-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Groove (paraduodenal) pancreatitis – a case series of six patients
Žliabková (paraduodenálna) pankreatitída – séria šiestich pacientov
Žliabková pankreatitída (groove pancreatitis – GP) je zvláštna forma chronickej pankreatitídy charakterizovaná vznikom inflamatórnych zmien a formáciou fibrózneho tkaniva v oblasti „žliabku“ medzi hlavou pankreasu, duodénom a žlčovodom. Na zobrazovacom vyšetrení sa manifestuje ako masa v žliabku v blízkosti malej papily, zhrubnutie alebo cystické formácie v žliabku alebo v stene duodéna. Klinicky sa prejavuje bolesťou brucha, váhovým úbytkom alebo zvracaním. Ochorenie môže imitovať karcinóm pankreasu, žlčovodu alebo duodéna, pričom odlíšenie od karcinómu hlavy pankreasu býva problematické. Konzervatívna liečba samotná, prípadne v kombinácii s endoskopickou, býva často úspešná. Chirurgická liečba je indikovaná pri neúspešnej konzervatívnej liečbe alebo nemožnosti vylúčiť karcinóm. Autori prezentujú vlastné skúsenosti so žliabkovou pankreatitidou na sérii šiestich pacientov liečených v jednom terciárnom gastroenterologickom centre počas obdobia šiestich rokov.
Kľúčové slová:
žliabková pankreatitida – paraduodenálna pankreatitida – chronická pankreatitida – karcinóm pankreasu –endosonografia
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Doručeno:
11. 1. 2015Přijato:
6. 4. 2015
Authors: M. Kliment 1,2; O. Urban 1,2; P. Fojtík 1; M. Straka 3,4; M. Loveček 5; D. Žiak 6; J. Krátký 7; P. Holéczy 8
Authors place of work: Research Institute AGEL, Digestive Disease Center, Hospital Vítkovice, Ostrava, Czech Republic 1; Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic 2; Department of Surgery, Hospital Nový Jičín, Czech Republic 3; J. G. Mendel Oncology Center, Nový Jičín, Czech Republic 4; 1st Surgical Clinic of the Palacky University Faculty of Medicine and Olomouc Teaching Hospital, Olomouc, Czech Republic 5; CGB Laboratory, Ostrava, Czech Republic 6; Department of Radiology, Hospital Vítkovice, Ostrava, Czech Republic 7; Department of Surgery, Hospital Vítkovice, Ostrava , Czech Republic 8
Published in the journal: Gastroent Hepatol 2015; 69(3): 195-200
Category: Digestivní endoskopie: původní práce
doi: https://doi.org/10.14735/amgh2015195Summary
Groove pancreatitis (GP) is a special form of chronic pancreatitis characterised by the development of inflammatory changes and fibrous tissue formation in the ‚groove‘ between the pancreatic head, duodenum and common bile duct. Imaging studies show a mass in the groove close to minor papilla, a thickening, or cyst formations in the groove or the duodenal wall. Clinical symptoms include abdominal pain, weight loss or vomiting. The disease may imitate pancreatic head cancer, bile duct cancer or duodenal cancer, and its differentiation from pancreatic head carcinoma may be difficult. Conservative treatment alone or in combination with endoscopy is often successful. Surgery is indicated if conservative treatment fails or if carcinoma cannot be ruled out. Authors present their own experience with groove pancreatitis on a series of six patients diagnosed and treated at a single tertiary referral gastroenterology centre over a period of six years.
Key words:
groove pancreatitis – paraduodenal pancreatitis – chronic pancreatitis – pancreatic cancer – endosonographyIntroduction
Groove pancreatitis (GP, also known as paraduodenal pancreatitis) is a special form of chronic pancreatitis characterised by the development of inflammatory and fibroproductive changes in the anatomical area of the ‚groove‘ between the pancreatic head, duodenum and common bile duct. Clinical symptoms and morphology can be identical to those of pancreatic head carcinoma. Nevertheless, with knowledge of the entity, certain clinical and morphological signs can be used to differentiate GP from pancreatic head carcinoma.
The condition was first described by Becker [1] in 1973 and the term ‚groove pancreatitis‘ was introduced by Stolte in 1983 to indicate pancreatitis characterised by fibrous scars in the anatomical space between the dorsocranial section of the pancreatic head, duodenum and common bile duct [2]. Becker and Mischke subdivided GP into ‚pure‘ and ‚segmental‘ form [3]. In the pure form of GP, changes are localised in the groove only, between the duodenal wall and the pancreatic head, without affecting the pancreatic parenchyma or the main pancreatic duct. In the segmental form, in addition to those in the groove, changes are also present in the adjacent parenchyma of the pancreatic head, affecting (stricturing) the pancreatic duct or the common bile duct, too. Fléjou et al described this condition in a series of patients as a “cystic dystrophy of heterotopic pancreas in the wall of the stomach or the duodenal wall” [4]. Adsay and Zamboni proposed the term ‚paraduodenal pancreatitis‘ [5]. Due to the frequent presence of cysts in the duodenal wall or in the groove in the pure form, and the presence of a mass in the segmental form, some authors prefer the terms cystic and solid form. The exact prevalence of GP is unknown. In three published studies, GP was present in 2.7%, 19.5% and 24.4% of resected tissue obtained by hemi-duodenopancreatectomy in patients with chronic pancreatitis [2,3,6]. Despite the apparently ‚high‘ incidence of GP in the last two of the above mentioned studies, it is a condition that is rarely clinically diagnosed, as attested by only six patients identified in the authors‘ centre over six years.
A case series of six patients
Over a period of six years we have diagnosed and treated six patients with groove pancreatitis in our tertiary gastroenterology centre. Three patients underwent surgery, with diagnosis confirmed histologically; the remaining three were diagnosed on the basis of history, imaging examinations and clinical development. The patients’ clinical characteristics are detailed in Tab. 1. Their mean age (± SD) was 47.5 ± 9.4 years. Abdominal pain andweight loss were the most common clinical symptoms. Based on the contrast enhanced computer tomography (CT) examinations, pancreatic head carcinoma (Fig. 1) was suspected in five out of the six patients, while the benign changes, corresponding to pancreatitis (Fig. 2) were described by the radiologist in only one patient. Endoscopic ultrasound (EUS) showed focal pancreatitis with calcifications in the head (Fig. 3) and intact parenchyma and duct in the rest of the gland (Fig. 4) in all patients. Also showing on endoscopic ultrasound was a thickened duodenal wall in all patients, and cysts in the duodenal wall or in the so-called groove in four patients (Fig. 5). EUS-guided fine needle aspiration biopsy (EUS-FNA) was performed in three patients, with either non-diagnostic cytology (n = 1, duodenal cyst aspiration) or confirming normal pancreatic and inflammatory cells (n = 2). The duodenal cyst aspiration showed a significant increase in amylase activity (1,852 µkat/ L) and low levels of carcinoembryonic antigen (CEA: 10.8). On the basis of the EUS scans, all patients were diagnosed with groove pancreatitis. Three patients underwent surgery. Proximal pylorus-preserving pancreaticoduodenectomy (PPDPE) was performed on the first patient, a deliberation of duodenum with intraoperative biopsy of the duodenal mucosa in the second, and laparotomy with intraoperative USG scan in the third. Histology confirmed fibrosing pancreatitis in the first patient and Brunner’s gland hyperplasia in the second. Patient four underwent two EUS-guided duodenal cyst evacuations. Due to relapsing cyst in the duodenal wall with epigastric pain, EUS-guided cystoduodenostomy (EUS-CDS) was finally performed by introducing a pigtail drain into the duodenal cyst (Fig. 6). This patient continues to receive endoscopic treatment for distal common bile duct stricture by means of multi-stenting, i.e. parallel introduction of several plastic duodenobiliary drains. All patients are followed-up by a gastroenterologist on an out-patient basis and show favourable clinical development, characteristic of a benign condition of the pancreas. The mean length of follow-up of the patients in the group was 19.0 ± 16.4 (SD) months.
Tab. 1. Clinical characteristics of patients. Tab. 1. Klinické charakteristiky pacientov. 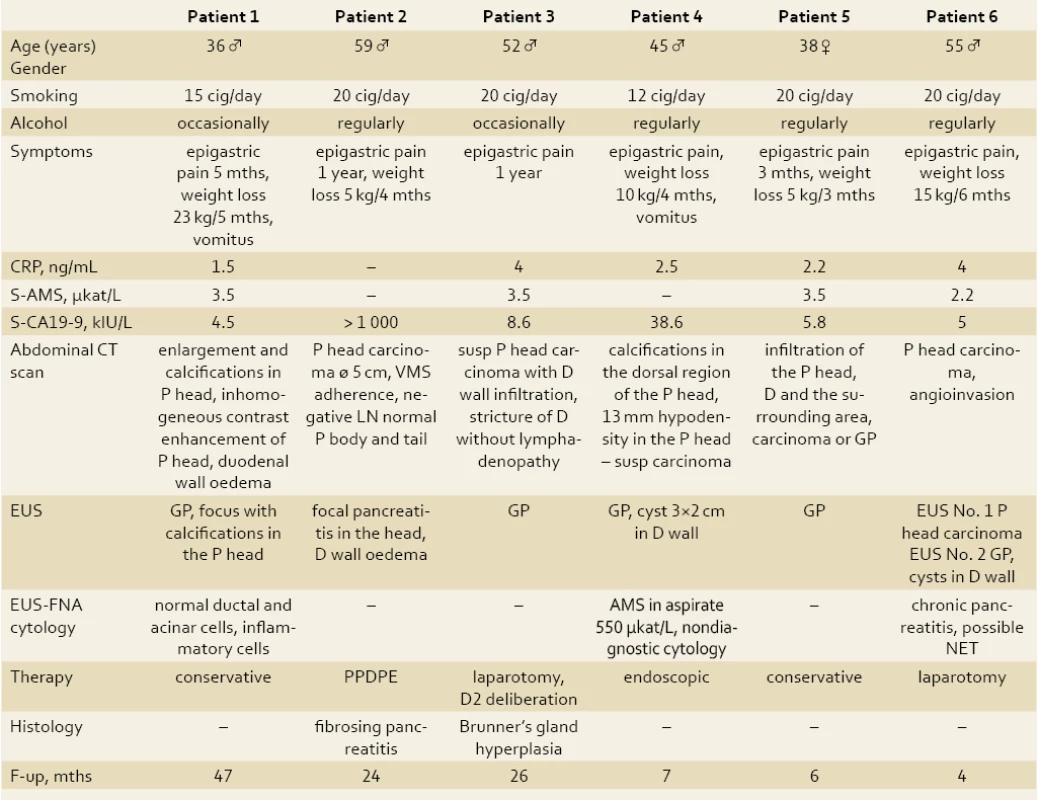
Abbreviations: P – pancreas, VMS – vena mesenterica superior, LN – lymph nodes, D – duodenum, NET – neuroendocrine tumor, S- AMS – serum amylase, CRP – C- reactive protein, F- up – follow‑up, mths – months, GP – groov pancreatitis, cig – cigarets, PPDPE – duodenopancreatectomy Fig. 1. CT scan of a patient with GP: suspected pancreatic head cancer (patient 2). Obr. 1. CT snímok pacienta s GP: vyslovené podozrenie na karcinóm hlavy pankreasu (pacient č. 2). 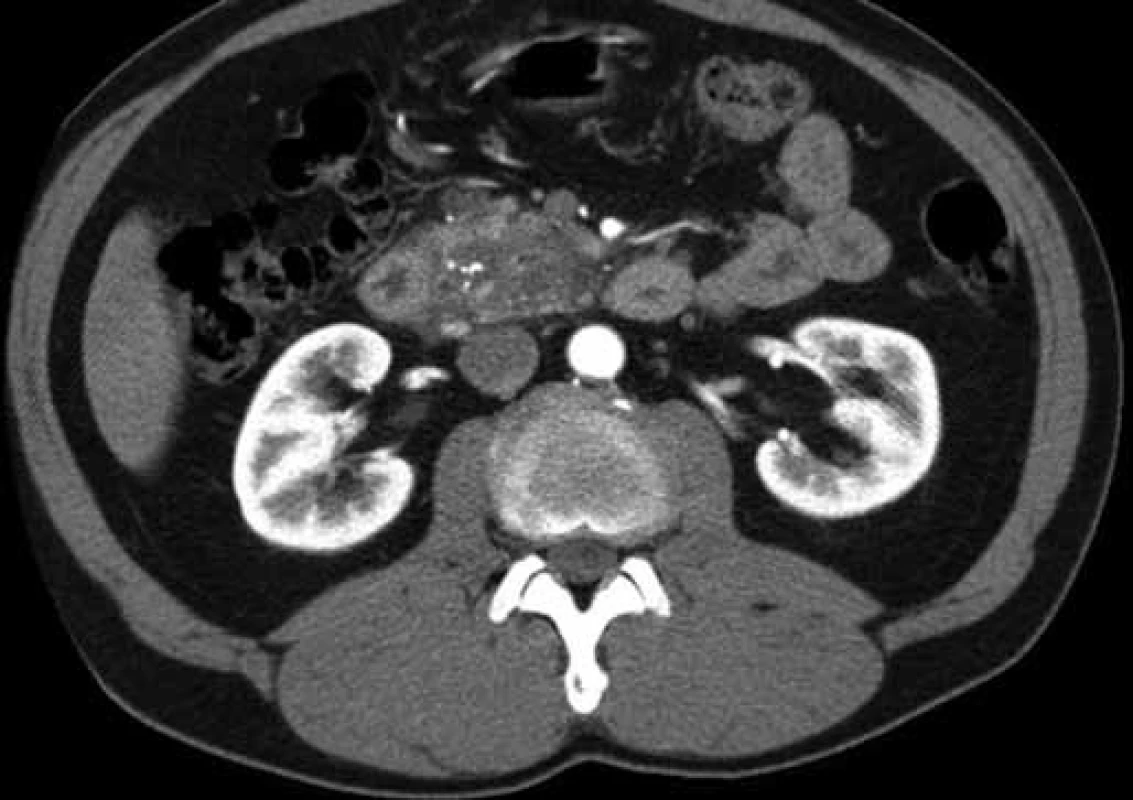
Fig. 2. CT finding of a focally changed pancreatic head in the ‚groove‘ region and the adjacent duodenal wall (patient 1). Obr. 2. CT obraz fokálne zmenenej hlavy pankreasu v oblasti „žliabku“ a priľahlej stene duodéna (pacient č. 1). 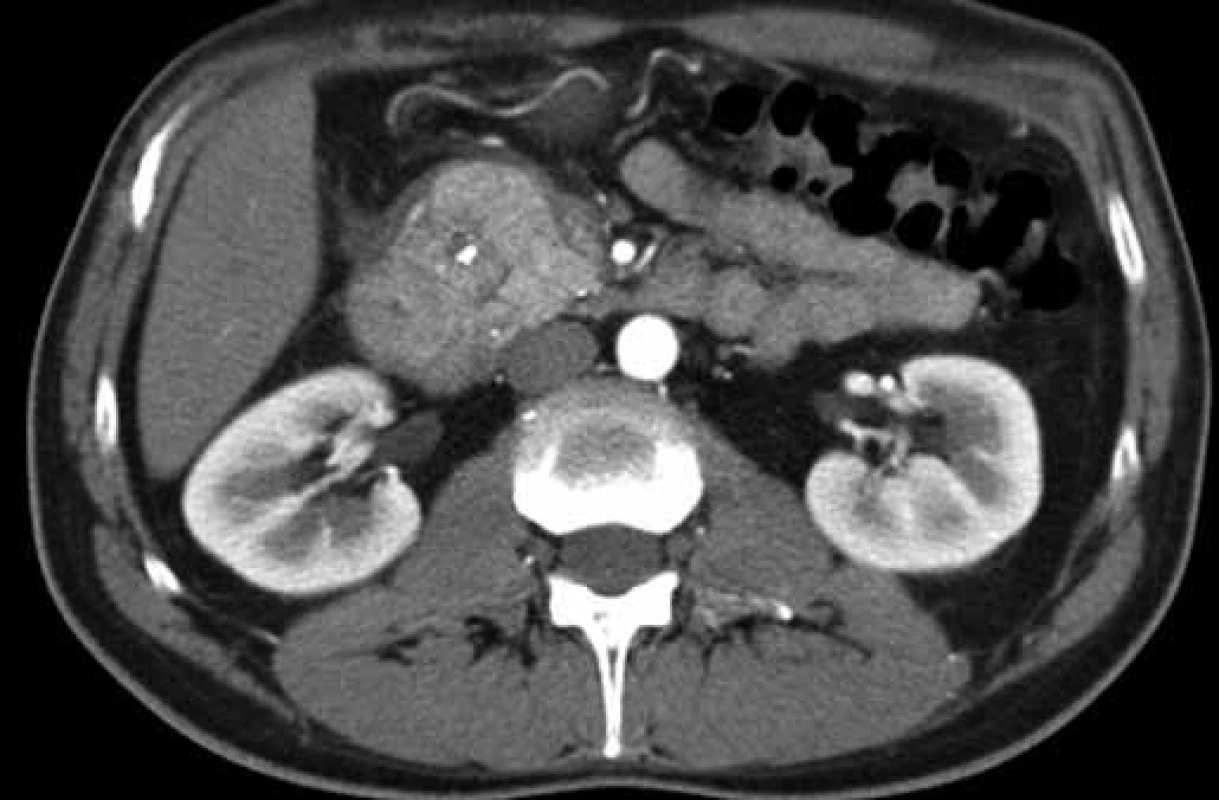
Fig. 3. EUS finding of GP: multiple calcifications in the ‚groove‘ and adjacent parenchyma of the pancreatic head (patient 1). Obr. 3. EUS obraz GP: viacpočetné kalcifikácie v „žliabku“ a naliehajúcom parenchýme hlavy pankreasu (pacient č. 1). 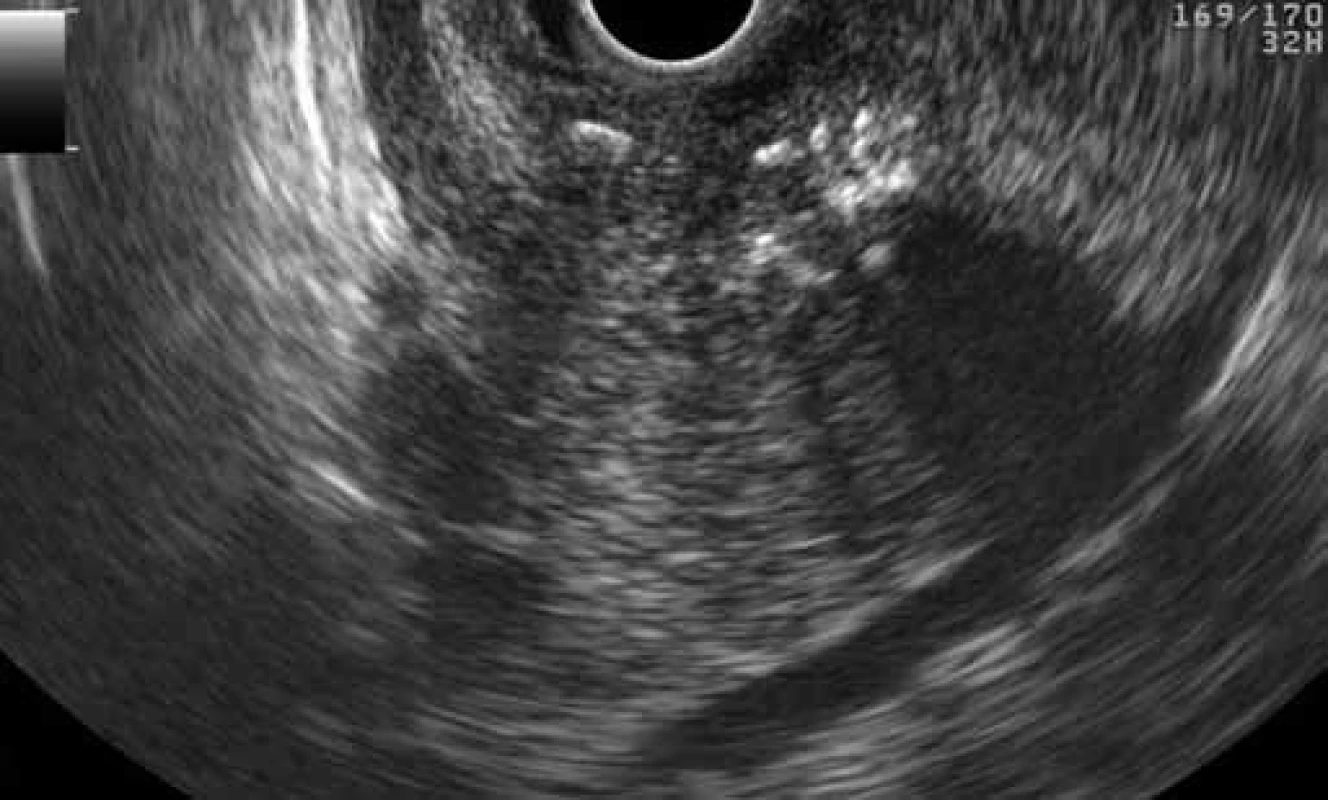
Fig. 4. Normal EUS finding of pancreatic body parenchyma with a nondilated main pancreatic duct in a patient with GP (patient 2). Obr. 4. Normálny EUS obraz parenchýmu tela pankreasu s nedilatovaným ductus Wirsungi u pacienta s GP (pacient č. 2). 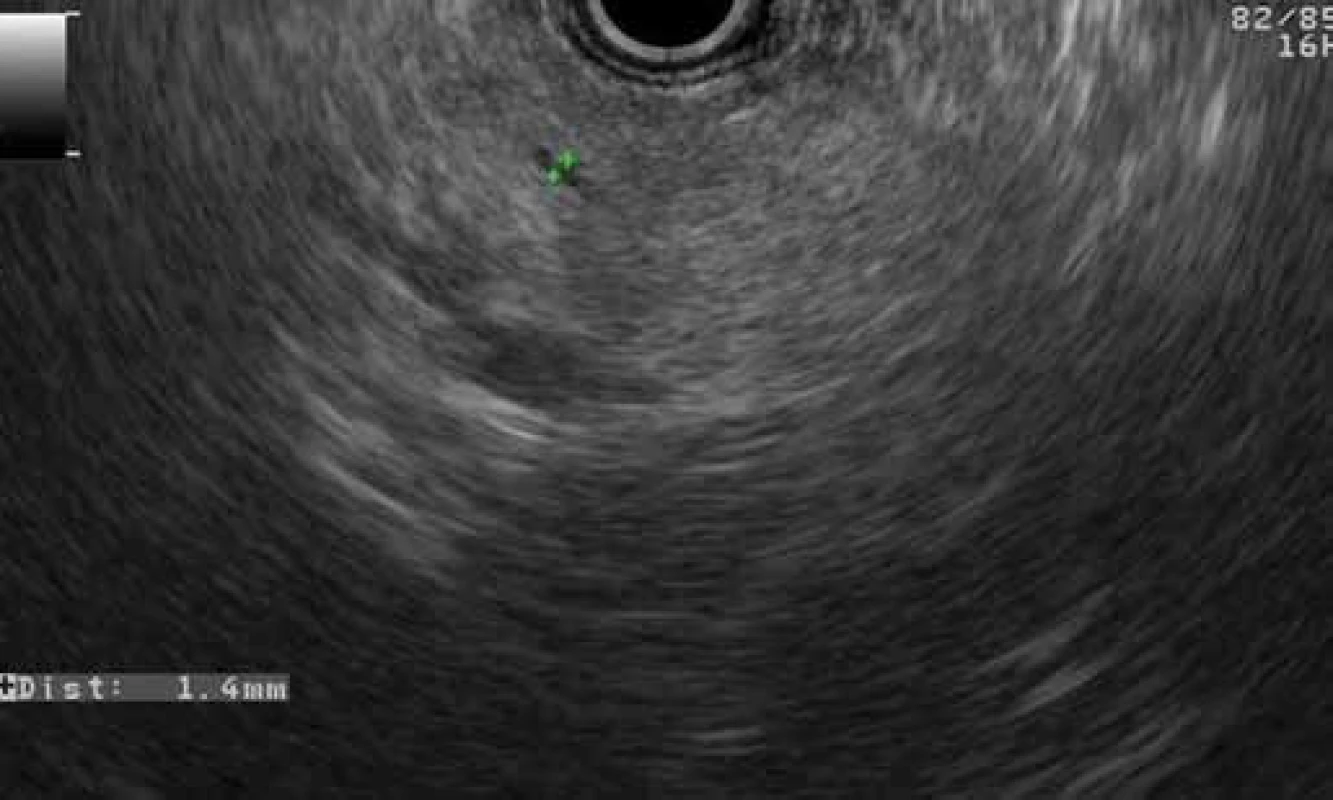
Fig. 5. EUS – groove pancreatitis: cyst in the duodenal wall (patient 4). Obr. 5. EUS – žliabková pankreatitída: cysta v stene duodéna (pacient č. 4). 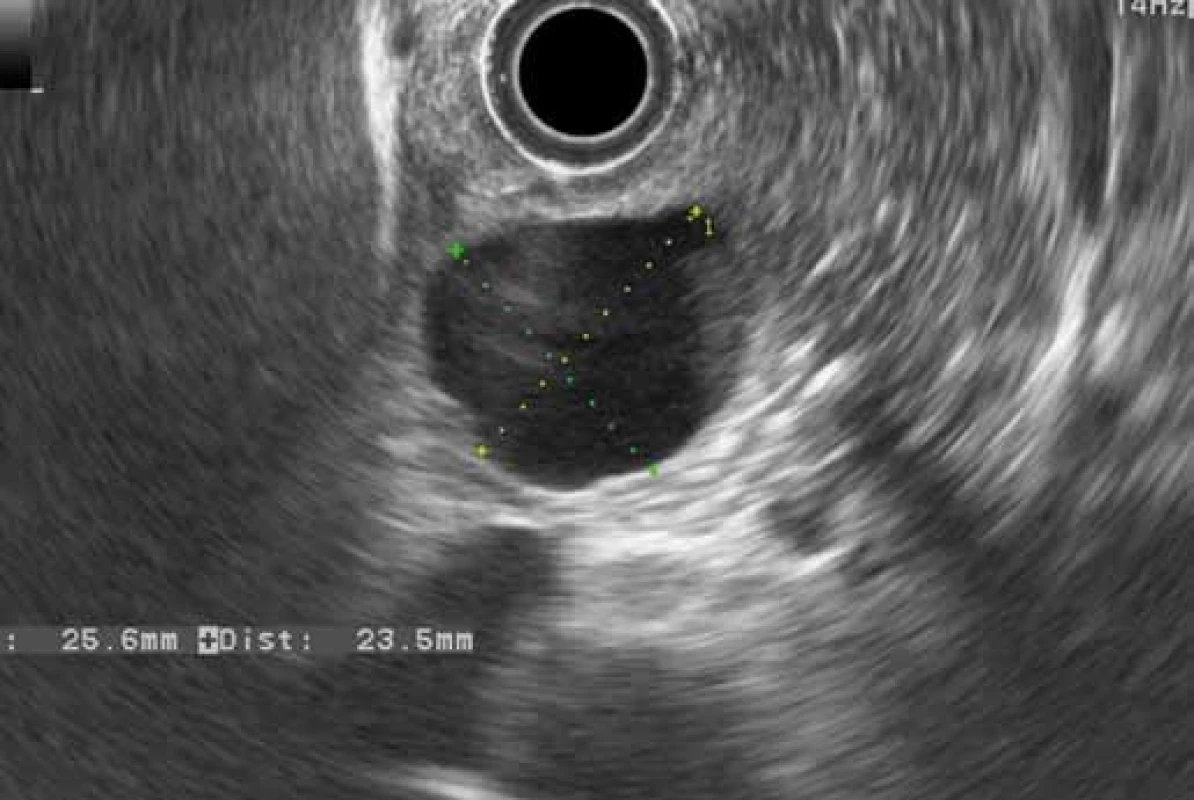
Fig. 6. EUS – cystography during EUS-CDS in a patient with GP with a cyst in the duodenal wall and a stricture of distal common bile duct, plastic stent in the common bile duct (patient 4). Obr. 6. EUS – cystografia počas EUS-CDS u pacienta s GP a cystou v stene duodéna a stenózou distálneho žlčovodu, plastikový stent v žlčovode (pacient č. 4). 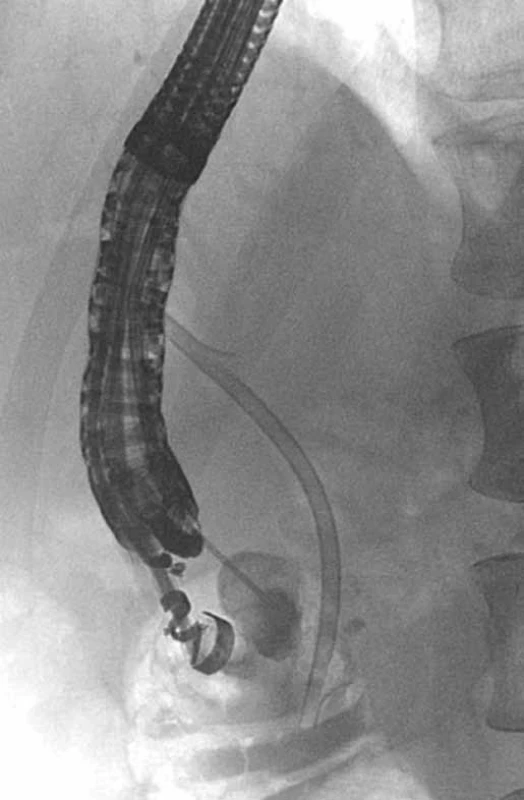
Discussion
Groove pancreatitis presents a considerable diagnostic problem, given that both its clinical picture and imaging examinations are very similar to pancreatic head carcinoma. The disease most commonly occurs in men aged 40 – 50 with a history of alcohol abuse and smoking. Its pathogenesis is unclear. The proposed predisposing factor for the development of GP is the incomplete involution of dorsal pancreas with embryonic remnants of pancreas glands present in the duodenal wall or possibly ectopic pancreas in the duodenal wall [5]. Chronicalcoholism as a potentiating factor may increase pancreatic juice viscosity, lead to Brunner’s gland hyperplasia and cause minor papilla occlusion or dysfunction [7,8]. The condition is often associated with pancreas divisum, a narrowing or absence of the duct of Santorini [9].
The most common clinical symptoms include abdominal pain and weight loss. In case of duodenal obstruction due to oedema of the duodenal wall, patients develop relapsing vomiting. Obstructive icterus is present if the pancreatic head is affected, with common bile duct stricture. Abdominal pain and weight loss was dominant in all patients in our series, with one patient loosing up to 23 kg, more suggestive of an advanced malignant condition rather than focal chronic pancreatitis.
Serum pancreatic enzymes activity is usually normal or slightly elevated, but never reaches levels as high as in acute pancreatitis. Tumor marker – carcinoembryonic antigen (CEA) and carbonic anhydrase 19 - 9 (CA 19 - 9) – levels usually fall within the normal range. Half of the patients in our set had elevated serum CA 19 - 9 levels, which in one patient reached the value of 1,000 – typical for pancreatobiliary malignancies.
Imaging techniques – contrast CT scans, magnetic resonance imaging (MRI) and EUS – are used to diagnose GP. The CT scan finding of the pure form of GP includes a hypodense lesion with delayed contrast enhancement between the duodenal wall and the pancreas near minor papilla, duodenal stricture with a widened duodenal wall, and a cyst in the duodenal wall or the adjacent part of the pancreatic head. Cysts may be small or large, and multilocular cystic ‚masses‘ also tend to occur. In the segmental form of GP, CT scan shows the presence of a hypodense mass in the pancreatic head near the duodenal wall, usually accompanied by the signs of advanced chronic pancreatitis with calcifications in the head around the minor papilla, stricture of the distal part of the pancreatic duct or common bile duct [3].
Distinguishing an adenocarcinoma of the pancreatic head in the groove area (groove adenocarcinoma) from GP is difficult. The absence of cysts in the duodenal wall and frequent sparing of the pancreatic duct in groove adenocarcinoma may be helpful in the diagnostic process [10]. Forceps biopsy from duodenal mucosa may contribute to a correct diagnosis but unless the groove adenocarcinoma invades the duodenal wall, establishing the correct diagnosis is a challenge. The finding of groove pancreatitis does not rule out a concomitant presence of adenocarcinoma. A markedly widened main pancreatic duct in combination with a mass in the groove area points to suspected groove adenocarcinoma. In contrast to adenocarcinoma, peripancreatic blood vessels are mostly intact in GP, without signs of thrombosis or infiltration. Despite advanced imaging techniques, it is often impossible to rule out carcinoma completely and unambiguously, and a large number of patients with GP undergo surgical resection [6]. In addition to carcinoma of the pancreas, common bile duct carcinoma and duodenal carcinoma must also be ruled out. Acute pancreatitis with periduodenal phlegmon or an acutely exacerbated chronic pancreatitis with a pseudocyst in the duodenal wall can also imitate the pure form of GP. In autoimmune pancreatitis, diffuse enlargement of the entire pancreas (‚sausage-like‘ pancreas) and hypodense capsule around the pancreas are often present, in contrast to GP. Cysts in the pancreas or the duodenal wall and isolated involvement of the ‘groove’ area do not occur in patients with autoimmune pancreatitis. In four out of five patients in our series, the diagnosis of suspected pancreatic head carcinoma was established on the basis of contrast CT examination and, consequently, three of the patients underwent surgical therapy.
In an endosonographic examination, GP manifests as a hypoechoic area between the duodenal wall and parenchyma of the pancreas (Fig. 7); in the cystic form, cysts are usually present in the groove or in the duodenal wall, while in the solid form there is usually a solid mass in the periampullary region of the pancreatic head, with focal calcifications near minor papilla. In order to arrive at a more specific diagnosis, EUS-FNA from a mass or a forceps biopsy from the macroscopically altered duodenal mucosa may be performed. When interpreting the results of cytology obtained from EUS-FNA, or the results of histology obtained from the forceps biopsy of the duodenum, it is important to bear in mind that a negative cytology or histology, or the presence of fibrosis in histology from forceps biopsy, does not rule out the presence of carcinoma (fibrosis may be the result of a desmoplastic reaction at the periphery of the carcinoma). In our set of patients, four out of five were diagnosed with GP on the basis of EUS examination. In patient six, pancreatic head carcinoma was suspected after the first EUS examination. Due to ambiguous cytology results (suspected NET), CT scan and the EUS finding of a mass in the pancreatic head, surgery was scheduled. However, findings obtained with laparotomy and intraoperative ultrasonography did not correspond with either carcinoma or NET, and resection was not performed. A control EUS examination four weeks after laparotomy showed the presence of the characteristic features of paraduodenal pancreatitis, including a cyst in the duodenal wall.
Fig. 7. EUS finding of GP: a hypoechoic area between the duodenal wall and pancreatic parenchyma with a cyst in the duodenal wall (patient 2). Obr. 7. EUS obraz GP: hypoechogénna oblasť medzi stenou duodéna a parenchýmom pankreasu s cystou v stene duodéna (pacient č. 2). 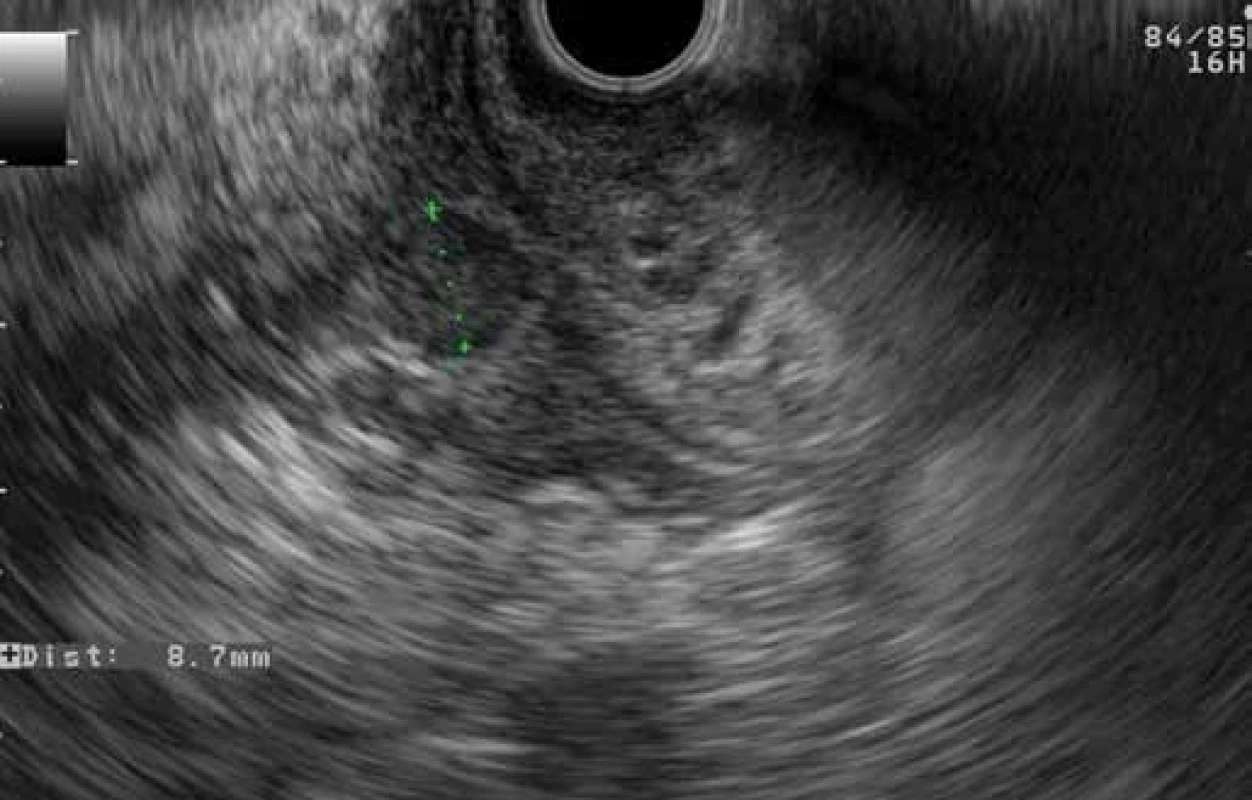
Distal common bile duct stricture, which may occur in some patients with GP, has a characteristic benign appearance in the cholangiogram, with regular smooth edges and a conical shape. Its correct interpretation may help to distinguish it from malignant common bile duct stricture in pancreatic head carcinoma.
The hypoechoic thickening of the duodenal wall, and the presence of one or more cysts in the duodenal wall or the groove, can be regarded as the most specific EUS sign of GP. Despite an EUS finding of GP, two more patients in our series underwent surgery, as pancreatic head carcinoma could not be ruled out completely (suspected CT finding, high serum CA19 - 9 levels).
Several approaches can be implemented in the treatment of GP, depending on the nature and the course of the disease. A successful treatment essentially requires the elimination of risk factors, i.e. alcohol abuse and smoking. Acute flare-ups of the disease are treated conservatively, in the same way as acute pancreatitis. The treatment consists of bed rest, discontinuation of intake per os, intravenous administration of crystalloid infusions, enteral or parenteral nutrition, and administration of analgesics. In our series of patients, patient one was successfully treated in this way. In cases of the much more common chronic relapsing course of the disease, which imitates the course of chronic pancreatitis, pharmacotherapy is used (analgesics, pancreatic enzymes if signs of exocrine insufficiency are present), along with endoscopic techniques for duodenal cyst drainage, transpapillary drainage in case of stricture of the duct of Wirsung or the duct of Santorini [11], or duodenobiliary drainage in case of common bile duct stricture. According to literary data, the success rate of endoscopic therapy is up to 70% [12].
The non-standard endoscopic transduodenal drainage of a cyst in the duodenal wall in patient four merits a more detailed comment. The treatment was indicated due to persisting abdominal pain; a stricture was present in the duodenum and after two EUS-guided aspirations the cyst relapsed. Reports on this treatment in global literature are limited. According to Arvanitakis et al [12], out of the total of 39 patients with paraduodenal pancreatitis who received endoscopic treatment, only 17 underwent endoscopic cystoduodenostomy as part of the initial treatment, and another four as part of retreatment due to relapsing symptoms. The authors do not specify whether the cystoduodenostomy involved a cyst in the duodenum or the pancreatic head, but given the number of patients diagnosed with one (n = 17) or more (n = 9) cysts in the duodenal wall, it may be inferred that they were referring to duodenal cysts. The treated cysts included cysts < 3cm in size, which is similar to our case [12].
Surgical therapy is indicated when conservative or endoscopic therapy is unsuccessful, or when carcinoma cannot be reliably ruled out.
In our series of patients, 50% underwent surgery, one patient (16.7%) received endoscopic treatment, and two (33.3%) were treated conservatively. Due to the limited number of papers published, the small number of patients in cohorts, and the retrospective nature of studies, it is difficult to evaluate the share of various therapies in the management of patients with GP. In the largest retrospective study published to date of 51 symptomatic, radiologically diagnosed GP patients, 19 (37.3%) initially received conservative pharmacological treatment, 39 (76.5%) initially received only endoscopic treatment in combination with pharmacotherapy, and three (5.9%) underwent urgery [12].
Conclusion
GP is a special form of a focal chronic pancreatitis affecting the anatomical region of the ‚groove‘ between the pancreatic head, duodenal wall and common bile duct. Both its clinical picture and imaging studies are very similar to pancreatic head carcinoma, making it difficult to diagnose. A contrast CT scan is not accurate enough to diagnose GP, and in our ground of patients only 17% were diagnosed correctly based on the CT scans. Endosonography is of significant help in the diagnostic process, and the presence of one or more cysts in the duodenal wall or in the groove, together with a hypoechoic thickening of the groove and adjacent duodenal wall, can be regarded as the most specific EUS signs of GP.
Depending on the patient‘s clinical conditions and on how reliably the condition was diagnosed, GP management involves conservative approach, endoscopic therapy, or surgical procedures. Even with sufficient knowledge of thediagnosis, half of the patients in our set underwent surgery because carcinoma could not be reliably ruled out. In order to be diagnosed correctly, the condition requires not just the availability of all imaging methods, including the EUS, but also sufficient awareness by the gastroenterologists, radiologists and surgeons of this clinical entity.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 11. 1. 2015
Accepted: 6. 4. 2015
MUDr. Martin Kliment, Ph.D.
Digestive Disease Center
Hospital Vítkovice, Ostrava
Zalužanského 1192/15 703 84 Ostrava-Vítkovice
martin.kliment@nemvitkovice.cz
Zdroje
1. Becker V. Bauchspeicheldrüse. In: Doerr W,Seifert G, Thlinger E (eds). Spezielle pathologische Anatomie. 4th ed. Berlin/ Heidelberg/ New York: Springer 1973 : 252 – 445.
2. Stolte M, Weiss W, Volkholz H et al. A special form of segmental pancreatitis: „groove pancreatitis“. Hepatogastroenterology 1982; 29(5): 198 – 208.
3. Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol 1991; 10(3 – 4): 173 – 182.
4. Fléjou JF, Potet F, Molas G et al. Cystic dystrophy of the gastric and duodenal wall developing in heterotopic pancreas: an unrecognized entity. Gut 1993; 334(3): 343 – 347.
5. Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying „cystic dystrophy of heterotopic pancreas“, „para-duodenal wall cyst“ and „groove pancreatitis“. Sem Diagn Pathol 2004; 21(4): 247 – 254.
6. Yamaguchi K, Tanaka M. Groove pancreatitis masquerading as pancreatic carcinoma. Am J Surg 1992; 163(3): 312 – 316.
7. Chatelai, D, Vibert E, Yzet T et al. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas 2005; 30(4): e92 – e95.
8. Malde DJ, Oliveira-Cunha M, Smith AM. Pancreatic carcinoma masquerading as groove pancreatitis: case report and review of literature. JOP 2011; 12(6): 598 – 602.
9. Shudo R, Obara T, Tanno S et al. Segmental groove pancreatitis accompanied by protein plugs in Santorini‘s duct. J Gastroenterol 1998; 33(2): 289 – 294.
10. Gabata T, Kadoya M, Terayama N et al. Groove pancreatic carcinomas: radiological and pathological findings. Eur Radiol 2003; 13(7): 1679 – 1684.
11. Isayama H, Kawabe T, Komatsu Y et al. Successful treatment for groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc 2005; 61(1): 175 – 178.
12. Arvanitakis M, Rigaux J, Toussaint E et al. Endotherapy for paraduodenal pancreatitis: a large retrospective case series. Endoscopy 2014(7); 46 : 580 – 587. doi: 10.1055/ s-0034-1365719.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2015 Číslo 3- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Digestivní endoskopie
- Kvíz
- Žliabková (paraduodenálna) pankreatitída – séria šiestich pacientov
- Torpidní průběh herpetické ezofagitidy u imunokompetentní pacientky – videokazuistika
- Biliární papilomatóza – raritní příčina obstrukčního ikteru
- 37. slovenské a české endoskopické dni Trnava, 28.– 29. máj 2015
- Doporučené postupy chirurgické léčby pacientů s idiopatickými střevními záněty – 2. část: Crohnova nemoc
- Budd-Chiariho syndrom u těhotné pacientky s Crohnovou chorobou
- Dysfunkce dolní části gastrointestinálního traktu u kriticky nemocných – současný pohled
- Těžký průběh kortikodependentní ulcerózní kolitidy u malého dítěte před érou biologické terapie
- Variabilita testů na okultní krvácení používaných praktickými lékaři ve screeningu kolorektálního karcinomu v České republice
- Správná odpověď na kvíz
- Zpráva o činnosti výboru Endoskopické sekce ČGS ČLS JEP za období 2011– 2015
- Udělení Kasafírkovy ceny za rok 2015
-
Cena pro nejlepší monografii v oboru gastroenterologie publikovanou v roce 2014
Cena pro nejlepší publikaci (pro autora do 35 let) publikovanou v roce 2014 - Trombotická mikroangiopatie z pohledu nefrologa
- Saccharomyces boulardii – probiotická kvasinka z Indočíny
- Autodidaktický test: digestivní endoskopie
- SOUTĚŽ O NEJLEPŠÍ KAZUISTIKU 2015
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Saccharomyces boulardii – probiotická kvasinka z Indočíny
- Žliabková (paraduodenálna) pankreatitída – séria šiestich pacientov
- Doporučené postupy chirurgické léčby pacientů s idiopatickými střevními záněty – 2. část: Crohnova nemoc
- Trombotická mikroangiopatie z pohledu nefrologa
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



