-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhysiological factors with impact on the drug behaviour in the gastrointestinal tract
Fyziologické faktory ovlivňující osud léčiva v gastrointestinálním traktu
Lék při perorálním podání prochází gastrointestinálním traktem (GIT), který ovlivňuje jeho další osud v organismu. Jde-li o systémové podání, léčivo se zde uvolňuje z lékové formy, rozpouští se a nakonec vstřebává, případně se jeho zbytky vylučují stolicí. Hlavními faktory, které ovlivňují podaný lék, jsou zejména hodnota pH, doba pasáže, solubilizační schopnost nebo oxido-redukční potenciál v jednotlivých částech GIT. Tyto faktory souvisejí přímo s uvolňováním, vstřebáváním eventuálně stabilitou léčiva a lze je využít v prostředí in vitro k simulaci prostředí GIT a k celkovému designu lékové formy in vivo. Jelikož některé literární údaje nebývají uvedeny v souvislostech a navíc se často liší, tato práce shromažďuje základní hodnoty výše zmiňovaných fyziologických parametrů ve formě přehledového článku.
Klíčová slova:
lék, trávení, vstřebávání, pH, GIT, motilita, trávící enzymy
Authors: Aleš Franc; Kateřina Dvořáčková; Martina Kejdušová; Roman Goněc
Authors place of work: Veterinary and Pharmaceutical University Brno ; Department of Pharmaceutics, Faculty of Pharmacy
Published in the journal: Čes. slov. Farm., 2013; 62, 243-248
Category: Přehledy a odborná sdělení
Summary
Orally administered drugs are passed through the gastrointestinal tract (GIT), which influences their next metabolism in the body. In the case of systemic administration, the drug is released from the dosage form, is dissolved and eventually absorbed. The residual amount is excreted in the faeces. The main factors influencing administered drugs are particularly pH, passage time, solubilizers or the oxido-reductive potential in different parts of the GIT. These factors are directly related to the release, absorption and stability of drugs. They can be used for simulation of the GIT environment in vitro and for the overall design of the dosage form in vivo. Because some literature data are not given in context and sometimes they are contradictory, this paper summarizes elementary values of the above-mentioned physiological parameters in the form of a review.
Keywords:
drug, digestion, absorption, pH, GIT, motility, digestive enzymesIntroduction
The gastro-intestinal tract has significant impact on the general fate of the drug in the human organism on its oral administration. The most significant physical-chemical parameters are pH, viscosity, ionic strength, and hydrophilic-lipophilic balance of digestive juices within particular parts of the GIT, i.e. the mouth, oesophagus, stomach, small intestine, colon and rectum. Further important parameters include the volume of these juices, passage time in particular GIT segments and the intensity of stomach and bowel peristaltics. Other biological and biochemical factors are also involved, depending on the amount and ratio of digestive enzymes, possible resorption area of particular organs, and species and amount of micro-organisms that inhabit the GIT and take part in the digestive process. All these factors contribute to the complex process which is influenced to a varying extent by many other variables, e.g. age, anamnesis, gender, quality and quantity of food, geography, race, etc. The most important factors and values concerning the GIT are summarized in Table 1. All these factors have to be taken into account by all professionals, medicinal chemists and technologists developing the active substance and dosage form, as well as medical practitioners prescribing the medicine, nurses administering the drug or pharmacists instructing the patient. Specific physiological and anatomical properties of particular GIT organs are not the subject of this paper.
Tab. 1. Transit times of particular immediate release dosage forms in selected segments of the GIT 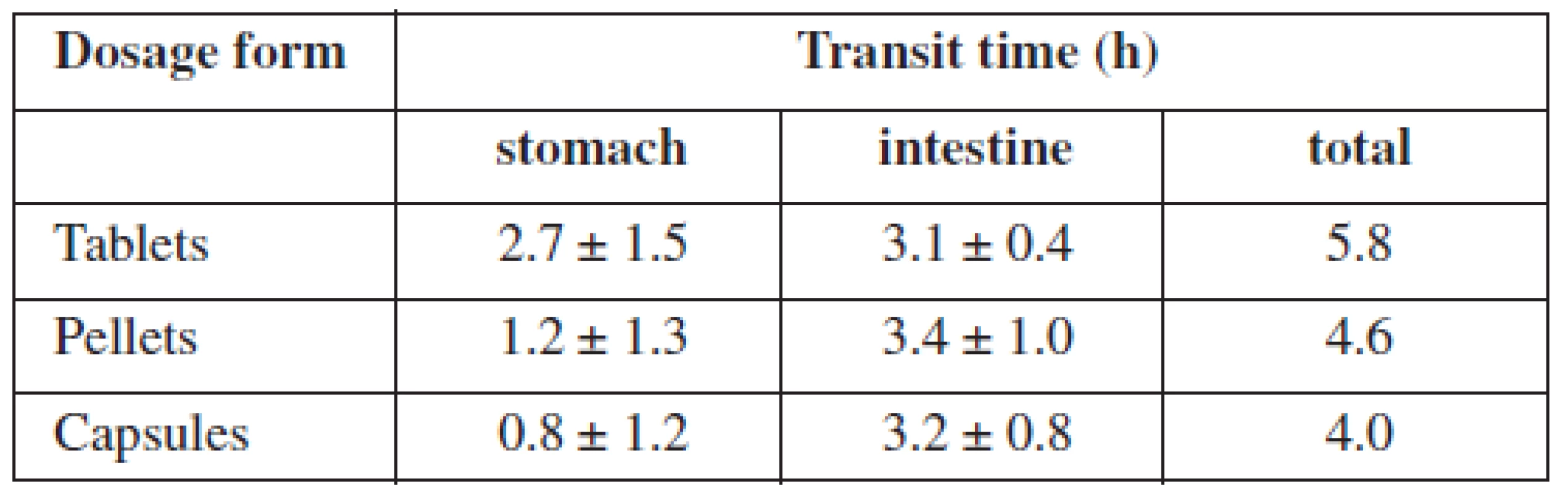
Significant factors
pH value
The first liquid the drug comes in contact with is saliva. It has neutral pH, about 7, usually given as 6.5–8. Slightly lower values were found in patients with diabetes1). The following passage through the stomach and bowels is characterized by pH values being dependent on the composition and amount of the ingested food, health status of the patient and the distance between the drug and the mucosa. Therefore, the individual parameters found in the literature can reach a whole range of values. General agreement claims that pH in the stomach ranges from 1.2 to 5.0, with higher values for the state immediately after eating. Significantly higher values were found in people of Japanese origin2). The pH value in the small intestine (duodenum, ileum, and jejunum) is 4.5–7.5 and in the large intestine, 5.5–8.03). The decrease in pH in the direction of the colon is caused by acids produced by colonic bacteria. A significantly lower pH in the colon was found in patients with non-specific bowel inflammatory disease. Because pH value is influenced by many factors within particular parts of the GIT, there is relatively large variability in the population4). Low pH value in the stomach is caused by the production of hydrochloric acid; on the other hand, high pH in the intestine is caused by hydrogen carbonate anions that protect the intestinal mucosa against the aggressive acidic chyme flowing from the stomach. Hydrogen carbonate ions create pH that is suitable for the activity of intestinal and pancreatic enzymes. In the colon, alkaline secret is formed, containing water, ions and mucus5).
With the exception of specific absorption mechanisms, each active substance has to dissolve prior to the absorption. The pH value is therefore very important in active substances that are administered as salts whose solubility depends on the pH value of the environment. The salts of weak acids and strong bases (e.g. NSAIDs) are more soluble in acidic environment because of dissociation. On the contrary, the salts of weak bases and strong acids (e.g. various alkaloids or amines) are more soluble in alkaline environment. Generally, the active substance is better absorbed from the environment in which it is better soluble6). However, it should be noted that drugs which are salts of strong bases can react with hydrochloric acid and get neutralized, resulting in such a form of substance which is less soluble, e.g. tablets containing warfarin sodium have significantly lower dissolution in acidic environment7).
Dissolution methods used both in formulation and quality control of drugs reflect actual pH values within the GIT. FDA guideline that regulates the production transfer or production changes of immediate release dosage forms lists pH range of 1.2–6.8, recommending not to exceed 88). Four individual pH values representing particular parts of the GIT are suggested: 1.2, 4.5, 6.8, and 7.59).
The solubility is not the only criterion. Active substances which are not stable in acidic environment (e.g. proton pump inhibitors) or should act locally in distal parts of the GIT have to be protected by an enterosolvent coat which usually consists of esters of polymeric organic acids insoluble in acidic environment10). If the dosage form which releases the drug with a constant liberation rate is required, neutral polymers are used as excipients. Thus the liberation of the active substance is slowed down independently of pH value. Ethylcellulose or polyacrylic polymers are frequently used11). If the active substance is poorly soluble in a particular GIT segment, the solubility can be improved by adding a pH modifier directly to the dosage form (e.g. tablet or pellet)12).
Digestive enzymes
All GIT fluids contain enzymes; however, their concentration and importance differ. The saliva contains α-amylase that splits polysaccharides, mainly starch5). Furthermore, there are lipases that are essential for lipid digestion in babies and some phosphatases and amidases of little importance. Lysozymes have antibacterial function because they are able to split glycosidic bonds in bacterial wall peptidoglycane13). In the stomach, the proteins are split by gastric proteases; the most important of which is pepsin. Chymosin coagulates milk proteins and urease is used to hydrolyse urea. Pepsin decomposes proteins to peptides or individual amino acids. Lipase, amylase and gelatinase are also present14). Pancreatic juice brings a whole range of enzymes to the small intestine. Trypsin, chymotrypsin, and carboxypeptidase split proteins to shorter chains, respectively end amino acids, elastase specialises in elastin. Lipase splits lipids, cholesterol-esterase hydrolyses cholesterol esters and phospholipase decomposes phospholipids. Last but not least, amylase splits surviving polysaccharides and ribonuclease and deoxyribonuclease break apart nucleic acids5). The small intestine produces enzymes by itself, too: lactase, saccharase, maltase and α-dextrinase decompose particular disaccharides15).
Digestive enzymes can play a significant role in decomposition of excipients that constitute the dosage form. Oils that have been more and more used to form “lipidic formulations” of poorly soluble drugs differ in their inclination to be digested by particular lipases, resulting in different drug liberation16). For this reason, the use of mineral oils is limited – mostly they can not be decomposed by enzymes. The impact of enzymes is significant in quality control as well. Dissolution medium for drugs formulated in gelatinous capsules should contain pepsin because gelatinous capsules do not dissolve easily in purely inorganic medium, on storage their solubility decreases even more. During dissolution, pellicules of gelatinous matter are formed and entrap the drug. This is easily prevented by the addition of pepsin17). During the development of the dosage form attention has to be paid to the fact that some excipients, e.g. saccharides, are digested whereas some, e.g. polymers or inorganic salts, remain unchanged. This can be convenient when formulating polymer matrix tablets where an addition of lactose ensures that the matrix eventually disintegrates to smaller parts because of combined forces of dissolution and digestion. Repeated administration does not cause any mechanic obstruction of narrow parts of the GIT, e.g. the ileum18). Whether macromolecules can be absorbed without their decomposition and reach systemic efficient blood levels without being deactivated by serum enzymes remains unclear and the question of oral administration of proteases is questionable19).
Solubilizers
The gastrointestinal tract contains substances that possess solubilizing properties, the most important being bile acids and their salts. Primary non-conjugated bile acids include cholic acid and chenodeoxycholic acid, conjugated bile acids include taurocholic acid and glycocholic acid. These acids help to solubilize lipids either by themselves or together with cholesterol and lecithin form bile micelles, in which lipophilic substances dissolve easily20). There are also secondary bile acids produced by colonic bacteria from primary bile acids, e.g. deoxycholic acid and lithocholic acid. Their ratio is significant in the diagnosis of colorectal carcinoma21). Pharmaceutical technology lists bile acids among true o/w anionic emulsifiers. They are able to emulsify lipids containing long chain fatty acids that are otherwise poorly absorbable22). A solubilizer by itself and a precursor of bile acids, hormones, and vitamin D synthesis, cholesterol is produced by the liver and excreted in the bile; however, exogenous cholesterol from food is important, too. Cholesterol is a true non-ionic o/w tenside23). Detailed description of cholesterol and its derivatives is not necessary for the purpose of this paper and can be found elsewhere. Lecithin is also an emulsifier, helping to form micelles containing emulsified lipids. Similarly to cholesterol, lecithin is found both in bile and food. A true amphoteric tenside able to form both o/w and w/o emulsions, lecithin was found to increase the bioavailability of some drugs24).
The importance of these substances for drug solubility and absorption was used by pharmaceutical technology, where they are used to formulate oral dosage forms. The impact of bile acids on drug liberation is convenient as well as the possibility to use lecithin to build liposome walls. The use of liposomes in oral dosage forms has increased lately25). Specific dissolution media, called bio-relevant, have appeared, helping to simulate the impact of digestive process on the liberation of poorly soluble substances26).
Bacterial microflora
Various parts of the gastrointestinal tract are colonised by numerous species of bacteria. This factor has to be taken into account. Bacteria in the oral cavity do not contribute to the digestive process. Similarly, bacteria in the stomach are not important from this point of view. As the pH value increases, the environment becomes more bacteria-friendly, towards the end of the small intestine Lactobacillus, Enterococcus, Bacteroides, Escherichia coli, and Candida can be found. In the distal ileum, there are more G-negative bacteria than G-positive bacteria. The large intestine is populated to a high degree, the microorganisms amounting up to 8% of faeces weight. More than 400 species were identified, 99% of them are anaerobic. Colonic bacteria metabolise saccharides that were not metabolised in the small intestine and produce short chain fatty acids. The most frequently bacteria belong to Escherichia coli, Bifidobacterium, Lactobacillus, Clostridium, Bacteroides, and Streptococcus species. Candides, such as Candida albicans, Candida parapsilosis, and Candida glabrata, inhabit the colon, too. Bacteria produce also many hydrolytic and reductive enzymes27): azoreductase, β-glucuronidase, β-xylosidase, dextranase, esterase, nitroreductase, α-arabinosidase, β-galactosidase, and deaminase28).
These enzymes are beginning to be used for colon-targeted drugs. Peptides, antiphlogistics or cytostatics to treat colon - and rectum-located diseases could profit from these locally available enzymes29). These drugs are formulated using specific polymers that are resistant to proximal GIT enzymes but decompose through bacterial enzyme hydrolysis and reduction. Consisting mainly of polysaccharides and/or azo-polymers, these excipients can be used to constitute both the matrix system and functional coating of the dosage forms30). When testing the drugs, some progressive dissolution methods evaluating colon-targeted administration in vitro use specific colonic bacteria31).
Passage time
Although passage time of food, respectively of the drug, is individual, and depends on age, health status, physiognomy, and food, there are some general prerequisites. It can be said that the passage through the stomach takes 1–5 hours on average; food containing mainly saccharides stays for 2–3 hours, food containing mainly fat can stay for as long as 7 hours. The passage through the complete small intestine covers 3–8 hours32) and the passage through the large intestine lasts approximately 11–72 hours33). By summing up all these data, we can see that the total passage time ranges from 15 to 84 hours. Out of these values, the passage time through the small intestine is the most stable and relatively unlikely to be influenced by external factors. The most frequent small intestine passage time is 3–4 hours4). Three hours correspond with the passage time of multiple dosage forms (e.g. pellets), 4 hours correspond with single dosage forms (e.g. tablets, capsules)34). However, other sources claim that there is no difference between these sorts of dosage forms35) (Table 2).
Tab. 2. Summary of selected parameters of particular segments of GIT 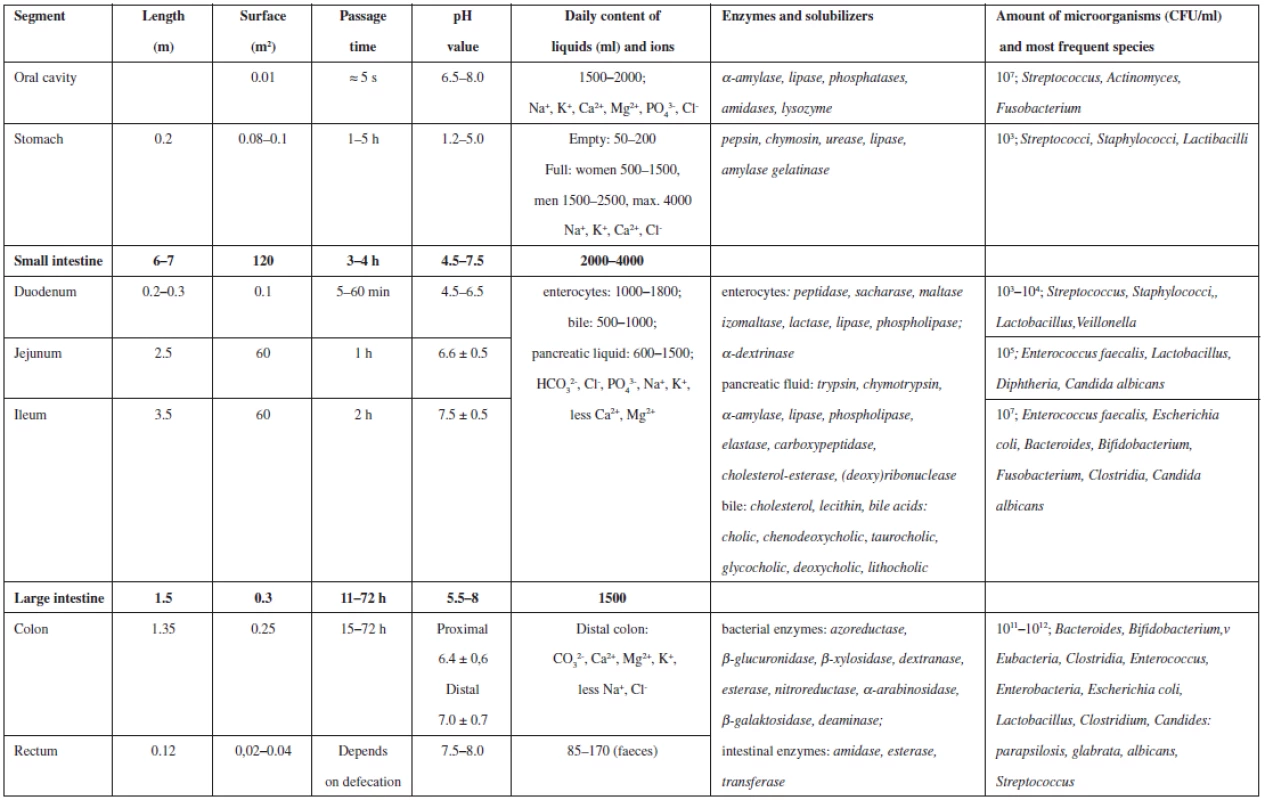
These passage times together with differing pH values throughout the GIT are important for any pharmaceutical technologist who is going to develop controlled release dosage form. He or she has to choose such technology and excipients that correspond to these factors. The position, environment pH value and enzymes are ever-changing variables. The knowledge of them is necessary for successful in vitro drug evaluation, e.g. the limit of the dissolution method for acid-resistant dosage forms, using simulated intestinal fluid, is usually not more than 10% of the active substance released in 120 minutes, then the dissolution medium is changed, pH value increased, usually to 6.8, other conditions can be changed as well36). There are several other methods that combine time, pH value and enzymes to simulate the specific conditions of the GIT. One of them suggests that colon-targeted drug should release not more than 5% of the active substance at pH of 1.2 in 2 hours, then not more than 60% of the active substance at pH of 6.8 in 6 hours and finally not less than 85% of the active substance at pH of 7.2 in 12 hours37).
Amount of fluid
In the oral cavity, the daily production of saliva amounts to 1.5–2 l; nevertheless, any actual amount is insignificant. The volume of gastric fluid of the empty stomach is only about 200 ml; that is the reason why any orally taken medication should be swallowed with 200 ml of water to improve the disintegration. Daily production of gastric fluid amounts to 0.5–2.5 l. The amount of fluid in the small intestine is not constant and the daily total is about 2–4 l. The large intestine receives daily about 1.5 of fluid from the small intestine and concentrates it with the help of bacteria to 85–170 ml of daily faeces. The rate of this concentration may depend on drugs, e.g. laxatives38).
With respect to the volume of administered dosage form, the volume of particular GIT organs is relatively high; therefore the position of the drug in the GIT is very important. Due to different density or adhesiveness, the drug can stick to the mucosa or float on the fluid surface. Such properties are sometimes suitable, as in mucoadhesive oral tablets39) or “floating” capsules40) with prolonged gastric passage time, but usually undesirable and avoided. The literature shows that if the same dose of the drug is divided in more parts, e.g. pellets, their distribution within GIT organs is more even and the absorption less varied41). The volume of particular GIT organs is reflected also in quality control. In vitro dissolution testing methods usually involve one of three most frequent volumes – 500, 900, or 1000 ml8). 200 ml is sometimes used for buccal tablets42).
Motility
Gastric and intestinal motility is the last of the most important factors. Motility has similar impact as the mixing intensity on the character of mixed dispersions. Particular organs use their movement to mix the food and this has impact on the liberation of the active substance, indeed. The most intensive mixing and mechanic destruction occurs already in the oral cavity. However, most drugs are swallowed intact, so this is insignificant. The transport through the oesophagus is too short, but in the stomach very intensive mixing follows. If the stomach is empty, its walls adhere partially, but when full, the inner space increases. Due to anatomical features of gastric muscles, the mixing is caused by four intensive concomitant contractions. Ring peristaltic wave is formed, going from the cardia to the pylorus. 2–4 of these contractions occur per minute and their strength grows with the load. The chyme is ejected in small amounts through the pylorus to the duodenum. Thin fluids only flow through the stomach. Bowel peristaltics is not so strong, it consists of movements that move and mix the chyme, which moves with the velocity of about 1 cm per second. The movement is caused by 3–5 cm long peristaltic waves moving with the speed of 0.5–2 cm per second. The movements are more intensive after meals because the stomach and duodenum are distended (gastrointestinal reflux). Spontaneous movements occur as well, they are frequent in the duodenum, peaking at 11–13 movements per minute, the frequency decreases towards the ileum5). The movements of the colon are even sparser. The contractions occur after several minutes in adjacent areas and last about 1 minute, moving slowly the chyme onwards. After meals, propulsive, i.e. more intense movement occurs with 2–3 minutes lasting repeating ring movements. The motility within the colon is different. In colon ascendens, mixing movements prevail and the chyme moves at the velocity of 5 cm per hour, respectively accelerates to 10 cm per hour after meals43).
Gastrointestinal movements are important in the evaluation of active substance liberation. The simplest and non-selective method is the disintegration method of solid dosage forms, supposing that the dissolution of the active substance in the test fluid is directly proportional to the disintegration time that is measured. This method is harmonized in major pharmacopoeias and it is based on mechanised repeated vertical movement of the dosage form in a liquid, mostly in water or simulated gastric fluid. The vertical movement imitates gastric peristaltics. Dosage form has to disintegrate from 15 to 60 minutes, depending on type44). Dissolution methods actually replace the previous method. US regulations demands paddles at 50 rpm for tablets and baskets at 100 rpm for capsules8). Oral suspensions are tested by paddles at 25 rpm only42). Suitable simulated dissolution methods have been sought to account for specific motility of particular parts of the GIT following the path of the drug as far as the colon31).
Conclusion
Gastrointestinal tract organs are the first that the orally administered active substance encounters. The knowledge of their physiology from the point of view of interaction with particular drugs including the ability to predict their behaviour because of their physical and chemical characteristics is essential for any pharmacist. Such knowledge is crucial for the research and development, clinical evaluation, quality control, and dispensation. The paper briefly summarizes some elementary factors, their relation to selected pharmaceutical aspects, and suggests detailed references.
Conflicts of interest: none.
Received 13 September 2013
Accepted 17 October 2013
A. Franc • doc. PharmDr. Kateřina Dvořáčková, Ph.D. (∗) • M. Kejdušová
Department of Pharmaceutics, Faculty of Pharmacy
Veterinary and Pharmaceutical University Brno
Palackého 1/3, 612 42 Brno, Czech Republic
e-mail: dvorackovak@seznam.cz
R. Goněc
Pharmacy, Masaryk Memorial Cancer Institute, Brno, Czech Republic
Zdroje
1. Goyal P., Kaur H., Jawanda M. K., Verma S., Parhar S. Salivary pH and dental caries in diabetes mellitus. Int. J. Oral. Max. Pathol. 2012; 4, 13–16.
2. Mitra A., Kesisoglou F. Impaired drug absorption due to high stomach ph: a review of strategies for mitigation of such effect to enable pharmaceutical product development. http:// pubs.acs.org/doi/ipdf/10.1021/mp400256h (22. 7. 20013).
3. Dvořáčková K., Bautzová T., Rabišková M. Dissolution study in the evaluation of oral preparations with controlled drug release. Chem. Listy 2011; 105, 50–54.
4. McConnell E. L., Fadda H. M., Basit A. W. Gut instincts: explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008; 364, 213–226.
5. Trojan S. Lékařská fyziologie, 4. vyd. Praha: Grada Publishing 2003.
6. Komárek P., Rabišková M., et al. Technologie léků: galenika. 3. vyd. Praha: Galén 2006.
7. McCormick T. J., Gibson A. B., Diana F. J. Development and validation of a dissolution method for warfarin sodium and aspirin combination tablets. J. Pharmaceut. Biomed. 1997; 15, 1881–1891.
8. Dissolution testing of immediate release solid oral dosage forms: Guidance for Industry. CDER 1997. http://www.fda.gov (4. 9. 2013).
9. Immediate release solid oral dosage forms scale-up and postapproval changes: chemistry, manufacturing, and controls, in vitro dissolution testing, and in vivo bioequivalence documentation. CDER. 1995. http://www.fda.gov (4. 9. 2013).
10. Pilbrant A., Cederberg C. Development of an oral formulation of omeprazole. Scand. J. Gastroentero. 1985; 20, 113–120.
11. Siepmanna F., Siepmanna J., Waltherb M., MacRaeb R. J., Bodmeier R. Polymer blends for controlled release coatings. J. Control. Release 2008; 125, 1–15.
12. Dvořáčková K. Drug release from oral matrix tablets containing hypromelose. Chem. Listy 2009; 103, 66−72.
13. Tenovuo J. O. Human saliva: clinical chemistry and microbiology. Boca Raton, Fla.: CRC Press 1989.
14. Ménard D. Development of human intestinal and gastric enzymes. Acta Paediatr. 1994; 83, 1–6.
15. Marschall S., Patterson C. Principles of molecular medicine. 2. vyd. NJ: Humana Press, 2006; 542–590.
16. Franc A., Vetchý D., Smilková L., Rabišková M., Kratochvíl B. Lipophilic formulations for increasing bioavailability of poorly water-soluble drugs. Chem. Listy 2012; 106, 3–9.
17. Singh S., Rama Rao K. V., Venugopal K., Manikandan R. Alteration in dissolution characteristics of gelatin-containing formulations: a review of the problem, test methods, and solutions. Pharm. Technol. 2002; 26, 36–58.
18. Růžicka D., Kiss F., Hájek E., Sládek T., Martínek A., Buriánek I., Novotný J., Seménkova L., Hanák A. Tablets with controlled release of dilthiazemium chloride. Lachema, a.s., CS19890005635, priorita 10. 4. 1989.
19. Heinicke R. M., Ito T., McCarthy L., Yokoyama M. Effect of bromelain on clinical laboratory tests after oral administration. Jpn. Heart J. 1971; 12, 517–527.
20. Carey M. C., Small D. M., Bliss A. C. Lipid digestion and absorption. Annu. Rev. Physiol. 1983; 45, 651–677.
21. Wen R. W., Thompson M. H., Hill M. J., Wilpart M., Mainguet P., Roberfroid A. M. The importance of the ratio of lithocholic to deoxycholic acid in large bowel carcinogenesis. Nutr. Cancer. 1987; 9, 67–71.
22. Dahan A., Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur. J. Pharm. Biopharm. 2007; 67, 96–105.
23. Rowe R. C., Sheskey J. P., Quinn M. E. Handbook of pharmaceutical excipients. 6. vyd. London: Pharmaceutical Press 2009.
24. Gupta P. Enhancement of oral bioavailability of non-emulsified formulation of prodrug esters with lecithin. Tap Pharmaceutical Products Inc. US20010867353, priorita 29. 5. 2001.
25. Rogers J. A., Anderson K. E. The potential of liposomes in oral drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 1998; 15, 421–480.
26. Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J 2010; 12, 397–406.
27. Guarner F., Malagelada J. R. Gut flora in health and disease. Lancet 2003; 360, 512–519.
28. Sinha V. R., Kumria R. Microbially triggered drug delivery to the colon. Eur. J. Pharm Sci. 2003; 18, 3–18.
29. Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv. Drug. Deliver. Rev. 2004; 491–509.
30. Mooter G. B., Maris C., Samyn P., Augustijns P., Kinget R. Use of azo polymers for colon-specific drug delivery. J. Pharm. Sci. 1997; 12, 1321–1327.
31. Yang L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J. Control. Release 2007; 125, 77–86.
32. Mercier G. T., Nehete P. N., Passeri M. F., Nehete B. N., Weaver E. A., Templeton N. S. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007; 25, 8687–8701.
33. Press A. G., Hauptmann I. A., Hauptmann L., Fuchs B., Fuchs M., Ewe K., Ramadori G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment. Pharm. Therap. 1998; 12, 673–678.
34. Chawla G., Gupta P., Koradia V., Bansal A. K. Gastroretention: a means to address regional variability in intestinal drug absorption. Pharm. Tech. 2003; 27, 50–51.
35. Zhao H., Cafiero S., Williams Z., Bynum K. C. Practical considerations for the development of a robust two-step dissolution test for enteric-coated immediate and extended release solid oral dosage formulations. Dissolut. Technol. 2011; 18, 6–10.
36. Franc A., Sova P. Oral pharmaceutical composition for targeted transport of active substance into colon. Pliva-Lachema, a.s. CZ20040001167, priorita 12. 1. 2004.
37. Hebden J. M., Gilchrist P. J., Perkins A. L., Wilson C. G., Spiller R. C. Stool water content and colonic drug absorption: contrasting effects of lactulose and codeine. Pharm. Res. 1999; 16, 1254–1259.
38. Bhople A., Chandewar A., Sheiakh S. Ingole S., Deshmukh M., Pawar S. Formulation and development of mucoadhesive microcapsule for delivery of clarithromycin and omeprazole used against helicobacter pylori infection. AJPLS. 2012; 2, 27–48.
39. Zhan J., Wang J., Zhang Y., Li W., Wu W. Method for preparing xanthan gum omeprazole intragastric retention floating sustained-release tablets. Jilin Institute of Chemical Technology. CN2012173347, priorita 3. 10. 2012.
40. Rangasamy M., Ganesan P. G., Gummudavelly S., Ayyasamy B., Natesan S. Multiparticlate drug delivery systems: pellet & pelletization technique. DIT 2010; 5, 233–237.
41. Dissolution Methods U. S. Food and Drug Administration Dissolution database – list of all drugs in the Database. http://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults_Dissolutions.cfm?PrintAll=1 (4. 9. 2013).
42. Hall J. E. Guyton and Hall textbook of medical physiology. 12. vyd. Philadelphia, PA: Saunders Elsevier 2010.
43. Silbernagl S., Despopoulos A. Atlas fyziologie člověka, 6. vyd. Praha: Grada Publishing 2004.
44. Český lékopis 2009. 1. vyd. Praha: Grada Publishing 2009.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
-
Všechny články tohoto čísla
- Physiological factors with impact on the drug behaviour in the gastrointestinal tract
- Cationic Eudragit® Polymers as Excipients for Microparticles Prepared by Solvent Evaporation Method
- Nové knihy
- Influence of quaternary ammonium salt on liberation of drug with antiseptic effect
- Monitoring of formation of diclofenac sodium salt hydrates and their influence on the drug dissolution from prepared tablets
- K životnímu jubileu doc. RNDr. PhMr. Milana Čeladníka, CSc.
- Alkalimetric titrations of salts of organic bases in the Pharmacopoeia
- Determination of varenicline in the preparation Champix® with the use of two-dimensional capillary electrophoresis in connection with UV detection
- A view on providing care in the field of medicines in Slovakia – the pharmacist and the patient
- A chapter from the history of the pharmacy of the Brothers of Mercy in Spišské Podhradí in the 19th and 20th centuries
- 42. sympozium klinické farmacie
- Padesát let seminářů a sympozií z dějin farmacie
- Rejstříky
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A view on providing care in the field of medicines in Slovakia – the pharmacist and the patient
- Alkalimetric titrations of salts of organic bases in the Pharmacopoeia
- Influence of quaternary ammonium salt on liberation of drug with antiseptic effect
- Physiological factors with impact on the drug behaviour in the gastrointestinal tract
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



