-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
RNA-Silencing Enzymes Pol IV and Pol V in Maize: More than one Flavor?
article has not abstract
Published in the journal: . PLoS Genet 5(11): e32767. doi:10.1371/journal.pgen.1000736
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000736Summary
article has not abstract
There are some puzzling genetic phenomena in nature that have kept geneticists busily searching for clues for decades. Among genetics' X-files are cases in which alleles of the same gene have the same sequence but very different functional states. Gene behaviors of this sort fall under the heading of “epigenetic phenomena.” Traits attributable to epigenetically altered alleles (epialleles) are often not transmitted between generations as independent, immutable characters, according to the rules that Mendel worked out with his garden peas. Instead, there are cases where alleles can change state if and when they find themselves in the same nucleus as epialleles that are locked into a different functional state. This is the case in paramutation, which describes the situation whereby a silent, paramutagenic allele can influence the expression of an active, paramutable allele of the same gene when the two alleles are exposed to one another within the same genome [1]. The silent allele, which is defined as being paramutagenic, somehow transfers its silent state to the other allele, causing that previously active allele to become silenced, too. Most interestingly, the previously active allele is now stably altered in such a way that it can retain the silenced state through meiosis and future generations. Moreover, the newly silenced allele is paramutagenic itself and will silence compatible paramutable alleles. All of this happens without any changes in the nucleotide sequence at the affected locus. This unusual allelic behavior is the genic analogue of the shape-shifting that occurs among proteins that can convert to a prion state and then cause other proteins of identical amino acid sequence to acquire the altered conformation [2],[3]. How alleles can influence one another to acquire or maintain a paramutated or paramutagenic state is not yet known. However, in this issue of PLoS Genetics, two interesting papers from the Hollick (Stonaker et al. [4]) and Chandler (Sidorenko et al. [5]) laboratories provide further evidence that paramutation in maize has an RNA basis. Specifically, the studies suggest that multiple alternative subunits of the plant-specific multi-subunit RNA polymerases, Pol IV and Pol V, first discovered in Arabidopsis [6],[7], might assemble into Pol IV or Pol V sub-types that have different roles in paramutation and gene silencing.
Paramutation and siRNA-Mediated DNA Methylation
Paramutation occurs in animals as well as plants, but was first described in maize in the 1950s based on the behavior of genes that affect pigmentation of the plants and/or seeds. Sidorenko et al. and Stonaker et al. based their studies on the B1 (booster1) and Pl1-Rhoades (purple plant1-Rhoades) genes, both of which are involved in red/purple pigmentation. Certain alleles, such as B-I (B-intense) and Pl1-Rh, are fully functional and cause dark pigmentation, but alternative epialleles of these genes are silent, paramutagenic and designated with apostrophes (B' and Pl'). B-I and Pl-Rh alleles can spontaneously convert to B' and Pl' epialleles, but it is generally a one-way street; once converted, B' and Pl' alleles do not readily revert to the active state. Moreover, exposure of a functional allele to a corresponding B' or Pl' allele silences the active allele and converts it to a B' or Pl' allele. The predictability of these allelic behaviors and interactions has allowed for genetic screens to find mutants that are defective for the establishment or maintenance of paramutation.
The outcome of the maize genetic screens, to date, is the revelation that paramutation requires components of the 24-nt siRNA-directed DNA methylation pathway defined in considerable detail in Arabidopsis thaliana [7]. This pathway is required for the transcriptional silencing of transposable elements, endogenous repeats, and foreign transgenes. The pathway involves two plant-specific DNA-dependent RNA polymerases [8]–[11], now abbreviated as Pol IV and Pol V [12],[13]; the RNA-dependent RNA polymerase, RDR2; the double-stranded RNA endonuclease, DICER-LIKE3 (DCL3); and at least two of the ten Argonaute proteins, AGO4 and AGO6 [7]. Other proteins of the pathway include two putative chromatin remodeling ATPases—CLSY1 and DRD1 [14],[15] —and the de novo cytosine methyltransferase, DRM2 [16]. Pol IV acts early in the pathway, presumably generating transcripts that are then used as templates by RDR2, thereby producing double-stranded RNAs that are cleaved into 24-nt double-stranded siRNAs by DCL3. CLSY1 is thought to assist transcription by Pol IV and/or RDR2 [14]. Independent of siRNA biogenesis, Pol V generates transcripts that overlap loci that are subjected to siRNA-directed DNA methylation [12]. DRD1 is required for the production of these Pol V transcripts [12]. AGO4 can be crosslinked to Pol V transcripts [17], suggesting that structural RNAs synthesized by Pol V act as scaffolds for the recruitment of AGO4-siRNA complexes and the transcriptional silencing machinery, including DRM2. However, the biochemical details of how transcriptional repression is actually established are not yet in hand.
Evidence that proteins of the RNA-directed DNA methylation pathway are required for paramutation first came in 2006 when the gene responsible for the mediator of paramutation 1 (mop1) mutation was shown by the Chandler lab to be the maize ortholog of Arabidopsis RDR2 [18]. Two important papers from the Hollick lab soon followed. First, the required to maintain repression 1 (rmr1) gene was shown to encode a putative chromatin remodeling ATPase related to Arabidopsis CLSY1 and DRD1 [19]. Next, mutations defining the rmr6 locus were shown to disrupt shown to disrupt the maize ortholog of Arabidopsis NRPD1, the largest subunit of nuclear DNA-dependent RNA polymerase IV [20].
The morphological consequences of rdr2/mop1 and nrpd1 disruption are more severe in maize than in Arabidopsis. In Arabidopsis, rdr2- and nrpd1-null mutants cause an essentially complete loss of 24-nt siRNAs, loss of siRNA-directed DNA methylation, and derepression of transposons. The rdr2 and nrpd1 mutants are also developmentally impaired, displaying delayed flowering time, especially when days are short and nights are long. However, gross morphological aberrations are not observed in Arabidopsis rdr2 and nrpd1 mutants. Maize mop1 and nrpd1 mutants are similarly deficient for 24-nt siRNA biogenesis, transposon silencing, and flowering but also display a high incidence of morphological abnormalities [18], [20]–[23]. The much higher transposon and retrotransposon content of the maize genome, compared to Arabidopsis, likely confers a greater requirement for Pol IV, RDR2/MOP1 and the siRNA-directed DNA methylation pathway to prevent transposon-induced misregulation of genes affecting morphological development.
The Possibility of Multiple Pol IV or Pol V Sub-Types with Nuanced Functions
In the current issue, Stonaker et al. and Sidorenko et al. independently report on paramutation-impaired mutants that are defective for a maize paralog of NRPD2/NRPE2, the gene that encodes the second-largest subunit of both Pol IV and Pol V in Arabidopsis. Interestingly, maize has three such genes, with distinctive intron–exon structures (Figure 1), all of which are expressed and are presumably functional. By contrast, Arabidopsis thaliana has only one functional NRPD2/NRPE2 gene. The three maize NRPD2/NRPE2-like genes must not be fully redundant in their functions given that Stonaker et al. and Sidorenko et al. both identified recessive mutants of the same NRPD2/NRPE2-like gene in their screens for paramutation-defective plants. If the different NRPD2/NRPE2-like genes were fully redundant, double - or triple-recessive loss-of-function mutants would have been needed to observe mutant phenotypes.
Fig. 1. Intron-exon structures of the three NRPD2-/NRPE2-like genes in maize. 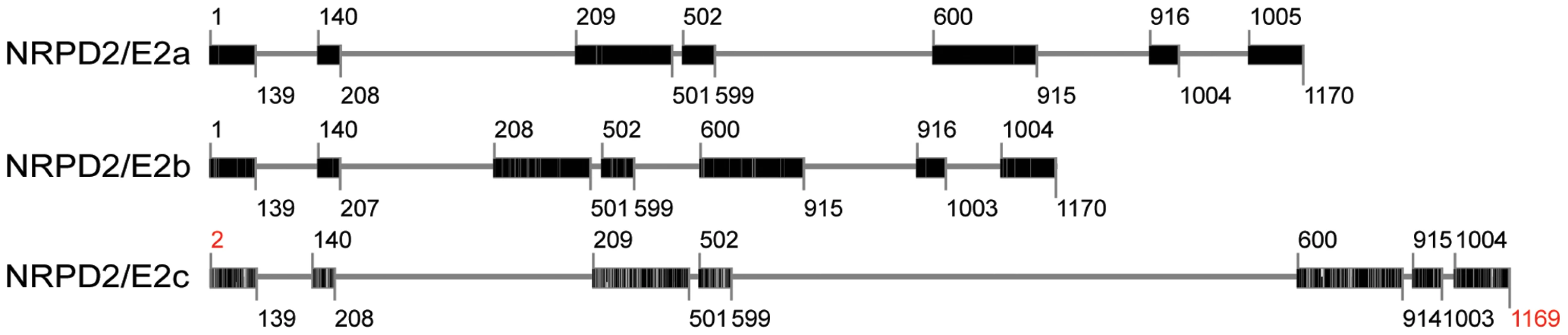
The gene models were made using TARGeT (http://target.iplantcollaborative.org/), with the Zea mays NRPD2/E2a amino acid sequence obtained from the NCBI Protein database (AAY45706.1) queried against the Z. mays pseudo-genome. Exonic regions are indicated by boxes; intronic regions are marked with lines. Darker bars in exons indicate increasing similarity to the query sequence. Numbers indicate amino acid positions relative to the query sequence (NRPD2/E2a). Positions with missing amino acids relative to the query sequence are marked in red. Despite the lack of full redundancy, the three maize NRPD2/NRPE2-like genes may be partially redundant for some Pol IV and/or Pol V functions. Evidence for this interpretation is indirect, but compelling. First, 24-nt siRNAs are severely depleted as a result of recessive loss-of-function mutations in the NRPD2/NRPE2-like gene that was identified in both studies; this is also true in nrpd1 mutants, suggesting that the NRPD2/NRPE2-like protein encoded by the gene is most likely a Pol IV subunit. Both the largest and second-largest subunits of Pol IV are required for polymerase activity, as in other multi-subunit RNA polymerases, because these are the catalytic subunits [24]. Therefore, one would expect loss-of-function mutations in the NRPD1 and NRPD2 genes to have the same phenotypes. However, the phenotypic consequences of recessive loss of function mutations in the NRPD2/NRPE2-like gene that was identified (termed NRPD2/E2a in the Chandler lab and NRPD2a by the Hollick lab) are not as severe as in nrpd1 mutants. These observations suggest that one or more of the other NRPD2/NRPE2-like proteins might also interact with NRPD1 to form Pol IV complexes with somewhat different functions, all of which are lost in an nrpd1 mutant. This interpretation is also supported by the fact that the Mop2-1 allele of the NRPD2/E2a gene identified by Sidorenko et al. is a dominant loss-of-function allele with more severe phenotypes than recessive nrpd2/e2 loss-of-function alleles. The authors suggest that the mutated second-largest subunit in Mop2-1 plants may assemble with other subunits to tie up NRPD1 in non-functional Pol IV complexes, thereby phenocopying nrpd1 mutants.
Predictions to Test, Questions to Answer
The Stonaker et al. and Sidorenko et al. papers raise a number of interesting questions and present some interpretations that will need to be further tested. First and foremost, it will be important to determine if the proteins encoded by the three NRPD2/NRPE2-like genes can truly serve as alternative catalytic subunits of Pol IV, Pol V, or both enzymes. Reciprocal co-immunoprecipitation studies could be one approach for revealing which of the three NRPD2/NRPE2-like proteins subunits associate with NRPD1 or NRPE1 in maize. It would be even more informative to affinity purify Pol IV or Pol V complexes containing each of the three alternative second-largest subunits and use mass spectrometry to determine the complete subunit compositions of the complexes. By this approach, Arabidopsis Pol IV and Pol V were recently shown to have 12 core subunits that are identical or paralogous to the 12 core subunits of Pol II [13]. For most of the smaller subunits of Arabidopsis Pol IV and Pol V, alternative versions of the proteins, encoded by paralogous genes, were detected in the complexes [13]. It is not yet known whether these alternative subunits are fully redundant and interchangeable in Arabidopsis or whether they co-associate in unique permutations within distinctive Pol IV or Pol V sub-types, but the latter is a distinct possibility. Therefore, it is possible that Pol IV and/or Pol V may come in multiple flavors, or sub-types in both maize and Arabidopsis, but using different sets of alternative subunits in the two species.
Another missing ingredient for interpreting the current papers is knowledge of bona fide Pol V mutant phenotypes in maize, which would best be defined by null mutations in the NRPE1 gene encoding the Pol V largest subunit. Unfortunately, a maize nrpe1 mutant is not yet available for comparison. In Arabidopsis, nrpd1 and nrpe1 mutants display similar losses in DNA methylation, such that DNA methylation assays do not distinguish between Pol IV and Pol V mutants. Although Pol IV is required for the biogenesis of virtually all 24-nt siRNAs in Arabidopsis, Pol V is also required for the biogenesis of approximately one-third of these siRNAs, is important for another one-third of the siRNAs, and is dispensable for siRNA production at the final one-third of Pol IV–dependent loci [25]. Therefore, depletion of siRNAs does not necessarily imply a Pol IV–specific function. It remains a possibility that Pol V and Pol IV might both be required for siRNA accumulation in maize, as at one-third of Arabidopsis loci.
The idea that Pol IV and Pol V will have the same functions in Arabidopsis and maize may be valid, and the three NRPD2/NRPE2-like proteins of maize may turn out to fit neatly within a Pol IV and Pol V classification due to limited overall subunit heterogeneity. But it is still early days in the study of these plant-specific RNA polymerases and their specialization in RNA-dependent silencing pathways. The possibility that maize may have an enzyme with sufficient functional and structural diversity to warrant being named Pol VI is not beyond imagination.
Zdroje
1. ChandlerVL
2007 Paramutation: from maize to mice. Cell 128 641 645
2. MooreRA
TaubnerLM
PriolaSA
2009 Prion protein misfolding and disease. Curr Opin Struct Biol 19 14 22
3. PerrettS
JonesGW
2008 Insights into the mechanism of prion propagation. Curr Opin Struct Biol 18 52 59
4. StonakerJL
LimJP
ErhardKFJr
HollickJB
2009 Diversity of Pol IV function is defined by mutations at the maize rmr7 locus. PLoS Genet 5 e1000706 doi:10.1371/journal.pgen.1000706
5. SidorenkoL
DorweilerJE
CiganAM
Arteaga-VazquezM
VyasM
2009 A dominant mutation in mediator of paramutation 2, one of three second largest subunits of a plant specific RNA Polymerase, disrupts multiple siRNA silencing processes. PLoS Genet 5 e1000725 doi:10.1371/journal.pgen.1000725
6. PikaardCS
HaagJR
ReamT
WierzbickiAT
2008 Roles of RNA polymerase IV in gene silencing. Trends Plant Sci 13 390 397
7. MatzkeM
KannoT
DaxingerL
HuettelB
MatzkeAJ
2009 RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21 367 376
8. HerrAJ
JensenMB
DalmayT
BaulcombeDC
2005 RNA polymerase IV directs silencing of endogenous DNA. Science 308 118 120
9. OnoderaY
HaagJR
ReamT
NunesPC
PontesO
2005 Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120 613 622
10. PontierD
YahubyanG
VegaD
BulskiA
Saez-VasquezJ
2005 Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev 19 2030 2040
11. KannoT
HuettelB
MetteMF
AufsatzW
JaligotE
2005 Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet 37 761 765
12. WierzbickiAT
HaagJR
PikaardCS
2008 Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135 635 648
13. ReamTS
HaagJR
WierzbickiAT
NicoraCD
NorbeckAD
2009 Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol Cell 33 192 203
14. SmithLM
PontesO
SearleI
YelinaN
YousafzaiFK
2007 An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell 19 1507 1521
15. KannoT
MetteMF
KreilDP
AufsatzW
MatzkeM
2004 Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr Biol 14 801 805
16. CaoX
JacobsenSE
2002 Role of the Arabidopsis DRM Methyltransferases in De Novo DNA Methylation and Gene Silencing. Curr Biol 12 1138 1144
17. WierzbickiAT
ReamTS
HaagJR
PikaardCS
2009 RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat Genet 41 630 634
18. AllemanM
SidorenkoL
McGinnisK
SeshadriV
DorweilerJE
2006 An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 295 298
19. HaleCJ
StonakerJL
GrossSM
HollickJB
2007 A novel Snf2 protein maintains trans-generational regulatory states established by paramutation in maize. PLoS Biol 5 e275
20. ErhardKFJr
StonakerJL
ParkinsonSE
LimJP
HaleCJ
2009 RNA polymerase IV functions in paramutation in Zea mays. Science 323 1201 1205
21. NobutaK
LuC
ShrivastavaR
PillayM
De PaoliE
2008 Distinct size distribution of endogeneous siRNAs in maize: Evidence from deep sequencing in the mop1–1 mutant. Proc Natl Acad Sci U S A 105 14958 14963
22. ParkinsonSE
GrossSM
HollickJB
2007 Maize sex determination and abaxial leaf fates are canalized by a factor that maintains repressed epigenetic states. Dev Biol 308 462 473
23. HaleCJ
ErhardKFJr
LischD
HollickJB
2009 Production and processing of siRNA precursor transcripts from the highly repetitive maize genome. PLoS Genet 5 e1000598
24. HaagJR
PontesO
PikaardCS
2009 Metal A and metal B sites of nuclear RNA polymerases Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing. PLoS ONE 4 e4110
25. MosherRA
SchwachF
StudholmeD
BaulcombeDC
2008 PolIVb influences RNA-directed DNA methylation independently of its role in siRNA biogenesis. Proc Natl Acad Sci U S A 105 3145 3150
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2009 Číslo 11- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- X Chromosome: Expression and Escape
- Stage-Specific Expression Profiling of Spermatogenesis Suggests that Meiotic Sex Chromosome Inactivation Drives Genomic Relocation of Testis-Expressed Genes
- RNA-Silencing Enzymes Pol IV and Pol V in Maize: More than one Flavor?
- 10 Reasons to be Tantalized by the B73 Maize Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- 10 Reasons to be Tantalized by the B73 Maize Genome
- X Chromosome: Expression and Escape
- Stage-Specific Expression Profiling of Spermatogenesis Suggests that Meiotic Sex Chromosome Inactivation Drives Genomic Relocation of Testis-Expressed Genes
- RNA-Silencing Enzymes Pol IV and Pol V in Maize: More than one Flavor?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



