-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Severe gastric ulcer as a first manifestation of systemic lupus erythematosus in a child
Žaludeční vřed jako první projev systémového lupus erythematodes u dítěte
Systémový lupus erythematodes (SLE) je autoimunitní onemocnění s velmi pestrým klinickým obrazem, které může napodobovat řadu jiných nemocí. Postihuje převážně mladé ženy ve věku mezi 20 a 40 lety, nicméně nevyhýbá se ani mladším věkovým kategoriím. V následujícím sdělení prezentujeme případ dětské pacientky s neprospíváním a recidivující cystitidou, u které došlo k náhlému vzniku závažných gastrointestinálních komplikací vyžadujících chirurgickou léčbu. Jako nejpravděpodobnější diagnóza byla zvažována Crohnova nemoc, přestože při histologickém vyšetření nebyl v bioptických vzorcích žaludeční tkáně nalezen žádný granulom. Navzdory intenzivní imunosupresivní terapii se hluboké žaludeční vředy nikdy zcela nezhojily. Pět let po manifestaci choroby došlo k rozvoji lupusové nefritidy s autoimunitní hemolytickou anémií. Následně byla u pacientky potvrzena diagnóza SLE. Zahájení adekvátní terapie vedlo k významnému zlepšení klinického stavu i k úpravě laboratorních parametrů. Tento případ jasně poukazuje na fakt, že při nálezu atypických žaludečních vředů je nutné jako možnou příčinu zvažovat i SLE. Závěrem tak lze konstatovat, že stanovení diagnózy SLE může být někdy velice obtížné, a to zejména v případech, kdy jsou přítomné pouze gastrointestinální příznaky a symptomy syndromu dolních močových cest.
Klíčová slova:
žaludeční vřed – Crohnova nemoc – autoimunitní hemolytická anémie – intersticiální cystitida – systémový lupus erythematodes
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Doručeno:
13. 10. 2017Přijato:
23. 10. 2017
Authors: J. Zieg; O. Hradský; J. Nevoral; J. Bronský
Authors place of work: Department of Paediatrics, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic
Published in the journal: Gastroent Hepatol 2017; 71(6): 483-486
Category: Dětská gastroenterologie a hepatologie: kazuistika
doi: https://doi.org/10.14735/amgh2017483Summary
Systemic lupus erythematosus (SLE) is an autoimmune disease with diverse clinical symptoms that can imitate a number of other diseases. It affects predominantly young women aged 20–40 years but cases diagnosed at a younger age are not unusual. We report a case of a young girl with failure to thrive and recurrent cystitis who presented abruptly with severe gastrointestinal manifestations requiring surgical treatment. Crohn’s disease of the stomach was the most likely diagnosis, although no granuloma was found in tissue biopsies. In spite of intensive immunosuppressive therapy, complete healing of the deep stomach ulcers was not achieved. Five years after the disease manifestation, the patient developed lupus nephritis with autoimmune haemolytic anaemia and finally a diagnosis of SLE was established. Adequate therapeutic management of the disease consequently led to a significant improvement in the patient’s clinical condition as well as laboratory results. This case report clearly points to the fact that in the case of atypical stomach ulcers, SLE must be considered in differential diagnosis. In addition, diagnosis of SLE may be extremely challenging, especially in cases with exclusive gastrointestinal and urinary symptoms.
Key words:
gastric ulcer – Crohn’s disease – autoimmune haemolytic anaemia – interstitial cystitis – systemic lupus erythematosusIntroduction
Gastrointestinal (GI) symptoms in patients with systemic lupus erythematosus (SLE) are common. Early recognition of SLE can be difficult because many diseases mimic SLE symptoms. In particular, GI symptomatology is not specific and may cause diagnostic ambiguity. Intriguingly, in patients with SLE, abdominal symptoms may be due to the disease itself or medications used to treat the disease. Timely diagnosis enables adequate disease management and improves long-term survival of patients with SLE [1]. Mucosal ulcers in the oral cavity and decreased salivation are the most common GI signs; however, other parts of the digestive system may be involved. The small and large intestine may be affected by small-vessel vasculitis. Furthermore, complications secondary to abdominal thrombosis, protein-losing enteropathy, fat malabsorption and pancreatitis or hepatitis due to SLE or medication may be observed [2]. However, SLE does not often present with severe GI symptoms at the beginning of the disease and typical SLE diagnostic criteria, apart from oral ulcers, are based on non-GI symptoms [3]. Keeping in mind the wide aetiology of GI symptoms in the paediatric population, SLE diagnosis becomes challenging when other diagnostic criteria are lacking.
Patient report
The patient was a 10-year-old Caucasian female who was admitted to the hospital with 4 kg weight loss over a 4-month period, growth retardation, abdominal pain over the previous 3 months and 2 weeks of intermittent hematemesis. Past medical history was significant for recurrent urinary tract infections (UTI) from the age of 3 years and frequent acute respiratory infections. On admission, the patient was asthenic, with normal blood pressure and temperature. The chest was clear to auscultation and abdominal palpation was painful in the epigastrium. Of note, erythrocyte sedimentation rate and IgA were slightly elevated and there was thrombocytosis in full blood count. Complement components, lymphocyte subpopulation levels and neutrophil function were within the normal range, autoantibodies were negative. Urine sediment was normal. The evolution of laboratory results is summarised in Tab. 1. Abdominal ultrasound revealed thickening of both the antral stomach wall (9 mm) and the urinary bladder wall (5 mm). Esophagogastroduodenoscopy (EGD) showed severe mucosal inflammation in the antrum and stomach body along with multiple deep ulcerations and significant stenosis of pylorus (Fig. 1). The rapid urease test was negative, and the level of gastrin was normal. The patient had normal macroscopic findings on ileocolonoscopy. However, histological examination revealed mild chronic mucosal inflammatory changes in the whole GI tract with a significant proportion of eosinophils in the lymphocytic infiltrate, but no specific features were seen. Despite atypical localisation, the diagnosis of Crohn’s disease (CD) was made and immunosuppressive treatment with prednisone (1.3 mg/kg/day) and omeprazol (1 mg/kg) was initiated. Two weeks after discharge, vomiting with abdominal pain reoccurred. An upper GI series, magnetic resonance and endosonography revealed infiltration of the stomach wall with multiple ulcerations of the stomach body, pylorus and duodenum. Because of the severe gastric inflammation with suspected perforation, a gastroduodenostomy (Billroth’s I operation) was performed. Histology of stomach biopsies showed inflammation with deep ulceration and a tumourous aetiology was excluded. The patient also suffered from further UTIs and frequent periods of dysuric pain without positive urine culture and was put on prophylactic antibiotic treatment. The decision to start infliximab and enteral feeding via nasojejunal tube for presumed severe CD was made. Follow-up EGD after the second dose of infliximab showed significant healing of ulcers and a reduction in stomach wall inflammation. However, enteral nutrition was reinitiated along with azathioprine treatment due to progression of GI inflammation on a regular diet. The patient tolerated the treatment well and gained weight accordingly. Suspected infusion reaction in combination with a high level of anti-infliximab antibodies required a switch to treatment with adalimumab 4 months after infliximab therapy initialisation. One year after disease manifestation, anti-thyroid antibodies with high thyroid-stimulating hormone (TSH) were found. Ultrasound showed a small struma and substitution of levothyroxine for autoimmune thyroiditis was started. Over the next 5 years, serial endoscopies showed exacerbation of inflammation with alternating periods of remission. Cystoscopy showed severe chronic cystitis with marked mucosal inflammation. At 15 years of age, our patient presented with nephrotic proteinuria, microscopic haematuria, hypertension and mild elevation of renal parameters. Her immunologic results were positive for antinuclear and anti-double stranded DNA, as well as for significantly decreased complement component levels. In addition, autoimmune haemolytic anaemia (AIHA) and mild thrombocytopenia occurred. Renal biopsy was performed, and histology results were consistent with global diffuse lupus nephritis class (IV-G) [4]. Induction treatment with corticosteroids and mycophenolate mofetil was started.
Tab. 1. Evolution of the patient’s laboratory results. Tab. 1. Laboratorní výsledky pacientky. 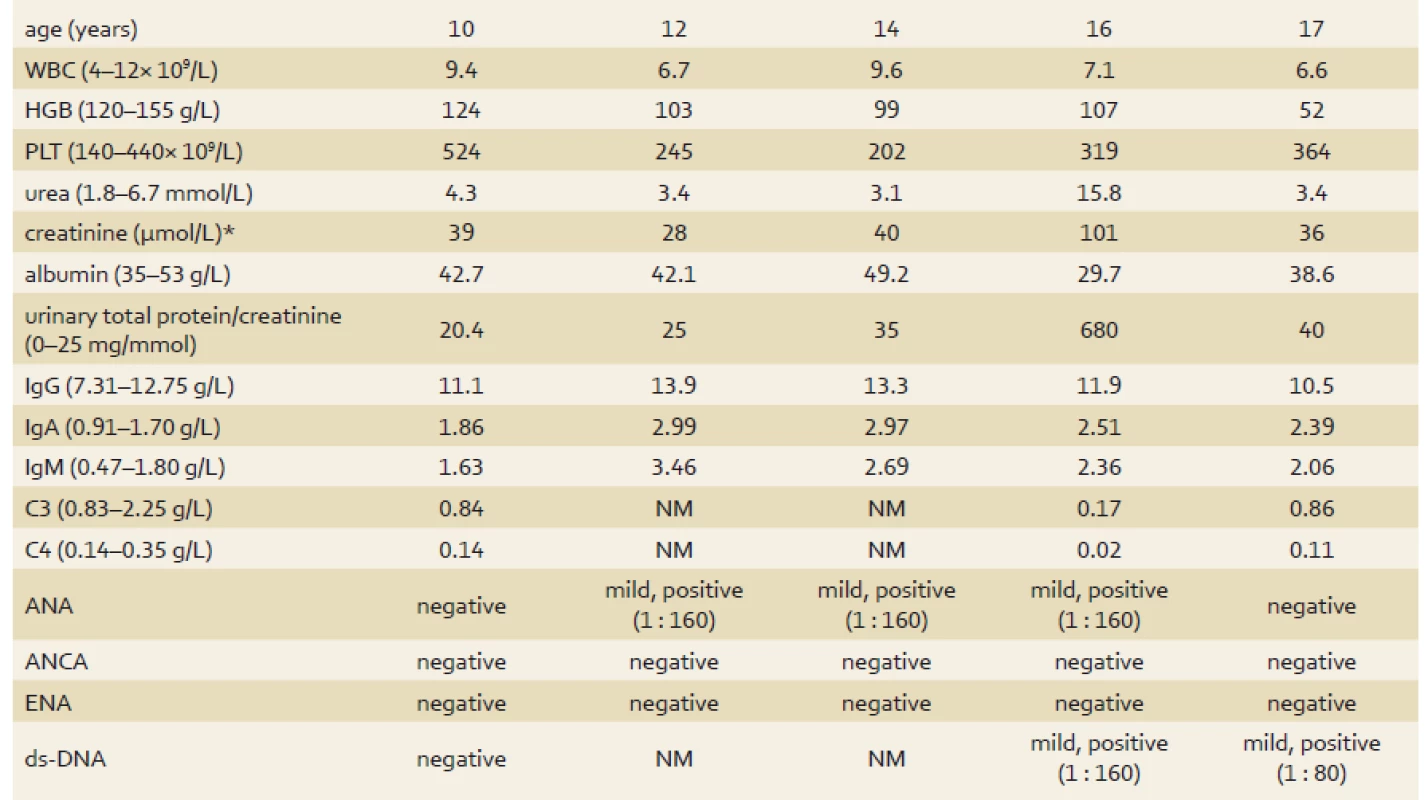
*normal serum creatinine varies with age WBC – white blood cells, HGB – haemoglobin, PLT – platelets, C3 – complement component 3, C4 – complement component 4, ANA – anti-nuclear antibodies, ANCA – anti-neutrophil cytoplasmic antibodies, ENA – extractable nuclear antigens, ds-DNA – double-stranded deoxyribonucleic acid, NM – not measured, normal range displayed in parenthesis Fig. 1. Endoscopic image of gastric ulcer. Obr. 1. Endoskopický nález žaludečního vředu. 
EGD 3 weeks after the induction of treatment for SLE nephritis showed healed lesions without new inflammatory changes or ulcers (Fig. 2). Proteinuria decreased; however, anaemia did not improve, and the patient required multiple transfusions. Finally, rituximab (4 × 500 mg per week) was administered and led to significant improvements in clinical conditions, including further decrease of proteinuria, complete normalisation of renal parameters and gradual resolution of anaemia with negative Coombs test.
Fig. 2. Healed gastric lesions after 3 weeks of induction SLE treatment. Obr. 2. Vyhojená léze žaludku po 3 týdnech indukční terapie SLE. 
Discussion
Here, we presented the case report of a patient with SLE with GI and urinary manifestations. Our first impression was that an atypical form of CD caused this GI affection. Consequently, the diagnosis of SLE was delayed because initial GI signs preceded manifestation of renal involvement and specific antibody positive results for 5 years. In patients with SLE, peptic ulcer disease is mainly associated with stress and non-steroid anti-inflammatory drug use. The association between corticosteroid use and peptic ulcer disease is much weaker [2,5]. Interestingly, it has been reported that SLE can also cause inflammatory changes in gastric and intestinal mucosa [6]. Pathophysiological mechanisms of GI involvement of SLE usually include small vessel vasculitis, immune complex deposition and complement activation. Thrombotic microangiopathy has also been reported [2,7]. In patients with concurrent genitourinary and GI manifestations, smooth muscle dysmotility secondary to immune complex-mediated damage is suspected [7]. Cystoscopy revealed typical findings associated with interstitial cystitis. Although a bladder wall biopsy was not performed, we presumed the diagnosis of interstitial cystitis related to SLE [8]. Our patient was thought to suffer from CD because endoscopic macroscopic findings in stomach, bowel microscopic inflammation and growth retardation, were all consistent with CD. On the other hand, the diagnosis of CD was questioned when histology revealed chronic mucosal inflammation that lacked CD-specific features. Interestingly, cases of SLE complicated by CD have also been reported [9]. It is known that anti-tumour necrosis factor alpha (anti-TNF-α) therapy dampens autoimmune reactions in autoimmune diseases, including SLE. This likely explains the effect of these agents on the reduction of the disease activity [9]. However, it has also been reported that anti-TNF-α drugs can induce SLE in adults [10]. However, the incidence of drug-induced SLE is very low and we do not think this occurred in our patient because initial GI and urinary features preceding biologic therapy were, in retrospect, the first signs of SLE.
This case report shows that lupus etiology needs to be sought in patients with atypical presentation of gastric and duodenal ulcer. It was not possible to ensure the correct diagnosis at the time of the disease manifestation, when specific immune tests were negative, and our patient did not present with other signs of SLE. However, seronegative lupus appears in 5–10% of SLE patients, so we have to be aware of this diagnosis despite negative immunologic testing. If we had at present a patient with similar disease presentation we would consider administration of immunosuppressive treatment which is also used for SLE patients (e. g. cyclosporine A, mycophenolate mofetil) to suppress the disease activity. In addition, we would also aim to avoid surgery in this patient. Interestingly, other features which pointed to SLE were the presence of autoimmune thyroiditis and highly suspicious interstitial cystitis. In conclusion, extensive search of other organ involvement is essential in children with predominant GI presentation and inconspicuous immunologic results to approach the correct diagnosis.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 13. 10. 2017
Accepted: 23. 10. 2017
Jakub Zieg, MD, PhD
Department of Paediatrics 2nd Faculty of Medicine
Charles University and
Motol University Hospital
V Uvalu 84
150 06 Praha 5
Czech Republic
jakubzieg@hotmail.com
Zdroje
1. Hallegua DS, Wallace DJ. Gastrointestinal manifestations of systemic lupus erythematosus. Curr Opin Rheumatol 2000; 12 (5): 379–385.
2. Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford) 1999; 38 (10): 917–932.
3. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40 (9): 1725.
4. Weening JJ, D‘Agati VD, Schwartz MM et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited: J Am Soc Nephrol 2004; 15 (2): 241–250.
5. Pecora PG, Kaplan B. Corticosteroids and ulcers: is there an association? Ann Pharmacother 1996; 30 (7–8): 870–872.
6. Musaev SN, Novikova AV, Shershevskaia A et al. The morphometric and immunohistochemical characteristics of the gastric and duodenal mucosa in systemic lupus erythematosus. Biull Eksp Biol Med 1991; 111 (2): 203–236.
7. Tan TC, Wansaicheong GK, Thong BY. Acute onset of systemic lupus erythematosus with extensive gastrointestinal and genitourinary involvement. Lupus 2012; 21 (11): 1240–1243. doi: 10.1177/0961203312455111.
8. Tanaka H, Waga S, Tateyama T et al. Interstitial cystitis and ileus in pediatric-onset systemic lupus erythematosus. Pediatric Nephrology 2000; 14 (8–9): 859–861.
9. Yamashita H, Ueda Y, Kawaguchi H et al. Systemic lupus erythematosus complicated by Crohn’s disease: a case report and literature review. BMC Gastroenterol 2012; 12 : 174. doi: 10.1186/1471-230X-12-174.
10. De Bandt M, Sibilia J, Le Loët X et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 2005; 7 (3): R545–R551.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2017 Číslo 6- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Pediatric gastroenterology and hepatology
- Bariatrics
- Evaluation and applicability of nonbiopsy criteria in children and adolescents according to ESPGHAN for diagnosis of celiac disease
- Wilson’s disease in a cohort of pediatric patients in Slovakia
- Severe gastric ulcer as a first manifestation of systemic lupus erythematosus in a child
- Interdisciplinary European guidelines on metabolic and bariatric surgery
- Epidemiology, hospitalization and migration of patients with IBD under specialized care in the Czech Republic
- Obesity and kidney
- Cytomegalovirus infection and the liver
- Magnesium sulfate saline laxatives in the preparation for colonoscopy – our experience
-
Ružinovský gastroenterologický deň 2017
10. november 2017 - 4th International Symposium on Pediatric IBD
- The selection from international journals
- Kreditovaný autodidaktický test: dětská gastroenterologie a hepatologie + bariatrie
- Thanks to reviewers
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus infection and the liver
- Magnesium sulfate saline laxatives in the preparation for colonoscopy – our experience
- Epidemiology, hospitalization and migration of patients with IBD under specialized care in the Czech Republic
- Obesity and kidney
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



