a truncated protein product. As ATRX mutations have been associated with alternative lengthening of telomeres (ALT), we specifically searched the exome sequencing data for possible mutations in death-domain associated protein (DAXX), as also these mutations have been associated with the ALT phenotype [13,14]. Indeed, one ULMS (LMS61) had a mutation in DAXX. This mutation was a nonsense mutation (Glu650Stop) most likely leading to a truncated or unstable protein product.
Four mutations (4/19; 21%) were observed in MED12 (S1 Fig). All these were missense changes affecting amino acids Gly44 (3 mutations) or Leu36 (1 mutation), which have previously been reported as mutational hotspots in uterine leiomyomas [15]. All mutations were predicted to have a deleterious effect on protein function (S2 Table). Neither MED12, TP53, nor ATRX mutations were mutually exclusive (Fig 1).
Alterations in FSIP2 (4/19; 21%), ABCA13 (3/19; 16%), and ANKRD26 (3/19; 16%) all represented missense changes that scattered along the gene lengths. Two tumors had the same Met487Ile substitution in ANKRD26. One alteration (Gln581Leu) in FSIP2 and all changes in ABCA13 were predicted pathogenic by both Polyphen-2 and SIFT, whereas none of the other variants were predicted damaging by both in silico tools.
Aberrant TP53, ATRX, and DAXX expression in ULMS
We evaluated the protein expression levels of TP53, ATRX, and DAXX in the 19 exome-sequenced ULMSs by immunohistochemistry and validated the results in a larger set of 33 additional tumors (S2 Fig and S3 Table). DAXX immunostaining was successful in all 52 tumors and interpretable results for TP53 and ATRX were obtained from 50 and 44 tumors (50/52, 96%; 44/52, 85%). Aberrant TP53 expression was observed in 33 out of 50 ULMSs (66%) (S3 Table). Twenty-three out of 44 successfully analyzed ULMSs (52%) showed loss of nuclear ATRX expression, including all immunohistochemically successful ATRX mutation-positive tumors. Clearly diminished DAXX expression was present in only one ULMS (1/52, 2%) (Fig 2A and 2C): a tumor with the nonsense mutation (S3 Table).
Fig. 2. Representative images of ATRX and DAXX expression and ALT phenotype in ULMS. 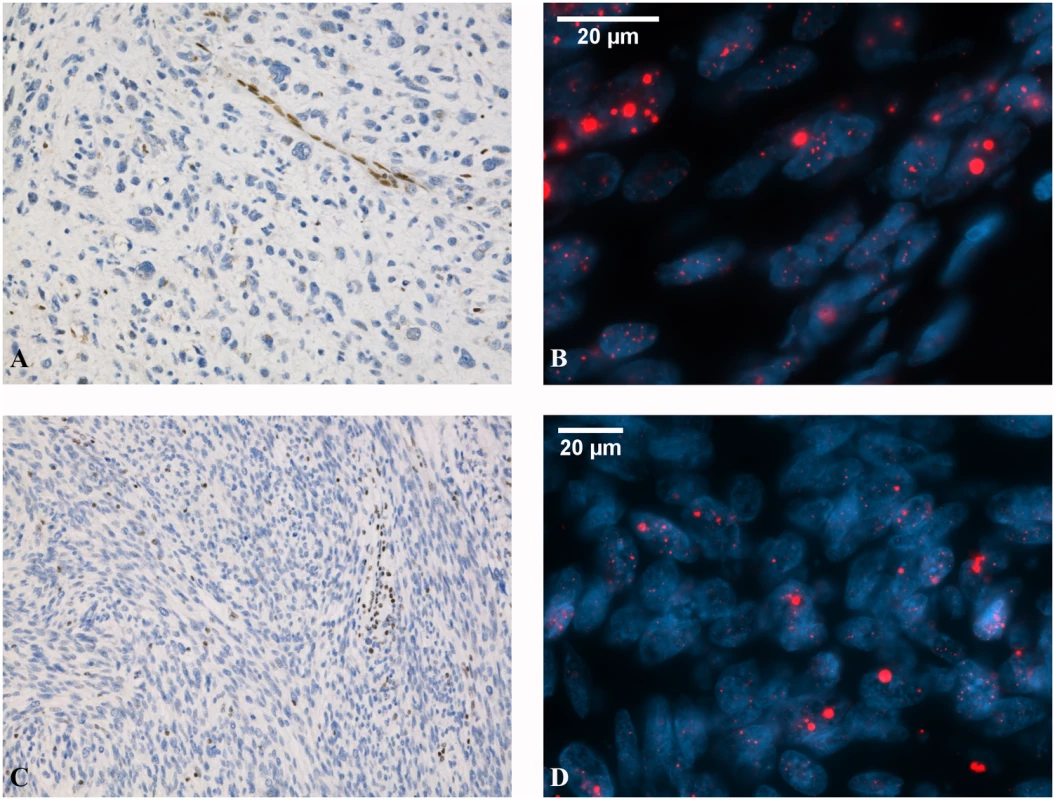
ATRX-mutated ULMS showing loss of ATRX expression (A) and positive ALT phenotype (B). Large, abnormally bright telomere FISH signals (red) are indicative of ALT. DAXX-mutated ULMS with reduced DAXX expression (C) and positive ALT phenotype (D). Immunohistochemical stainings are shown with 10×20 magnification and FISH stainings with 10×63 magnification. Fluorescence in situ hybridization shows alternative lengthening of telomeres
Telomere-specific fluorescence in situ hybridization (FISH) was carried out to assess the potential effect of ATRX and DAXX mutations on telomere length (Fig 2B and 2D). Twelve out of 19 exome-sequenced ULMSs (63%) were ALT-positive (S3 Table). This included four out of five ATRX mutation-positive tumors (80%) as well as the one DAXX mutation-positive tumor. Also seven out of 13 cases (54%) without detectable ATRX or DAXX mutations showed ALT positivity. Loss of ATRX or DAXX expression seems to correlate very well with the ALT phenotype.
The effect of aberrant TP53 and ATRX expression on patient survival
Kaplan-Meier survival curves were generated to study the association between TP53 and ATRX expression and overall survival time. The median overall survival time for all patients was 65 months (95% confidence interval 31.7–98.3 months). Only the number of Stage I tumors was large enough for the analyses. Neither aberrant TP53 or ATRX expression associated with poor survival (P = 0.261, P = 0.127) (Fig 3). Of note, TP53 and ATRX expression statuses correlated with each other (P = 0.005).
Fig. 3. Overall survival of patients with Stage I ULMS according to TP53 and ATRX expression status. 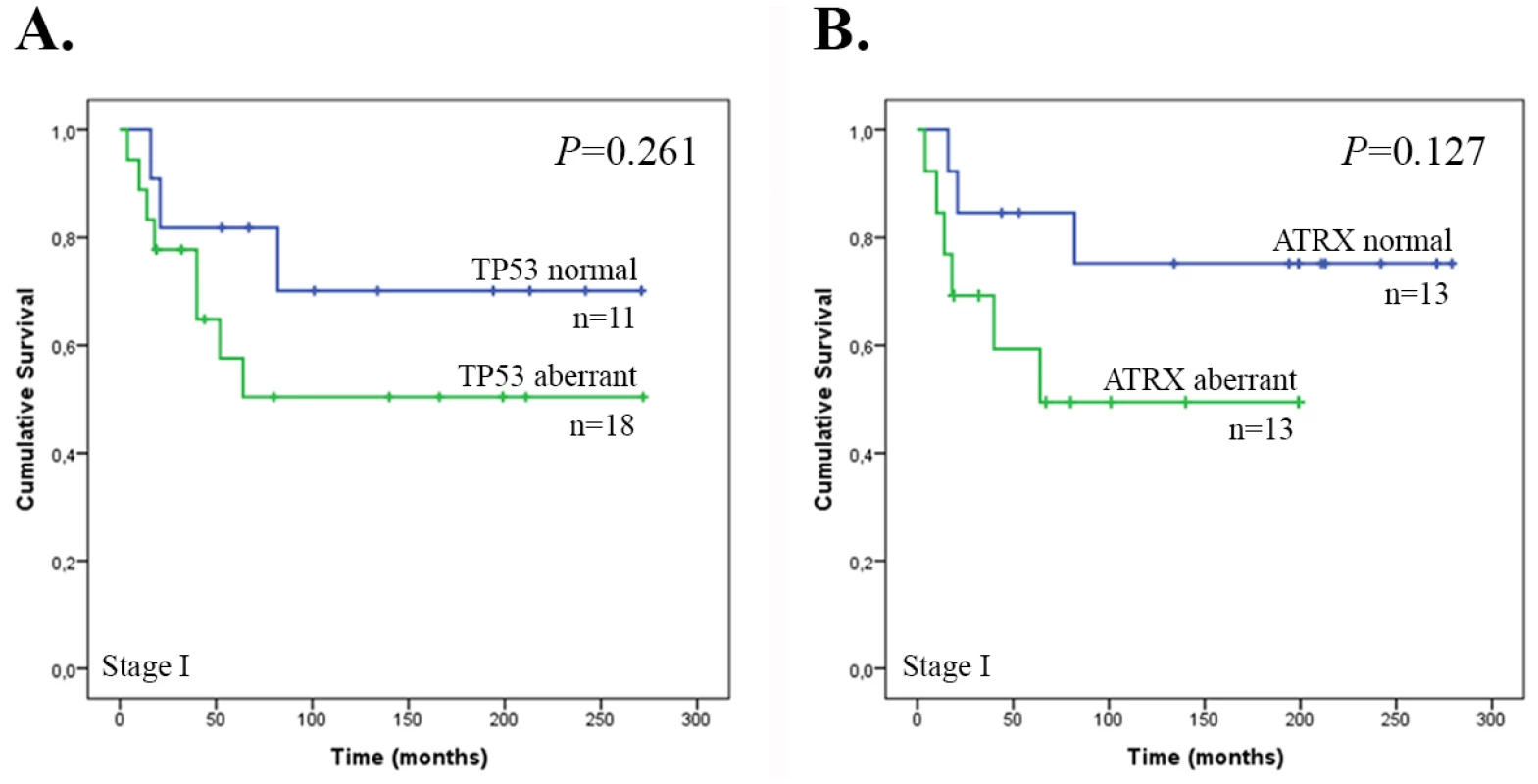
(A) TP53 expression (n = 29) and (B) ATRX expression (n = 26). Discussion
In this study, we examined somatic variation in 19 ULMSs by exome sequencing. We focused on genes, which were mutated in at least two tumors; altogether 43 such genes were identified. The most frequently mutated genes included TP53, ATRX, MED12, FSIP2, ABCA13, and ANKRD26. TP53 was the most commonly mutated gene with 32% of the tumors harboring mutations. Alterations in TP53 have been previously implicated in leiomyosarcomas and suggested to play a role in leiomyosarcoma pathogenesis [16–18]. In this study, most mutations (67%) were missense changes located in exons 4–8. This is in line with previous studies, where the majority of mutations have been missense mutations in exons 5–8, the most highly conserved region of the gene [9,17,19]. These mutations are known to alter the protein structure, inhibit its tumor suppressor function, and result in its prolonged half-life. Immunohistochemical analysis including 50 ULMSs confirmed altered expression in the majority of tumors, highlighting the role of TP53 in ULMS development.
ATRX was the second most frequently mutated gene with mutations observed in five tumors (26%). All mutations were either nonsense or frameshift alterations most likely leading to a truncated protein product. Loss of ATRX expression has been reported in leiomyosarcomas of various sites [20–22] and a recent meeting abstract on ULMSs reported genomic alterations of this gene in 32% (8/25) of the studied tumors, supporting our findings [23]. We successfully analyzed ATRX protein levels in 44 ULMSs and showed that 52% of the tumors, including all reliably analyzed mutation-positive lesions, had clearly reduced expression. ATRX encodes a transcriptional regulator that contains an ATPase/helicase domain, and is thus a member of the SWI/SNF family of chromatin remodelling proteins. Loss of ATRX expression has been associated with ALT [13,24], which prompted us to analyze the telomeres with telomere-specific FISH. The ALT phenotype was confirmed in all ULMSs with diminished ATRX expression. Some exome-sequenced tumors with reduced ATRX expression and ALT positivity did not show ATRX mutations, suggesting that there are regulatory or larger structural alterations undetectable by exome sequencing, or that the quality of FFPE samples was inadequate to reveal the underlying mutation. Interestingly, the only ULMS with two ATRX mutations did not show ALT.
ATRX is known to functionally cooperate with DAXX and DAXX mutations have been associated with ALT [13,14]. We therefore scrutinized the exome data for possible DAXX mutations. One nonsense mutation was identified, and FISH confirmed the ALT phenotype. Overall, these results show that ALT is very common in ULMS and that in addition to ATRX, also DAXX mutations contribute to the phenotype. Importantly, ALT was recently suggested to render cancer cells hypersensitive to ATR inhibitors [25]. These inhibitors might provide a novel treatment for ULMS, in which chemotherapeutic options have thus far been limited.
MED12 was mutated in four ULMSs (21%). All mutations were in exon 2, which is a known mutational hotspot in MED12. These mutations were first observed in uterine leiomyomas [15], and subsequently they have been identified in other tumor types [26]. Previous screening studies have reported recurrent MED12 mutations also in ULMS with similar frequencies as observed here [26]. It may be that a subset of ULMSs arises through a leiomyoma precursor, or alternatively MED12 mutations may provide growth advantage to ULMSs. MED12 is part of a multi-protein complex Mediator, which plays a key role in global transcription regulation in eukaryotic cells [27]. Based on our results, MED12 mutations can co-occur with TP53 and ATRX mutations.
FSIP2, ABCA13, and ANKRD26 were mutated in at least three tumors and additional 37 genes had mutations in two tumors. Most alterations were missense changes and gave either neutral or controversial results in in silico predictions. The possible role of these genes in ULMS development cannot be directly assessed as in addition of providing growth advantage to the cell, the observed alterations may represent rare germline polymorphisms or passenger mutations with no functional significance.
Although aberrant expression of both TP53 and ATRX in Stage I ULMSs, the only group of tumors large enough for the analyses, did not associate with poor overall survival, a trend toward poorer survival was seen in the patients. The limited number of samples in the survival analyses and the observation that expression statuses were associated with each other makes it difficult to draw conclusions regarding prognostic implications of TP53 or ATRX expression levels. In general, TP53 alterations are the most common genetic changes in human cancers and they are particularly associated with an aggressive phenotype. Recently, loss of ATRX expression was associated with poor clinical outcome in ULMS [21,22]. Larger sample series with information on both TP53 and ATRX are required to confirm these findings.
ULMSs are rare and aggressive cancers. In most cases the diagnosis is made only at surgery, and many patients thus present with an advanced disease. Here, we have utilized exome sequencing and identified several recurrently mutated genes, including TP53, ATRX, and MED12. While MED12 mutations are the most common alterations in benign conventional leiomyomas, TP53 or ATRX mutations have not been observed in these tumors. Specifically, identification of inactivating ATRX mutations and their association with the ALT phenotype in the substantial proportion of tumors may be translatable into clinical practice should the suggested effect of ATR inhibitors prove effective.
Materials and Methods
Ethics statement
This study was approved by the appropriate ethics review board of Hospital District of Helsinki and Uusimaa, Finland (408/13/03/03/2009).
Patient samples
Fifty-two archival FFPE ULMS tissue samples were derived from the Department of Pathology, Hospital District of Helsinki and Uusimaa, Finland, according to Finnish laws and regulations by permission of the director of the respective health care unit. These specimens represented diagnostic ULMS samples collected during surgery in 1985–2013. Simultaneously with the sample collection, clinical data were obtained for these cases (Table 1) after which the samples were anonymized for the study. Nineteen ULMSs (diagnosis 2003–2013) entered exome sequencing, while the remaining 33 tumors were available on a tissue microarray for immunohistochemistry.
Tab. 1. Clinical data of 52 ULMSs. 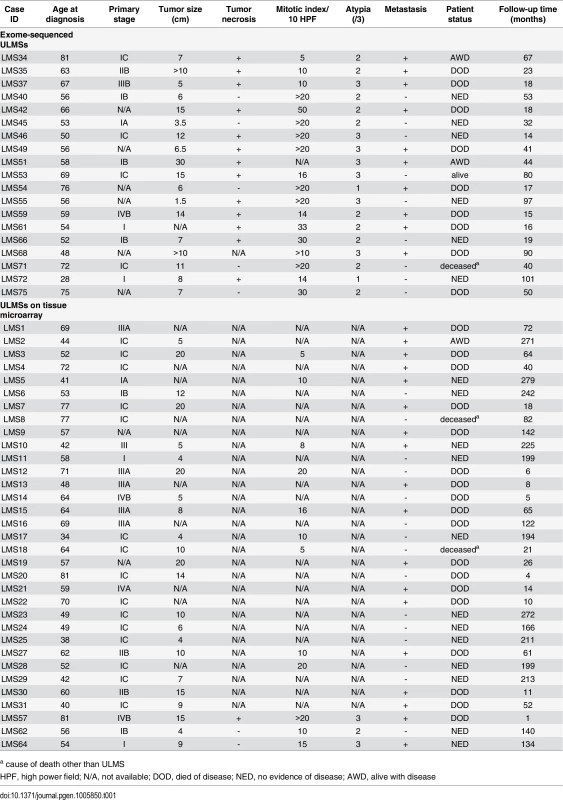
a cause of death other than ULMS Histological evaluation
Before exome sequencing, hematoxylin-eosin-stained sections from each specimen were re-evaluated by a pathologist (RB) and verified as ULMSs according to the WHO criteria [28]. Tumor percentage was ≥90% in all samples.
Exome sequencing
Genomic DNA was extracted with a standard phenol-chloroform method. Sample libraries were prepared using NEBNext DNA Library Prep Reagent Set for Illumina (New England Biolabs Ltd. catalog# E6000) and subjected to exome capture with NimbleGen SeqCap EZ System (Roche NimbleGen). Paired-end short read sequencing was performed with HiSeq 2000 (Illumina Inc.) at Karolinska Institutet, Sweden.
Somatic variant calling
Read mapping and somatic variant calling were carried out as previously described [29]. Additionally, single duplicate reads were removed with an in-house script.
Variant identification
Exome data was analyzed with an in-house analysis and visualization tool RikuRator. The requirements to call a variant included a minimum coverage of six reads and the mutated allele to be present in at least 20% of the reads. The Genome Analysis Toolkit (GATK) quality score of variants was required to be 25 or above. Both exonic regions and sequences within three base pairs of the exon-intron boundaries were included in the study. Synonymous changes, variants present in the dbSNP database (release 138), and variants in Exome Aggregation Consortium Server with a frequency over 0.1% were disregarded. To remove other potential germline variants, the exome data was filtered against data from 2315 Finnish controls (93 individuals from the 1000 Genomes Project, 1941 individuals from The Sequencing Initiative Suomi (SISu) (http://www.sisu.fimm.fi), and 281 in-house control exomes or genomes). Recently, it has been estimated that about 400 control samples remove germline variation (single-nucleotide variants and indels) from a tumor sample at least as efficiently as the matched normal sample [30]. Lastly, all the remaining variants were individually visualized with Rikurator to exclude those only present in the same direction reads as likely artifacts. The functional effects of the variants were predicted by two independent in silico tools: SIFT (http://sift.jcvi.org/) and Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/).
Direct sequencing
All candidate variants in genes mutated in at least three tumors were validated by direct sequencing. Oligonucleotide primers were designed with Primer3Plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) (S4 Table). PCR products were sequenced directly utilizing Big Dye Terminator v.3.1 sequencing chemistry (Applied Biosystems) on an ABI3730 Automatic DNA Sequencer.
Immunohistochemistry
TP53, ATRX, and DAXX immunolabeling was performed on FFPE sections of all 19 exome-sequenced ULMSs and on a tissue microarray containing 33 additional tumors. For TP53, immunostaining was performed as previously described [31]. For ATRX and DAXX, heat-induced antigen retrieval was carried out in a microwave using citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase blocking was followed by overnight incubation with the primary antibody at 4°C (anti-ATRX 1 : 500 dilution, Sigma-Aldrich catalog# HPA001906; anti-DAXX 1 : 500 dilution, Sigma-Aldrich catalog# HPA008736). The primary antibody was detected with DAB Plus Substrate System (Thermo Fisher Scientific catalog# TA-060-HDX). Immunohistochemical scoring was assessed by a pathologist (RB). Only nuclear labeling of the proteins was evaluated. The loss of nuclear staining in tumor cells together with retained expression in non-neoplastic cells (endothelial or inflammatory cells) was considered loss of expression. The scoring was done without knowledge of the clinical outcome data.
Telomere-specific FISH and microscopy
Detection of large, abnormally intense, intra-nuclear telomere DNA aggregates via telomere-specific FISH is considered the most sensitive and specific marker for identifying ALT phenotype in fixed tissue samples [13].
FFPE sections were deparaffinized at room temperature with xylene (3x10 min) and 100% EtOH (2x10 min) and air-dried. Subsequently, the slides were rinsed in phosphate-buffered saline (PBS) at 37°C (2x5 min) followed by RNAse A treatment (Sigma-Aldrich catalog# R4642) at 37°C for an hour. After a series of washes at room temperature with saline-sodium citrate (pH 7.0; 3x5 min) and deionized water (2x5 min), the slides were digested with Digest All 3-pepsin (Invitrogen/Life Technologies catalog# 00–3009) at 37°C for 10 min and rinsed with PBS at room temperature (2x5 min). Next, the slides were dehydrated and hybridized with Cy3-labeled peptide nucleic acid (PNA) probe (Panagene Inc. catalog# F1006-5). The denaturation took place at 85°C for 10 min and hybridization in dark at room temperature for an hour. Post-hybridization washes with saline-sodium citrate/0.1% Tween-20 (pH 7.0; 2x10 min) at 55°C and at room temperature for 10 min were followed by nuclear counterstaining with DAPI. The slides were imaged with a Zeiss Axio Imager epifluorescence microscope and image acquisition took place through Hamamatsu Orca Flash 4.0 LT camera and Zen software. The assessment of FISH slides was carried out independently by two authors (NM, MA). ULMSs were classified as ALT-positive if ≥5% of 300 assessed neoplastic cells displayed ALT-associated, abnormally bright telomeric DNA aggregates. In all cases, regions of necrosis and overlapping cells difficult to interpret were excluded from consideration.
Statistical analyses
Statistical analyses were performed using SPSS statistical software for Windows version 22.0 (SPSS Inc.). Here, survival was defined as overall survival time from the time of diagnosis. Survival curves related to TP53 and ATRX expression were generated using the Kaplan–Meier method, and median survival times with 95% confidence intervals were given. Comparison of survival curves between normal and aberrant expression was performed using the log-rank test. P-value <0.05 was considered statistically significant. Association between TP53 and ATRX expression statuses was evaluated using cross tabulation and Fisher’s exact test.
Supporting Information
Zdroje
1. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer 2006;119(12): 2922–2930. 17013893
2. Koivisto-Korander R, Martinsen JI, Weiderpass E, Leminen A, Pukkala E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas 2012;72(1): 56–60. doi: 10.1016/j.maturitas.2012.01.021 22377186
3. Reed NS, Mangioni C, Malmstrom H, Scarfone G, Poveda A, Pecorelli S, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 2008;44(6): 808–818. doi: 10.1016/j.ejca.2008.01.019 18378136
4. Hensley ML. Role of chemotherapy and biomolecular therapy in the treatment of uterine sarcomas. Best Pract Res Clin Obstet Gynaecol. 2011;25(6): 773–782. doi: 10.1016/j.bpobgyn.2011.06.003 21752717
5. Mayerhofer K, Obermair A, Windbichler G, Petru E, Kaider A, Hefler L, et al. Leiomyosarcoma of the uterus: a clinicopathologic multicenter study of 71 cases. Gynecol Oncol 1999;74(2): 196–201. 10419731
6. Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54(3): 355–364. doi: 10.1111/j.1365-2559.2009.03231.x 19236512
7. Pelmus M, Penault-Llorca F, Guillou L, Collin F, Bertrand G, Trassard M, et al. Prognostic factors in early-stage leiomyosarcoma of the uterus. Int J Gynecol Cancer 2009;19(3): 385–390. doi: 10.1111/IGC.0b013e3181a1bfbc 19407564
8. Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008;112(4): 820–830. doi: 10.1002/cncr.23245 18189292
9. Sandberg AA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: leiomyosarcoma. Cancer Genet Cytogenet. 2005;161(1): 1–19. 16114134
10. Yang J, Du X, Chen K, Ylipaa A, Lazar AJ, Trent J, et al. Genetic aberrations in soft tissue leiomyosarcoma. Cancer Lett. 2009;275(1): 1–8. doi: 10.1016/j.canlet.2008.06.013 18649996
11. Kobayashi H, Uekuri C, Akasaka J, Ito F, Shigemitsu A, Koike N, et al. The biology of uterine sarcomas: A review and update. Mol Clin Oncol. 2013;1(4): 599–609. 24649216
12. Garraway LA, Lander ES. Lessons from the cancer genome. Cell 2013;153(1): 17–37. doi: 10.1016/j.cell.2013.03.002 23540688
13. Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011;333(6041): 425. doi: 10.1126/science.1207313 21719641
14. Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331(6021): 1199–1203. doi: 10.1126/science.1200609 21252315
15. Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011;334(6053): 252–255. doi: 10.1126/science.1208930 21868628
16. de Vos S, Wilczynski SP, Fleischhacker M, Koeffler P. P53 Alterations in Uterine Leiomyosarcomas Versus Leiomyomas. Gynecol Oncol 1994;54(2): 205–208. 8063247
17. Jeffers MD, Farquharson MA, Richmond JA, McNicol AM. P53 Immunoreactivity and Mutation of the P53 Gene in Smooth Muscle Tumours of the Uterine Corpus. J Pathol 1995;177(1): 65–70. 7472782
18. Zhai YL, Kobayashi Y, Mori A, Orii A, Nikaido T, Konishi I, et al. Expression of steroid receptors, Ki-67, and p53 in uterine leiomyosarcomas. Int J Gynecol Pathol 1999;18(1): 20–28. 9891238
19. Muller PA, Vousden KH. P53 Mutations in Cancer. Nat Cell Biol 2013;15(1): 2–8. doi: 10.1038/ncb2641 23263379
20. Lee JC, Jeng YM, Liau JY, Tsai JH, Hsu HH, Yang CY. Alternative lengthening of telomeres and loss of ATRX are frequent events in pleomorphic and dedifferentiated liposarcomas. Mod Pathol. 2015;28(8): 1064–1073. doi: 10.1038/modpathol.2015.67 26022452
21. Liau JY, Tsai JH, Jeng YM, Lee JC, Hsu HH, Yang CY. Leiomyosarcoma with alternative lengthening of telomeres is associated with aggressive histologic features, loss of ATRX expression, and poor clinical outcome. Am J Surg Pathol. 2015;39(2): 236–244. doi: 10.1097/PAS.0000000000000324 25229770
22. Slatter TL, Hsia H, Samaranayaka A, Sykes P, Clow W, Devenish CJ, et al. Loss of ATRX and DAXX expression identifies poor prognosis for smooth muscle tumours of uncertain malignant potential and early stage uterine leiomyosarcoma. J Path: Clin Res. 2015;1(2): 95–105.
23. Huho A, Sheehan CE, Otto GA, Wang K, Palmer G, Yelensky R, et al. Evaluation of Uterine Leiomyosarcoma by Next Generation Sequencing Reveals Actionable Genomic Abnormalities and New Routes to Targeted Therapies. USCAP 2014, #1184, Mod. Pathol. 2014;27(S2): 287A.
24. Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8(7): e1002772. 22829774
25. Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015;347(6219): 273–277. doi: 10.1126/science.1257216 25593184
26. Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102(3): 621–629. doi: 10.1016/j.fertnstert.2014.06.050 25106763
27. Conaway RC, Conaway JW. Function and regulation of the Mediator complex. Curr Opin Genet Dev. 2011;21(2): 225–230. doi: 10.1016/j.gde.2011.01.013 21330129
28. Hendrickson MR, Tavassoli FA, Kempson RL, McCluggage WG, Haller U, Kubik-Huch RA. Mesenchymal tumours and related lesions. In: Tavassoli FA, Devilee P, editors. Tumours of the Breast and Female Genital Organs. Lyon: IARC; 2003. pp. 233.
29. Mäkinen N, Vahteristo P, Bützow R, Sjöberg J, Aaltonen LA. Exomic landscape of MED12 mutation negative and positive uterine leiomyomas. Int J Cancer 2014;134(4): 1008–1012. doi: 10.1002/ijc.28410 23913526
30. Hiltemann S, Jenster G, Trapman J, van der Spek P, Stubbs A. Discriminating somatic and germline mutations in tumor DNA samples without matching normals. Genome Res 2015;25(9): 1382–1390. doi: 10.1101/gr.183053.114 26209359
31. Koivisto-Korander R, Bützow R, Koivisto AM, Leminen A. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: expression and prognostic importance of ten different markers. Tumour Biol. 2011;32(3): 451–459. doi: 10.1007/s13277-010-0138-1 21161468
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2016 Číslo 2- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
Nejčtenější v tomto čísle- Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies
- 2015 Reviewer Thank You
- EEPD1: Breaking and Rescuing the Replication Fork
- Exome Sequencing of Uterine Leiomyosarcomas Identifies Frequent Mutations in , , and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



