-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLeksell gamma knife past, present and future
The first prototype of the Leksell Gamma Knife was installed in 1968 at Sophiahemmet Hospital in Stockholm, Sweden. Since that this system underwent significant improvement affecting quality and safety of patient’s treatment. Initial Leksell Gamma Knife systems called U and B were later on replaced by models C and 4C. Finally, the newest system called Leksell Gamma Knife Perfexion was introduced in 2006. The first and currently only gamma knife unit in the Czech Republic was installed in Prague in 1992. Initial Leksell Gamma Knife model B was later upgraded to the Leksell Gamma Knife model C which was finally replaced by the latest Leksell Gamma Knife Perfexion installed at Na Homolce Hospital in December 2009. The source of radiation in the Leksell Gamma Knife is 60Co. Half life of 60Co is 5.26 years. Two energies of gamma photons are emitted with energy of 1.17 MeV and 1.33 MeV. High number of multiple 60Co beams directed in one focus point are used. This geometry leads to a high dose in focus point and very low dose to surrounding tissue. There are 201 60Co beams in the case of Leksell Gamma Knife models U, B, C and 4C and 192 60Co beams in the case of Perfexion, respectively. Radiosurgical treatment is done in one single fraction. Leksell stereotactic frame which is attached to patient’s head is used for exact imaging and targeting. The whole procedure involves typically only one single fraction of radiation and is done in one treatment day. Main diagnosis involve following intracranial lesions: arteriovenous malformations, meningiomas, acoustic neuromas, pituitary adenomas, single and multiple metastasis, glial tumors and functional radiosurgery such as trigeminal neuralgia. This review article describes how this system evolved since the first prototype, provides technical specifications of different Leksell Gamma Knife systems and makes comparison between different systems.
Keywords:
Leksell Gamma Knife, stereotactic radiosurgery, radiation oncology
Authors: Josef Novotný Jr., Ph.D.
Authors place of work: Na Homolce Hospital, Roentgenova 2, Prague 5 150 30, Czech Republic
Published in the journal: Lékař a technika - Clinician and Technology No. 3, 2012, 42, 5-13
Summary
The first prototype of the Leksell Gamma Knife was installed in 1968 at Sophiahemmet Hospital in Stockholm, Sweden. Since that this system underwent significant improvement affecting quality and safety of patient’s treatment. Initial Leksell Gamma Knife systems called U and B were later on replaced by models C and 4C. Finally, the newest system called Leksell Gamma Knife Perfexion was introduced in 2006. The first and currently only gamma knife unit in the Czech Republic was installed in Prague in 1992. Initial Leksell Gamma Knife model B was later upgraded to the Leksell Gamma Knife model C which was finally replaced by the latest Leksell Gamma Knife Perfexion installed at Na Homolce Hospital in December 2009. The source of radiation in the Leksell Gamma Knife is 60Co. Half life of 60Co is 5.26 years. Two energies of gamma photons are emitted with energy of 1.17 MeV and 1.33 MeV. High number of multiple 60Co beams directed in one focus point are used. This geometry leads to a high dose in focus point and very low dose to surrounding tissue. There are 201 60Co beams in the case of Leksell Gamma Knife models U, B, C and 4C and 192 60Co beams in the case of Perfexion, respectively. Radiosurgical treatment is done in one single fraction. Leksell stereotactic frame which is attached to patient’s head is used for exact imaging and targeting. The whole procedure involves typically only one single fraction of radiation and is done in one treatment day. Main diagnosis involve following intracranial lesions: arteriovenous malformations, meningiomas, acoustic neuromas, pituitary adenomas, single and multiple metastasis, glial tumors and functional radiosurgery such as trigeminal neuralgia. This review article describes how this system evolved since the first prototype, provides technical specifications of different Leksell Gamma Knife systems and makes comparison between different systems.
Keywords:
Leksell Gamma Knife, stereotactic radiosurgery, radiation oncologyTHE EVOLUTION OF GAMMA KNIFE TECHNOLOGY
The Gamma Knife was developed by Lars Leksell and Borje Larsson, to achieve their goal of an efficient, precise, hospital-based stereotactic radiosurgery system for intracranial targets4. The first prototype of the Leksell Gamma Knife was installed in 1968 at Sophiahemmet Hospital in Stockholm, Sweden (Figure 1). The first patient was treated for craniopharyngioma. Subsequently, gamma knife surgery was performed in patients with pituitary tumors, vestibular schwannomas, vascular malformations, and functional disorders such as intractable pain. In 1975, a series of surgical pioneers at the Karolinska Hospital, Stockholm began to utilize a new Gamma Knife, redesigned to create a more spheroidal dose-profile better suited for the treatment of intracranial tumors and vascular malformations. Units 3 and 4 were placed in Buenos Aires and Sheffield England in the early 1980’s. Lunsford et al. introduced the first clinical 201-source Gamma Knife unit to North America (the fifth gamma unit worldwide) and the first patient was managed in August 1987 at the University of Pittsburgh Medical Center6.
Figure 1 The first prototype of the Leksell Gamma Knife was installed in 1968 at Sophiahemmet Hospital in Stockholm, Sweden 
The first and currently only gamma knife unit in the Czech Republic was installed in 1992. Initial Leksell Gamma Knife model B was later upgraded to the Leksell Gamma Knife model C which was finally replaced by the latest Leksell Gamma Knife model called Perfexion in December 2009. Na Homolce Hospital is one of the busiest gamma knife centers worldwide treating currently close to one thousand patients per year. Over ten thousand patients were treated at Na Homolce Hospital since 1992 up today.
The encouraging results of radiosurgery for benign tumors and vascular malformations led to an exponential rise of radiosurgery cases and installations of radiosurgical units. Up today there are almost 300 installations of the Leksell Gamma Knife. In recent years metastatic brain tumors have become the most common indication of radiosurgery. Brain metastases now comprise 30-50% of radiosurgery cases at busy centers.
Leksell Gamma Knife models U, B and C
The source of radiation in the Leksell Gamma Knife is Cobalt-60 (60Co). Diagram for the decay of 60Co is given in Figure 2.
Figure 2 Diagram for the decay of <sup>60</sup>Co used as a radioactive source in gamma knife. Half life of <sup>60</sup>Co is 5.26 years. Two energies of gamma photons are emitted with energy of 1.17 MeV and 1.33 MeV 
Half life of 60Co is 5.26 years. Two energies of gamma photons are emitted with energy of 1.17 MeV and 1.33 MeV. When using single beam of 60Co maximum of dose is deposited very close to surface rather than deeply in brain tissue where potential radiosurgical targets can be located. This fact is simply given by absorption characteristic of photon beam. To overcome this unfavorable property of photons, high number of multiple 60Co beams directed in one focus point are used. This geometry leads to a high dose in focus point and very low dose to surrounding tissue. The situation is illustrated in Figure 3.
Figure 3 Basic principle of gamma knife radiosurgery. A) Depth dose curve in water for <sup>60</sup>Co for standard conditions. It is obvious that maximum of dose is deposited very close to surface. B) Combination of multiple <sup>60</sup>Co collimated beams that are directed in one focus point. This geometry leads to a high dose in focus point and very low dose to surrounding tissue. 
There have been numerous changes to the Gamma Knife® since the original 1968 design. In the first models (Model U) 201 Cobalt sources were arranged in a hemispheric configuration. These units presented challenging 60Co loading and reloading issues. To facilitate reloading, the unit was redesigned so that sources were arranged in a circular configuration (Model B, C and 4C) (Figure 4 and Figure 5A).
Figure 4 Schematic drawing of the Leksell Gamma Knife 4C. A) Cross section of the Leksell Gamma Knife 4C. B) Collimator helmet and four (4, 8, 14, 18 mm) color coded collimators. C) Schematic drawing of Automatic Positioning System (APS) and patient head fixation. 
Figure 5 Two currently most frequently used Leksell Gamma Knife systems. A) Leksell Gamma Knife 4C. B) Leksell Gamma Knife Perfexion. 
Gamma Knife radiosurgery usually involves the use of single or multiple isocenters of different beam diameters to achieve a treatment plan that conforms to the 3-dimensional volume of the target. The total number of isocenters may vary depending upon the size, shape, and location of the target. Each isocenter has a set of three Cartesian (X, Y, Z) stereotactic coordinates corresponding to its location in three-dimensional space as defined using a rigidly fixed stereotactic frame. When multiple isocenters are used, the stereotactic coordinates will need to be set individually. In 1999, the Model C Gamma Knife was introduced. This technology combined dose planning advances with robotic engineering. The unit incorporated an automatic positioning system (APS) with submillimetric accuracy, used to move the stereotactic frame with patient head to each coordinate (Figure 4). This technology obviates the need to manually adjust each set of coordinates in a multiple-isocenter plan. The robot eliminates the time spent removing the patient from the helmet, setting the new coordinates for each isocenter and repositioning the patient in the helmet. This has significantly reduced the total time spent to complete the procedure and also increased accuracy and safety1,3,7-9. The other features of the Model C unit include an integral helmet changer, dedicated helmet installation trolleys, and color-coded collimators. In 2005 the fourth generation Leksell Gamma Knife® model 4C was introduced.
The model 4C is equipped with enhancements designed to improve workflow and provide integrated imaging capabilities. The planning information can be viewed on both sides of the treatment couch. The helmet changer and robotic Automatic Positioning System® are faster and reduce total treatment time.
Leksell Gamma Knife Perfexion
The newest iteration of Gamma Knife technology is the Perfexion unit (Figure 5B and Figure 6). Beginning in 2002, an invited group of neurosurgeons, radiation oncologists and medical physicists was asked by the manufacturer to define specifications for a new Leksell Gamma Knife system. The group agreed on five critical features for a new system: 1) best dosimetry performance, 2) unlimited cranial reach, 3) best radiation protection for patient and stuff, 4) full automation of the treatment process, 5) patient and staff comfort, and 6) similar dosimetry for smallest collimator as prior units. The new unit was first installed in Marseille, France in 2006. The installation and commissioning of the Perfexion system in Prague, Czech Republic was performed in 2009.
The radiation unit was redesigned. A total of 192 60Co sources were arranged in a cylindrical configuration in five concentric rings. This differs substantially from the previous hemispherical arrangements and results in different source to focus distances for each ring varying from 374 to 433 mm. The primary and secondary collimators have been replaced by a single large 120 mm thick tungsten collimator array ring (Figure 6). Consequently no collimator helmets are needed for the Perfexion system.
Figure 6 Leksell Gamma Knife Perfexion radiation unit and collimator system. A) Cross section of the Leksell Gamma Knife Perfexion radiation unit. B) Detailed view of sectors, each sector holds 24 <sup>60</sup>Co sources and can be moved independently of other sectors in desired position to define a collimator size or block beams. C) Sector position which defines a 4 mm collimator. D) Sector position which defines a 8 mm collimator. E) Sector position which defines a 16 mm collimator. (Pictures taken with permission from Elekta Instrument AB, Stockholm, Sweden). 
Three collimators are available for the Perfexion system. The 4 mm and 8 mm collimators remain, and a new 16 mm collimator replaces the prior 14 mm and 18 mm collimators. The tungsten collimator array is subdivided into eight identical but independent sectors, each containing 72 collimators (24 collimators for 4 mm, 24 collimators for 8 mm, 24 collimators for 16 mm). The collimator size for each sector is changed automatically by moving 24 sources over the selected collimator set. Each sector with 24 sources can be moved independently into five different positions: 1) sector in home position when system is standby, 2) 4 mm collimator, 3) 8 mm collimator, 4) 16 mm collimator, and 5) sector off position (defined as the position between the 4 and 8 mm collimators providing blocking of all 24 beams for that sector) (Figure 6). Sector movement is performed by servo-controlled motors with linear scales located at the rear of the radiation unit.
The radiation cavity has been increased by more than 300% compared to previous models. However, due to an improved collimation system (120 mm tungsten ring), the average distance from source to focus is very close to previous models. This results in similar output for the prior 18 mm and new 16 mm administrations. The increase in the volume of the radiation cavity to more than three times allows for a greater mechanical treatment range in X/Y/Z stereotactic coordinates. It is (160/180/220mm) for the Perfexion system compared to (100/120/165mm) for other gamma knife models. This provides virtually unlimited cranial reach, so crucial in the care of patients with multiple brain metastases5.
The Automatic Positioning System (APS) used in the C units was replaced by the Patient Positioning System (PPS). Rather than just the head, the whole couch moves into pre-selected stereotactic coordinates. This provides better patient comfort and allows complete of the majority of radiosurgery treatments in one single run. Docking of the patient into the PPS is done by means of an adaptor that attaches to the standard stereotactic Leksell G frame with three clips. The adapter is then directly docked to the PPS. A patient can be attached in three different positions, with gamma angles of 70, 90 or 110 degrees reflecting neck flexion or extension. The gamma angle is the only treatment parameter that requires manual set up. The PPS has repeatability better than 0.05 mm. Comparison of LGK Perfexion with LGK 4C in various technical parameters is given in Table 1. Comparison of dose profiles for LGK Perfexion with LGK 4C is given in Figure 7.
Tab. 1. Comparison of different technical parameters between LGK PFX and LGK 4C. 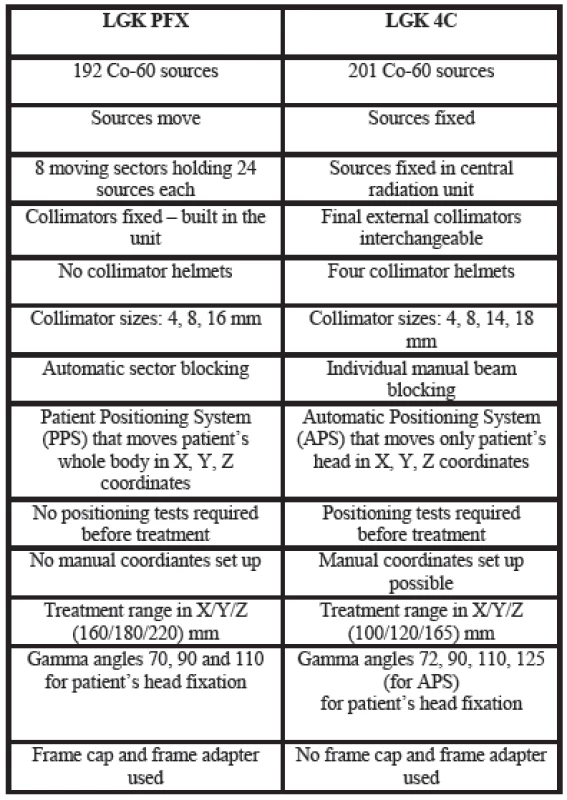
Figure 7 Comparison of Leksell Gamma Knife Perfexion and 4C profiles calculated for 4, 8, 14, 16, and 18 mm collimators for all stereotactic X, Y, Z axes. Profiles for LGK 4C are given as solid lines whereas those for LGK Perfexion are given as dotted lines. Figure A shows the profiles along the X axis whereas those for the Y and Z axes are shown in Figures B and C, respectively. 
The redesigned hardware of the Perfexion unit has had significant impact on the planning software Leksell GammaPlan (LGP), a new version of the LGP running on a PC platform with the Linux operating system. There are in principle three possible approaches in the treatment planning: 1) use of classical combinations of 4, 8, and 16 mm isocenters (shots), 2) use of composite shots containing combinations of 4, 8 16 mm or blocked sectors and 3) dynamic shaping using blocked selected sectors to protect volumes defined as critical structures. The most revolutionary change in the treatment planning is the ability to generate a single isocenter composed of different beam diameters. Such a composite shot design allows an optimized dose distribution shape for each individual shot. The setup of any sectors, combinations of different collimators, or blocking takes only minimal time (a few seconds done automatically). The latest version of the treatment planning software also allows automatic treatment planning. Thus after target and critical structures volume definition the software is able automatically generate treatment plan. Example of two treatment plans is given in Figure 8.
Figure 8 Example of treatment plan for patient with A) acoustic neuroma and B) multiple metastases. 
The new Perfexion system provides further improvements in patient and staff radiation shielding. The sectors are always in the off position (blocked) during patient transportation in the treatment position, transition into new stereotactic coordinates, pause or emergency interrupt. These results in significantly (about 5-10 times) lower extracranial irradiation to the patient compared to models B and C. Our preliminary comparison study shows that for a patient with ten brain lesions, the total time saved is about 1.5-2.0 hours compared to other systems. The new Leksell Gamma Knife Perfexion provides excellent dosimetry performance, unlimited cranial reach, enhanced radiation protection for patient and staff, full automation of the treatment process and better patient and staff comfort compared to previous models (Table 2).
Tab. 2. Technical specifications of different Leksell Gamma Knife Units. 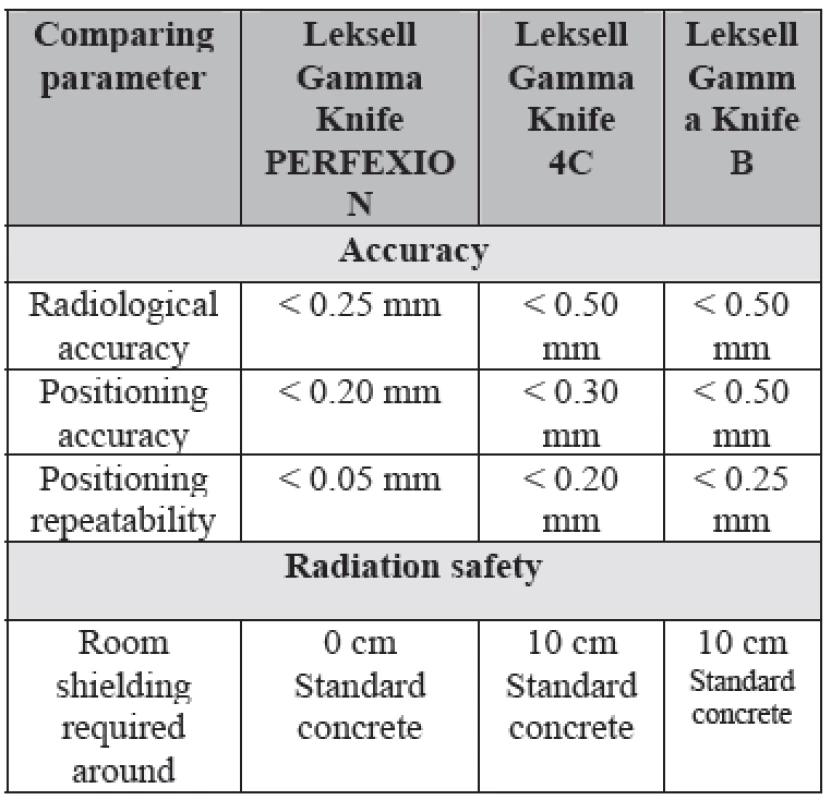
Thus, the PERFEXION unit provides the potential to increase the spectrum of treatable indications including multiple brain metastasis, access to the upper cervical spine, and other pathologies of the head and neck. The use for the lower to mid-cervical spine will require the development of a new fixation device, imaging and patient position monitoring system.
THE RADIOSURGERY PROCEDURE
Application of the stereotactic frame
For Gamma Knife® radiosurgery, appropriate stereotactic frame placement is an initial critical part of the procedure. Leksell stereotactic frame is basically Cartesian coordinate system with three X, Y and Z coordinates attached to patient’s head. The center of the coordinate frame is denoted as 100, 100, 100 (Figure 9D). Prior to frame placement, the radiosurgery team should review the preoperative images and discuss optimal frame placement strategy. An effort should be made to keep the lesion as close to the center of the frame as possible. The possibility of collision by the frame base ring, the posts/pins assembly, or the patient’s head with the collimator helmet during treatment should also be considered prior to the frame application. Steps to avoid a possible collision should be taken during frame placement. These collisions should be minimal with the LGK Perfexion.
Figure 9 Basic principles of stereotactic imaging. A) Leksell stereotactic frame positioned on the phantom head. B) Plastic magnetic resonance imaging (MRI) indicator box with copper sulfate filled fiducial markers. C) Stereotactic definition of the MRI image. D) Schematic drawing of stereotactic coordinate system with image definition principle. 
Techniques of Stereotactic Imaging
Imaging is crucial in radiosurgery. Magnetic resonance imaging (MRI) is the preferred imaging modality for all gamma knife radiosurgery patients. Computed Tomography (CT) is used when MR imaging is not possible. Angiograms are used in conjunction with MRI for arteriovenous malformation (AVM) radiosurgery. Angiography is the gold standard for AVM radiosurgery planning. It should be used in conjunction with MRI or CT imaging. The orthogonal images (instead of oblique or rotated) are preferred but are not necessary. For AVM nidi that are not properly visualized in orthogonal planes a rotation of up to 100 in two dimensions or aspects can be used without compromising the accuracy of radiation delivery.
Stereotactic imaging has to be done after frame fixation. Special indicator boxes with fiducial markers are attached for different imaging modalities. Thus there is a special indicator box for MRI, CT and angiography. After stereotactic imaging is done anatomical information is accompanied by fiducial markers that are used for accurate image spatial definition (Figure 9).
Determination of Target Volume(s)
Target determination is an important step in order to make a conformal plan. Target volume can be outlined using the LGP® software (manual or semiautomatic mode). Although experienced surgeons can create conformal dose plans without outlining the target, the target outline allows for a more quantitative assessment of the plan. For new centers especially where physicists assume the initial responsibility for planning, target definition and outlining by the surgeon or oncologist becomes an important step. The surgeon’s input is required to define radiosurgery targets for patients with AVMs, tumors and functional neurosurgery as used by some centers. By defining the target volume and volumes of critical structures better evaluation and quantification of the treatment plan can be done. Various parameters such as dose volume histograms for the target volume and critical structures plus conformity indexes can be obtained.
Techniques of Conformal Dose Planning
In the process of treatment planning, several strategies can be used. The Model C allows treatment using robotic automatic patient positioning system (APS mode), manual positioning (trunnion mode) or mixed treatment (some isocenters in APS mode and some in trunnion mode). Most users will select isocenters (shots) and directly place them over the target. Isocenter can be imagine in very simple way as almost spherical volume with diameter given by a collimator size. Thus at 50 % isodose line we have diameters of 4 mm, 8 mm, 14 mm and 18 mm for LGK B and C models or/and 4 mm, 8 mm and 16 mm for LGK Perfexion, respectively. Beginners can also use the inverse dose-planning algorithmto create a plan and then optimize it manually. The conformal dose planning is enhanced by the use of multiple small collimators.
Three different approaches in the treatment planning can be applied when using LGK Perfexion. The first is to use the same strategy as described for 4C system above. Since only 4, 8 and 16 mm collimators are available only combination of these three different collimators can be used to cover the entire target volume. The second approach is to use dynamic shaping that is new feature in the treatment planning introduced for the Perfexion system. This automatic procedure will provide solutions to block selected sectors to protect volumes defined as critical structures. Different levels to reduce dose delivered to critical structures can be selected. The treatment planning system then automatically calculates which sectors should be blocked for each individual shot. One should be aware that each blocking will significantly increase total exposure time. The third approach is to use single isocenters composed of different beam diameters or blocked sectors. Any pattern of sectors including 4, 8, 16 mm collimators and blocks can be generated. This can help significantly for shaping dose distribution especially for irregular volumes. Example of treatment plan for patient with acoustic neuroma and multiple metastases is given in Figure 8.
Radiation Administration during Stereotactic Radiosurgery
The Model 4C Gamma knife allows radiation delivery using trunnion mode (manual patient positioning) or APS mode (Robotic positioning) or a combination of the two (mixed treatment). In trunnion treatment, the X, Y, and Z of each isocenter are set manually and triple-checked to avoid errors.
The APS plan is transferred directly from the planning computer to control computer. The operator selects the run (a combination of isocenters of same beam diameter) that matches the collimator helmet on the gamma unit. The APS is moved to the dock position and the patient’s head frame is fixed into the APS. The accuracy of the docking position is checked. The system prompts the user to perform clearance checks first for all those planned isocenters in which the pins, posts, frame or patient’s head would be less than 12 mm away from the inner surface of the collimator helmet (even though they may not match with the collimator size which is being used for first run). The clearance check is performed by moving the patient to those positions under APS manual control and by visual check of collision with the collimator helmet. After the clearance check, the system prompts the surgeon to carry out position checks. In the position checks, all the isocenters using the same helmet are checked, one by one, by moving the patient’s head to these positions using APS manual control to make sure patient will handle all head position changes with sufficient comfort. All personnel then leave the room, and the radiosurgical dose is administered. The APS moves the patient to all planned positions, one by one, until the isocenters using that size collimator helmet are completed. The team monitors the patient and the coordinates of different isocenters on the control computer. If other runs using a different gamma angle but using the same helmet are planned, then the patient is taken out, next run is selected, APS is moved to the dock position and patient’s head is again fixed in the APS using the planned angle (720, 900, 1100, or 1250).
Radiosurgery with the LGK Perfexion is a fully automated process for all aspects of the procedure including set up of the stereotactic coordinates, set up of different sector positions defining collimator size or blocked beams and set up of exposure times. All treatment data are exported to the operating console. The only manual part of the procedure is the positioning of the patient’s head in the docking device and adjustment of the couch height for optimal comfort. After confirmation of the patients’ identity, most Perfexion treatments are administered in one single run (about 95%). Rarely a clearance check is needed. For this, a special test tool simulating the shape and dimensions of the inner collimator is attached and rotated around patient’s head. Once radiosurgery begins, the team monitors the patient and the set up of coordinates, exposure times and sector set up of different isocenters on the control computer of operating console. The system allows audio-visual communication with the patient during irradiation and the process can be interrupted at any time if needed.
FUTURE OF GAMMA KNIFE TECHNOLOGY
It can be expected that Perfexion system will slowly replace existing gamma knife systems model B and C. Currently there are almost three hundred Leksell gamma knife systems installed world wide. Out of these there are about one hundred Perfexion gamma knifes.
Since Perfexion system hardware can also offer extracranial reach (C spine, head and neck), it can be expected that technical evolution of the Perfexion will address these requirements. It will be necessary to design different fixation than currently used Leksell stereotactic frame. Also patient imaging and position monitoring during treatment will be necessary in order to be able to perform accurate patient set up. Future indications could thus beside intracranial lesions involve also head and neck cancer and C spine lesions.
CONCLUSION
In the past two decades, we have witnessed dramatic improvements in stereotactic radiosurgery technologies. Gamma knife radiosurgery now offers better image-handling features, faster and more compact software platforms that make the calculations almost real time, automated and robotic patient positioning thus reducing the potential for human error, inverse treatment planning, and expanded indications.
Institute of Biophysics and Informatics,
First Faculty of Medicine, Charles University in Prague,
Salmovská 1, Prague 2, 120 00, Czech Republic,
E-mail: josef.homolkaml@homolka.cz.
Phone: +420 257 272 919
Zdroje
1. Goetsch SJ: Risk analysis of Leksell Gamma Knife Model C with automatic positioning system. Int J Radiat Oncol Biol Phys 52 : 869-877, 2002
2. Kondziolka D, Maitz AH, Niranjan A, Flickinger JC, Lunsford LD: An evaluation of the Model C gamma knife with automatic patient positioning. Neurosurgery 50 : 429-431; discussion 431-422, 2002
3. Kuo JS, Yu C, Giannotta SL, Petrovich Z, Apuzzo ML: The Leksell gamma knife Model U versus Model C: a quantitative comparison of radiosurgical treatment parameters. Neurosurgery 55 : 168-172; discussion 172-163, 2004
4. Leksell L: The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102 : 316-319, 1951
5. Lindquist C, Paddick I: The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery 61 : 130-140; discussion 140-131, 2007
6. Lunsford LD, Flickinger J, Lindner G, Maitz A: Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery 24 : 151-159, 1989
7. Regis J, Hayashi M, Porcheron D, Delsanti C, Muracciole X, Peragut JC: Impact of the model C and Automatic Positioning System on gamma knife radiosurgery: an evaluation in vestibular schwannomas. Journal of Neurosurgery 97 : 588-591, 2002
8. Tlachacova D, Schmitt M, Novotny J, Jr., Novotny J, Majali M, Liscak R: A comparison of the gamma knife model C and the automatic positioning system with Leksell model B. J Neurosurg 102 Suppl:25-28, 2005
9. Yu C, Jozsef G, Apuzzo ML, MacPherson DM, Petrovich Z: Fetal radiation doses for model C gamma knife radiosurgery. Neurosurgery 52 : 687-693; discussion 693, 2003
Štítky
Biomedicína
Článek Editorial
Článek vyšel v časopiseLékař a technika

2012 Číslo 3-
Všechny články tohoto čísla
- ECMO ambulance and advanced emergency medical system
- Measurement of thermal symmetry of the human spine by the use of medical thermography
- Editorial
- Determination of Human Gait Phase by Zero-moment Point
- Stanovení pohybové aktivity na základě výsledků zátěžového vyšetření
- Vliv fotodynamické terapie na cytomechaniku nádorové buněčné linie HeLa
- Inovace praktických úloh ve výuce lékařské biofyziky Lékařské fakulty univerzity palackého
- Leksell gamma knife past, present and future
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Leksell gamma knife past, present and future
- Stanovení pohybové aktivity na základě výsledků zátěžového vyšetření
- Vliv fotodynamické terapie na cytomechaniku nádorové buněčné linie HeLa
- Inovace praktických úloh ve výuce lékařské biofyziky Lékařské fakulty univerzity palackého
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání







