-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Some immunological properties of female saliva and its effect on sperm motility
Imunologické vlastnosti ženských slin a jejich vliv na pohyblivost spermií
Východisko.
Cílem studie bylo sledovat pH, hladiny IgA, IgG, IgM, lyzozymu a spermaglutinační protilátky ve slině zdravých dívek s nebo bez hormonální antikoncepce, a účinek slin na motilitu spermií.Metody a výsledky.
Sliny 59 dívek byly odebrány ihned po probuzení. Změřili jsme pH v jejich sérech a slinách, IgG, M a A radiální imunodifuzí jen ve slinách. Spermie – slina kapilární penetrační test sloužil k vyšetření pohyblivosti spermií, nepřímý MAR-test k určení protilátek proti spermiím v sérech a ve slinách sledovaných pod inverzním mikroskopem. Průměrné pH nativní sliny rozdělené podle dnů menstruačního cyklu byly: mezi 2–5 dnem 6,896, mezi 6–17 dnem 7,027, od 18. dne a výše pak 7,17. Rychlý pokles pohyblivosti spermií jsme zjistili v kyselejší slině ve srovnání se zásaditější. Průměrná koncentrace IgA, IgG, IgM a lyzozymu se u dívek s hormonální nebo bez hormonální antikoncepce velmi málo liší. Mírné snížení motility spermií jsme zaznamenali ve slinách s vyššími hladinami IgA. Nezjistili jsme žádné spermaglutinační protilátky ve slinách ani v sérech u sledovaných dívek.Závěry.
pH ve slinách je ovlivňováno různými factory (hormonální, imunologické, mikrobiální, ale i jídlem, pitím, kouřením apod.). Poukázali jsme na to, že motilita spermií je nizší v kyselejší slině. pH slin se výrazně mění během menstruačního cyklu. Prokázali jsme individuálně vysoké hladiny IgA ve slinách. Přirozené lidské sliny používané jako koitální lumbrikans snížují pohyblivost spermií, ale nenahrazují lokální přípravky kontracepce.Klíčová slova:
lidská slina – imunoglobuliny – lysozym – pH – pohyblivost spermií – protilátky proti spermiím
Authors: Nadia Gonçalves Ferreira 1; Lenka Černá 1; Miroslava Čedíková 2; Katarina Bibková 4; Zdenka Mičanová 4; Zdenka Ulčová-Gallová 1,4
Authors place of work: Department of Gynecology and Obstetrics, Charles University, Pilsen, Czech Republic 1; Department of Histology and Embryology, Faculty of Medicine Charles University, Pilsen, Czech Republic 2; Biomedical Centre, Faculty of Medicine, Pilsen, Charles University, Prague, Czech Republic 3; Genetics, Pilsen, Czech Republic 4
Published in the journal: Čas. Lék. čes. 2014; 152: 86-90
Category: Původní práce
Summary
Background.
In this study, we investigated pH, levels of IgA, IgG, IgM, lysozyme and spermagglutinating antibodies in human saliva of healthy women, with or without hormonal contraception (HC) and their effect on sperm motility.Methods and results.
Saliva was collected immediately after waking up from 59 healthy randomly selected women. We measured pH in sera and in saliva as well as immunoglobulin G, A and M levels in saliva by radial immunodiffusion. Sperm - saliva capillary penetration test in native saliva served for examination of sperm motility, indirect-MAR test for detection of sperm antibodies in sera, and in saliva, both observed by inverted microscope. The average pH values in native saliva according to the menstrual cycles were: from 2–5 days 6.896, from 6–17 days 7.027, and from 18 and more days 7.17. Rapid decrease of sperm motility was registered in acidic saliva in comparison with alkaline. The average concentration of IgA, IgG, IgM and lysozyme slightly differed in women with or without hormonal contraception. Moderate decreasing of sperm motility was found in saliva with higher levels of IgA. No sperm agglutinating antibodies in saliva and in serum were detected in all subjects.Conclusions.
The pH of saliva is influenced by several causes (hormonal, immunological, microbiological factors, by meal and drinks, smoking, etc.). We demonstrated that sperm motility is lower in acidic saliva. pH is markedly changed during various days of menstrual cycles. We proved individual high levels of saliva IgA. Human saliva used as coital lubricant decreases sperm motility, but does not replace a local form of contraception..Keywords:
human saliva – immunoglobulins – lysozyme – pH – sperm motility – sperm antibodiesINTRODUCTION
Saliva is a complex aqueous fluid composed of electrolytes (bicarbonate, potassium, sodium chloride), mucopolysaccharides and glycoproteins, some antibacterial components such as hydrogen peroxide, thiocyanate or secretory immunoglobulin A and several enzymes like alpha-amylase, lingual lipase, lysozyme, salivary lactoperoxidase, lactoferrin, beta-glucuronidase, esterases, phosphatases, kallikrein, ribonucleases (1). Immunoglobulins of all isotypes are also detected in human saliva (2, 3). Saliva is easily available through non-invasive methods and as a body fluid is constantly exposed to external factors such as smoking, food intake and internal aggressors such as microbes in dental caries that easily disturb salivary biochemical composition (3–5). Due to its properties, saliva can be involved in several important physiological mechanisms such as digestion. Properties of saliva are also influenced by disinfection and hormones (6). Currently, 15% of the couples of reproductive age suffer from infertility. Some of them use saliva as “natural lubricant”, often recommended during the initial phase of sexual intercourse. However, saliva has a negative effect on sperm motility (7, 8).
Our experimental study assesses selected immunological properties of human saliva on sperm motility in young women with and without hormonal contraception.
STUDY AND METHODS
In this study we included blood and saliva samples from 59 randomly selected healthy female students aged 14–30 years (average age 22 years); 13 of them were on HC, 46 without hormonal contraception; 50 girls were sexually active and 9 still virgin. The questionnaire given to any girl informed about basic gynaecological information. This study was approved by the Czech Institutional Ethics Committee. A written informed consent and a questionnaire were obtained from each subject participating in the study. Table 1 and 2 provide additional anamnestic data. All samples of sera and saliva were collected under fasting conditions and under abstinence of alcohol and cigarette smoking for at least 9 hours before collection. Saliva samples were collected in sterile containers and immediately after the student woke up. The saliva was then transported within half hour into laboratory.
Tab. 1. Basic gynaecological data from 59 questionnaires of female students 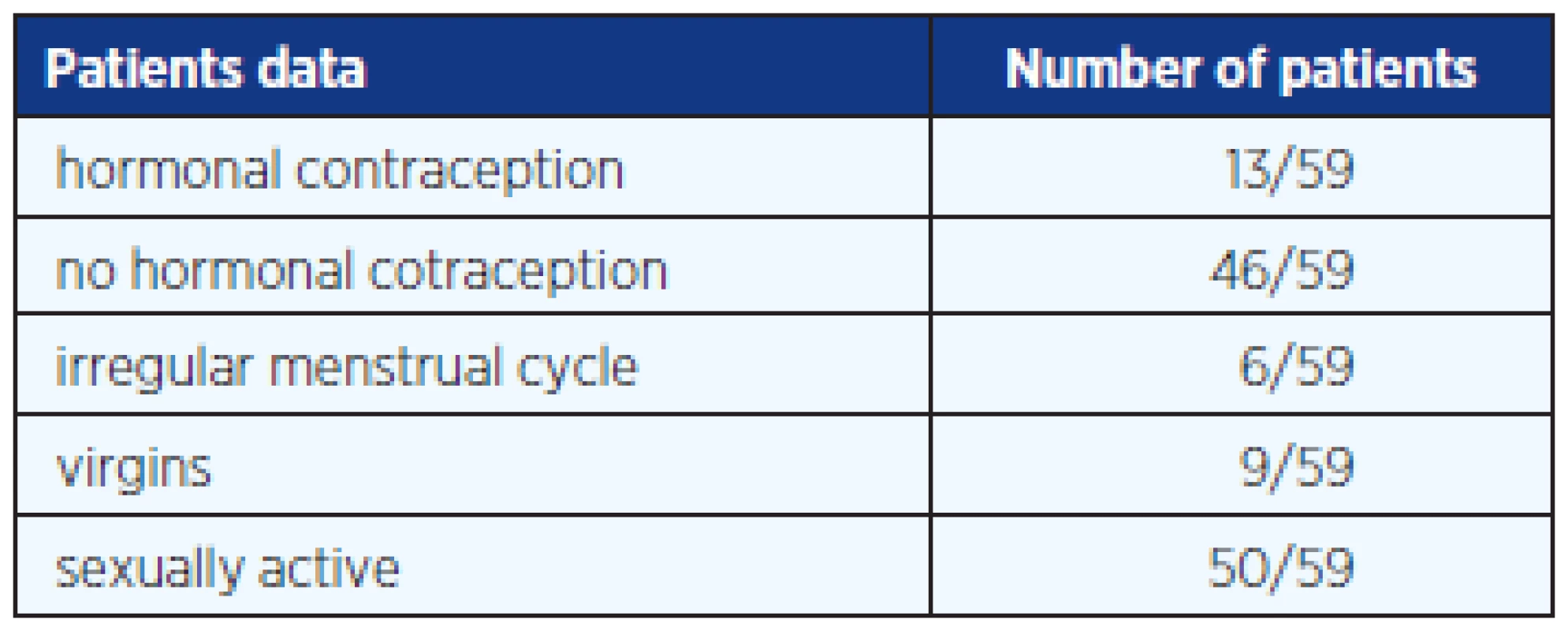
Tab. 2. Social history and up to date information from student questionaire 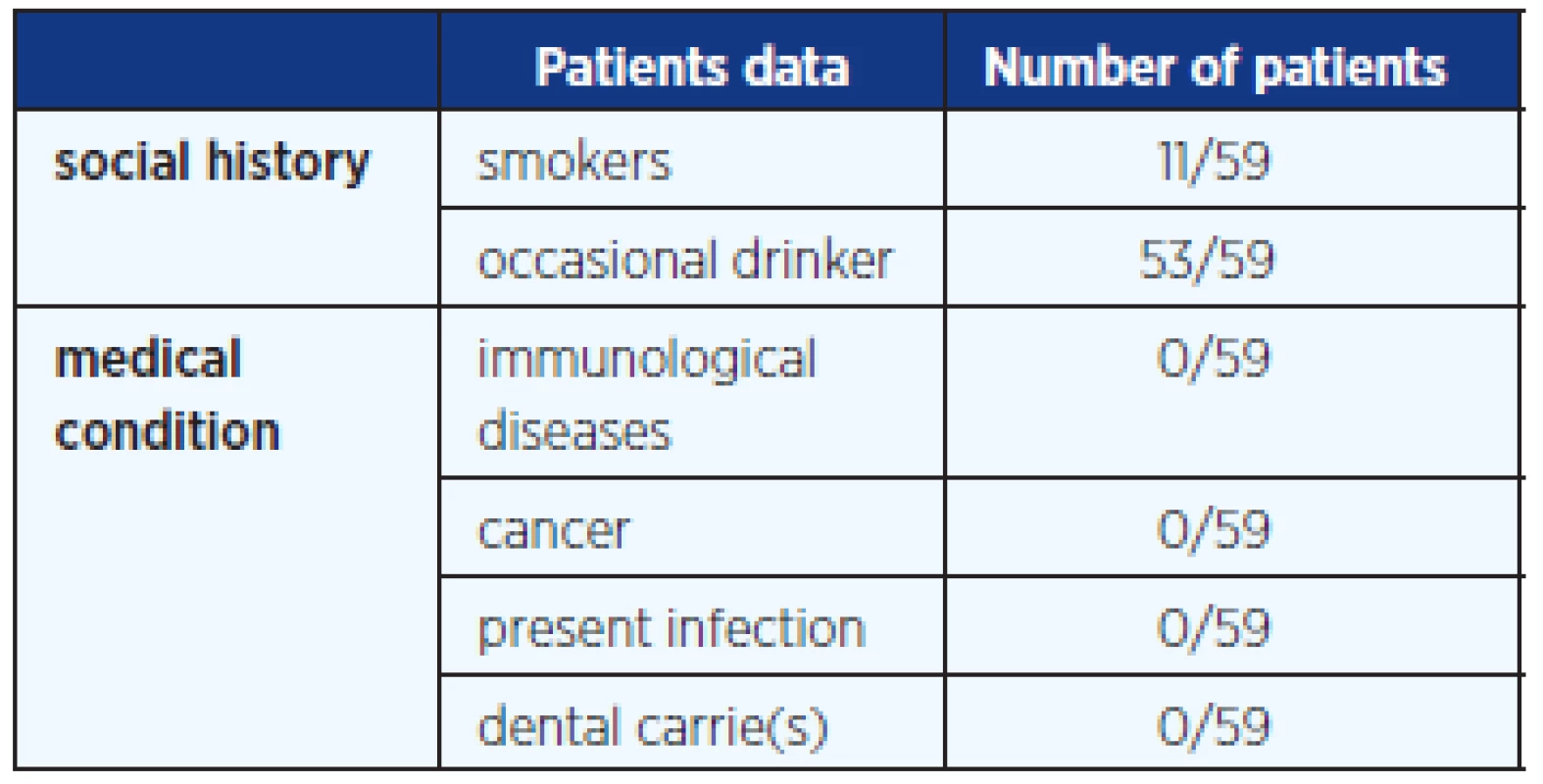
Each sample was centrifuged (5000 rpm for 10 minutes), to remove mucin. Supernatants were stored at –80 ºC for later immunological examination. We took also 5 ml of blood and serum was obtained and stored at –20 ºC.
Fresh semen samples without spermagglutinating antibodies were collected from two healthy fertile men by masturbation for next study.
Our following experiments were performed at room temperature.
pH measurement
pH meter (Hannah Instruments, Hamilton-electrodes) was used for determination of the pH of native and supernatant of saliva, and blood serum in each sample. The pH values were distributed according to the day of the menstrual cycle, and hormonal contraception. All measurements were repeated twice and the mean value was plotted on a graph for further analysis.
Radial immunodiffusion technique
BIND A RID (The Binding Site, UK) was used for detection of IgA, IgG, IgM isotypes, and lysozyme levels in the 59 supernatants of saliva only.
Sperm motility in saliva
We used a simple sperm-saliva capillary penetration test, modification of the Kremer test for ovulatory cervical mucus (9). For our experiments, we have chosen 2 saliva samples with different pH (acidic and alkaline), and another 2 samples with different values of IgA.
A vertical tube was filled with saliva supernatants and put to a reservoir filled with 200 µl of fresh semen with a cell concentration of 60.000.000/ml from a healthy donor. Under inverted Microscope (Olympus, Japan), sperm progressive motility and vitality was measured at the intervals of start 0, 2, 5, 10 and 15–20 minutes.
Indirect mixed antiglobulin reaction test (i-MAR)
We examined sperm agglutinating antibodies in donors semen by direct MAR and in all supernatants of saliva samples by i-MAR-test).
The i-MAR test was performed to analyze the anti-sperm response in IgG and IgA class. One microliter of native sperm suspension, 1 µl of inactivated saliva, 1 µl of glutaraldehyde-fixed sheep erythrocytes pre-coated with human IgG, and IgA, were mixed together. Then, 1 µl of the corresponding antiserum anti-IgG, anti-IgA (Behringer, Hannover, Germany) was added. Finally, the mixture was covered with coverslip and incubated in humid Petri chamber for 5–10 min. The result of the sperm agglutination reaction was observed under the inverted microscope at 200× magnification. The i-MAR test was considered as positive, if more than 49% of motile spermatozoa were involved in mixed agglutinates (spermatozoa and sheep erythrocytes coated by the corresponding immunoglobulin).
RESULTS
Native saliva sample had a lower pH (average 7.10) value compared with supernatant saliva (7.4). Average pH of sera was 7.35 (Table 3).
Tab. 3. Average pH of saliva and blood serum in all examined samples 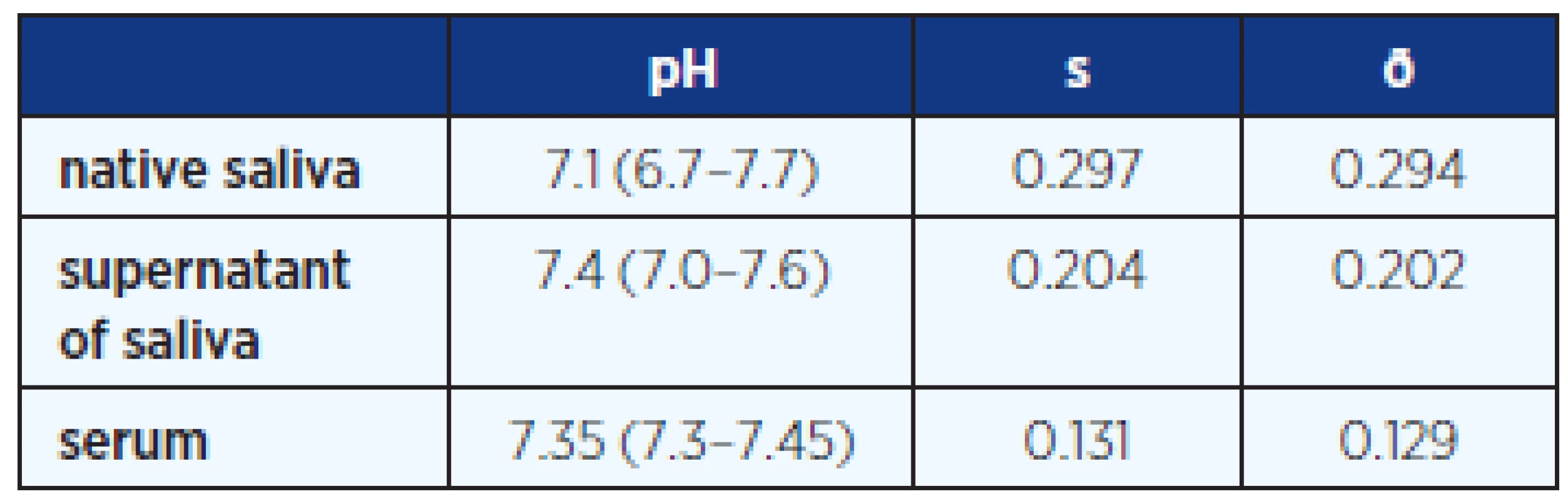
ð – standard deviation of the population of data The average pH values during menstrual cycle from 0–5 days 6.896, from 6–17 days 7.027, and in secretory phase, from 18 and more days 7.17 in all girls are shown in Figure 1.
Fig. 1. pH values of native saliva according to the menstrual cycle in all studied samples 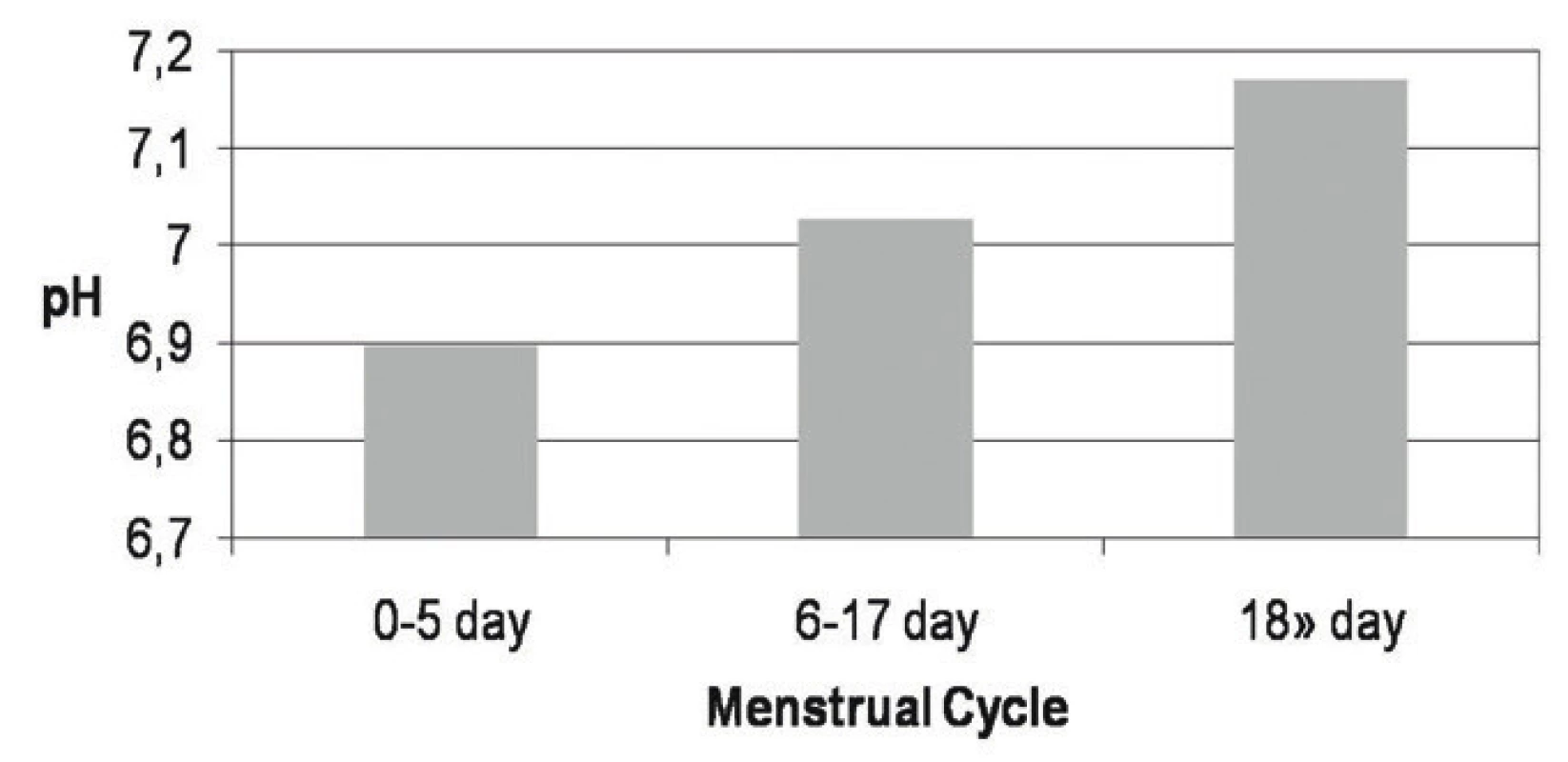
Women using hormonal contraception have a lower (more acidic) pH value compared with no hormonal contraception Figure 2.
Fig. 2. pH values of native saliva from patients using HC and without HC according to the menstrual cycle NHC – not used HC, HC – hormonal control 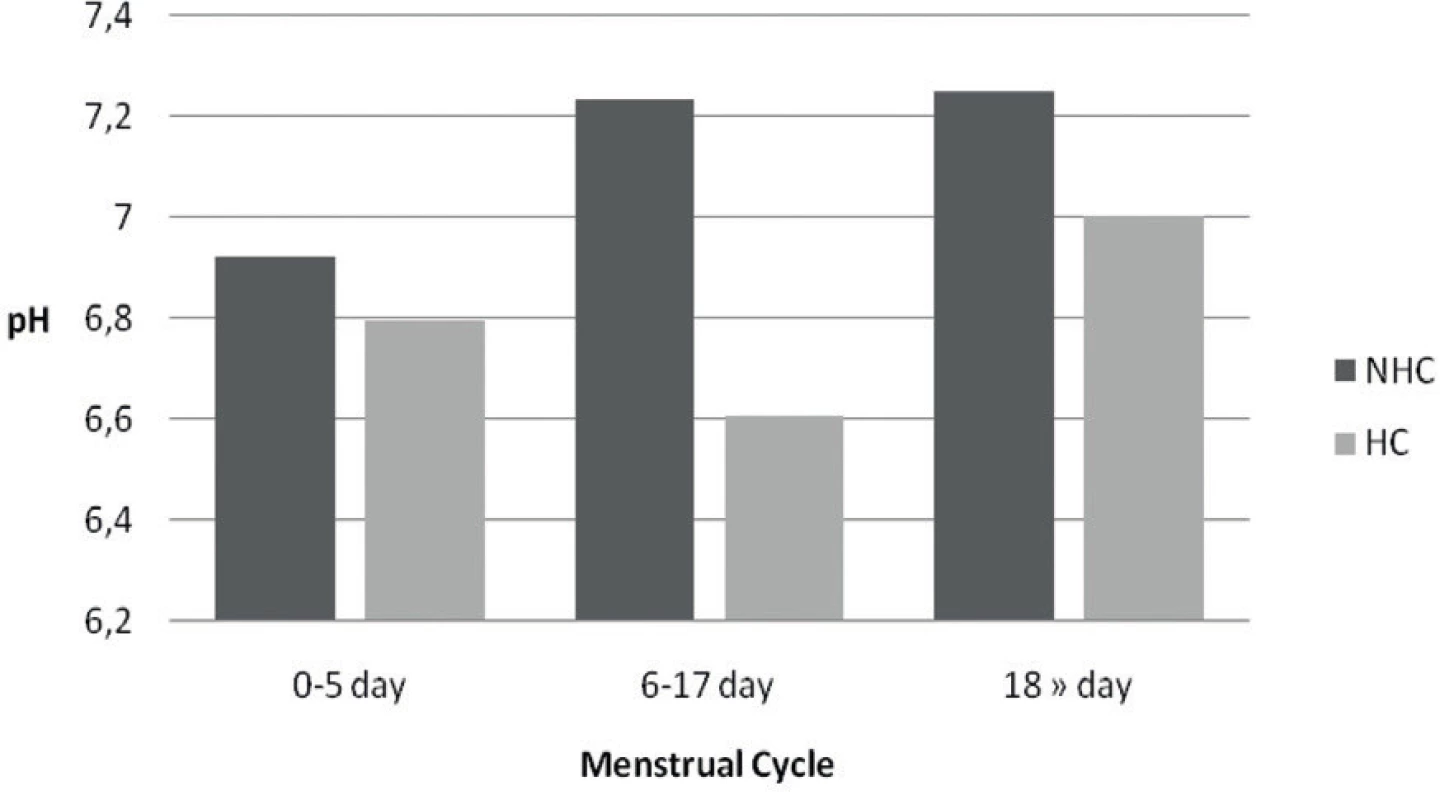
Two saliva samples chosen for experiment show decreased sperm motility and viability in acidic saliva (pH 5.545) compared with alkaline saliva (pH 7.1) in sperm – saliva capillary penetration test (Figure 3). The sperm motility was reduced by 50% in one minute with the more acidic saliva and after 5 min no sperm motility was observed.
Fig. 3. Sperm motility according to various pH. Two saliva samples were chosen according to various pH (alkaline and acidic) for the experiment. Results are expressed in percentage of decreased viability and progressive movement of sperm (used concentration of 60 millions per ml). 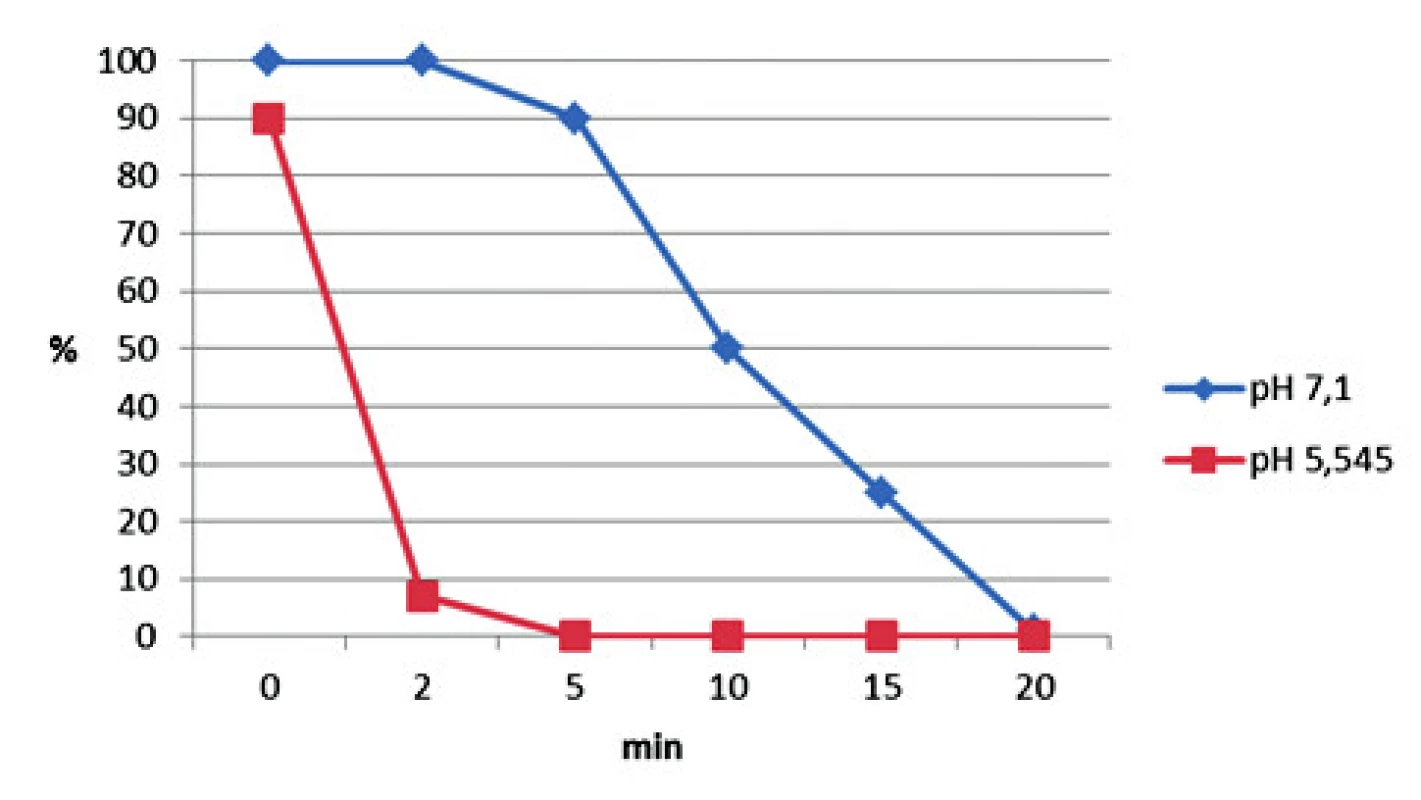
The average levels of IgA, IgG, IgM isotypes and lysozyme were almost the same in women with or without HC (Fig. 4, Table 4).
Fig. 4. Average levels of IgA, IgG, IgM and lysozyme in all supernatants of saliva samples 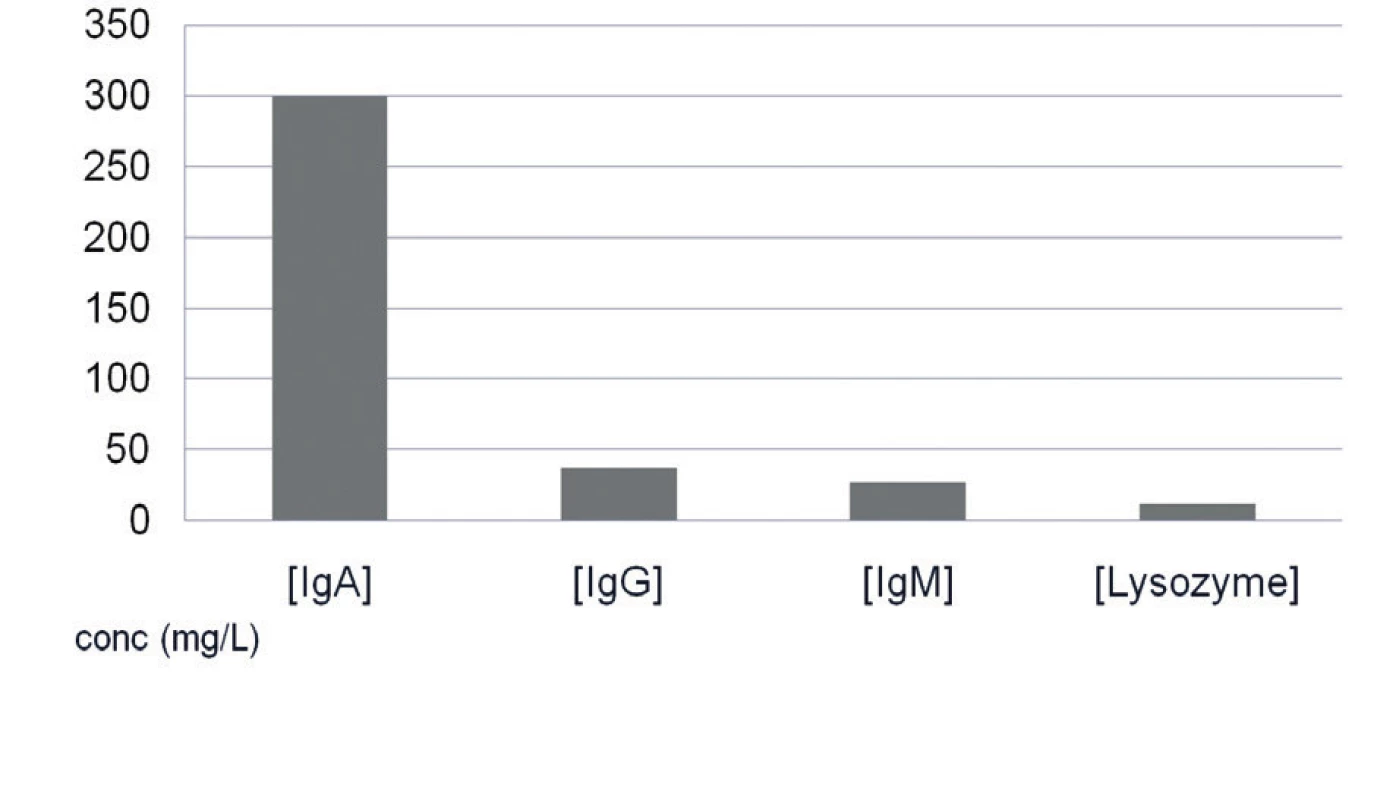
Tab. 4. Concentration of IgA (78–983 mg/l), IgG (15–176 mg/l), IgM (18.7–27.4 mg/l) and lysozyme (2.6–15.2 mg/l) with standard deviation in native saliva samples 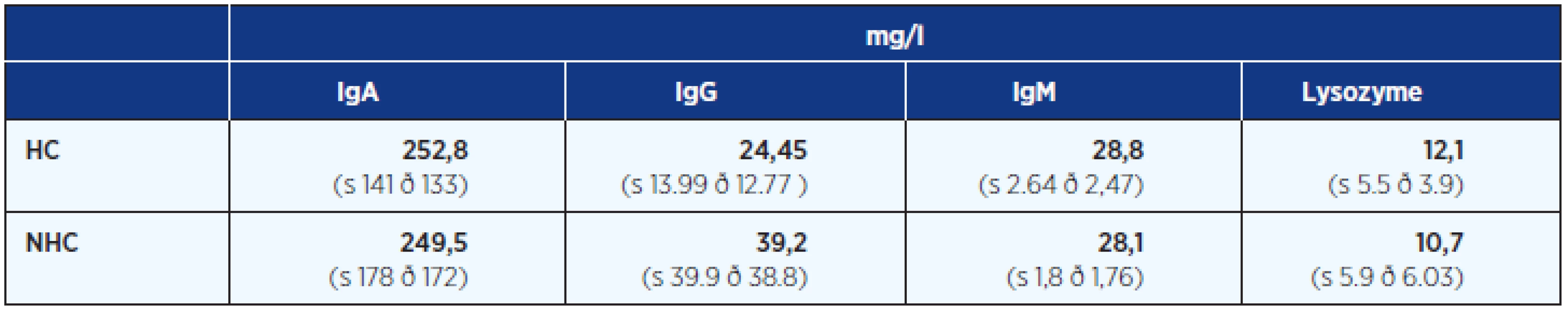
HC – hormonal contraception (13 girls), NHC – non hormonal contraception (46 young women) s – standard deviation of the sample of data, ð – standard deviation of the population of data In all semen samples, saliva reduced sperm progression and motility. For example Figure 5 shows the result of reduced sperm motility in a saliva sample with higher and lower IgA levels (very similar results also for IgG, IgM isotypes and lysozyme).
Fig. 5. Sperm motility according to IgA levels into two individual samples chosen according to the higher and lower levels of IgA in saliva supernatant. Decreased sperm progressive motility is expressed as a percentage of the sperm and in time. 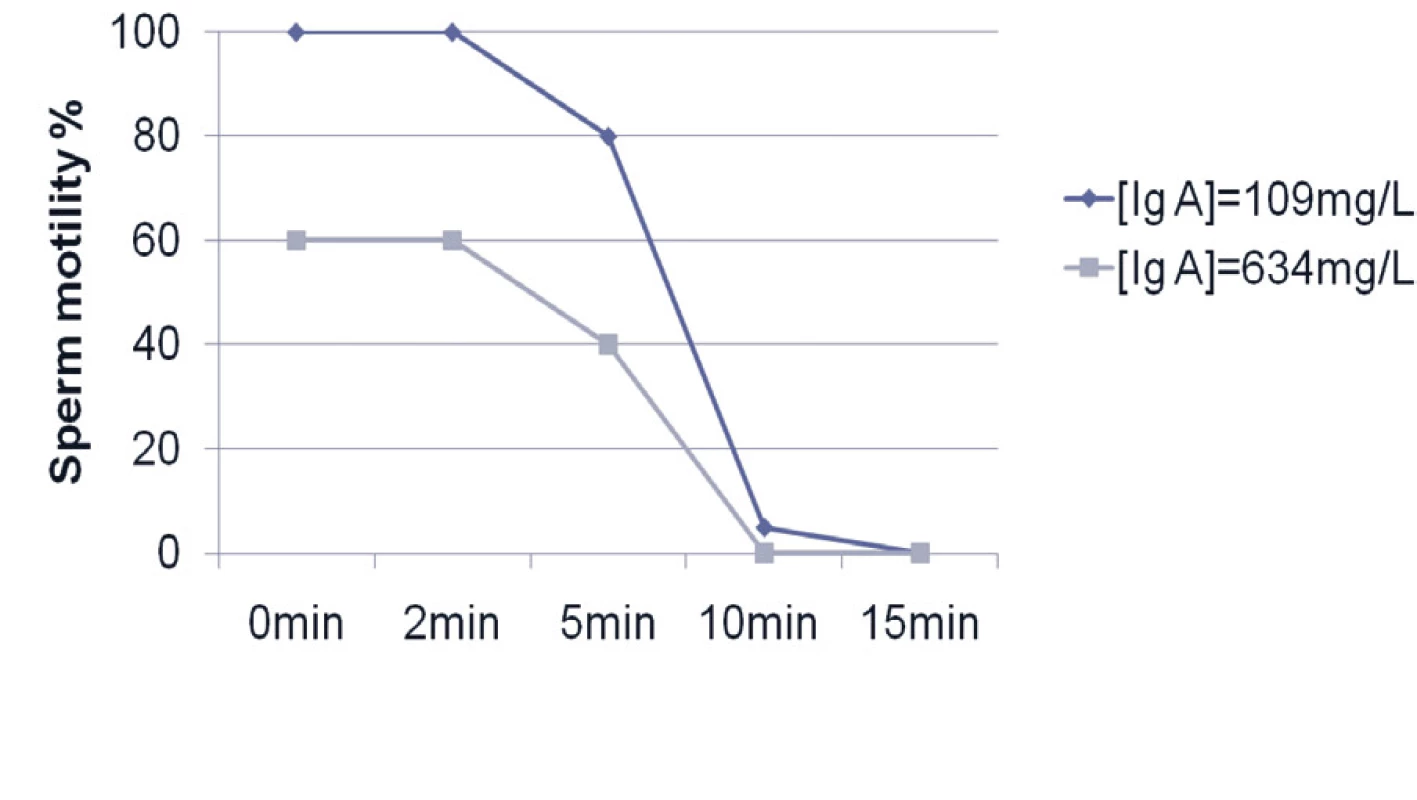
DISCUSSION
Previous studies (e.g. 2, 5, 10) highlight the fact that human saliva contains direct antibacterial components such as various antimicrobial peptides as thiocyanate ions, even increased amylase levels, lactoperoxidase, and lysozyme, immune cells as well as secretory IgA and other immunoglobulin isotypes, which participate in the mechanism decreasing sperm motility. Whole saliva is composed of the secretions of the parotid glands, the submandibular, the sublingual, and the minor salivary glands.
We found that the pH of saliva increases during the menstrual cycle. The fact can be explained by hormonal changes not only in physiological cycle but also by hormonal contraception. We noted a small difference in pH in supernatant and native saliva. Slightly acidic native saliva is affected by mucin (e.g. 3).
Our study demonstrates that young women with hormonal contraception have nearly the same levels of IgG, IgA, IgM isotypes and lysozyme in saliva compared with those without artificial cycles. Identification of basic immunoglobulins suggests an IgA predominance, which is powerfully secreted directly by salivary glands.
Saliva is a mirror of our body (4, 11–17) allowing detection of many hormonal and metabolic disease as diabetes, thyroid disorder, adrenal disease, cancer, viral infections etc. Our group of young women were healthy, without oral acute or chronic periodontal pathologies such as gingivitis and /or other autoimmune disease as e.g. Sjögren’s syndrome.
We have shown in this work some properties of human saliva and their differences in women with and without hormonal contraception. We modeled the situation of unprotected intercourse (using human saliva as lumbricant) as simple sperm - saliva capillary penetration test. We proved that human saliva impairs motility and progressive movement of sperm. Our data highlights to two facts:
- The effect of saliva as lubricant for couples trying to be pregnant is not suitable because the movement of sperm into vagina and uterine cervix will be slower as we have shown in our experiments.
- Saliva cannot be used as a local contraceptive “treatment” for fertile couples even though some spermicidal properties on the sperm are proven.
CONCLUSION
Saliva with its chemical and immunological properties used as a coital lubricant could not use as a nature form of contraception even if sperm progressive motility is impaired.
This work was supported by the project ED2.1.00/03.0076 from European Regional Development Fund and by the grant from Charles University Grant Agency no. 969212.
ADRESA PRO KORESPONDENCI:
prof. MUDr. Zdenka Ulčová-Gallová, DrSc.
Genetika Plzeň
Parková 11A, 326 00 Plzeň
e-mail: ulcova-gallova@email.cz
Zdroje
1. Beeley JS. Clinical applications of electrophoresis of human salivary proteins. Chromatography 1991; 569 : 261–280.
2. Brandtzaeg P. Salivary immunoglobulins. In Tenovuo JO (ed.) Human Saliva: Clinical Chemistry nad Microbiology, Vol II.Boca Raton, Florida: CRC Press 1989; 1 – 55.
3. Fos P. Saliva somposition abd its importance in dental health. Compend Contin Educ Dent 1989; 13 : 457–460.
4. Chiappin S, Antonelli G, Gatti R, de Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 2007; 383 : 30–40.
5. Dougherty KA, Cockett ATK, Urry RL. Effect on amylases on sperm motility and viability. J Urol 1978; 120 : 426–430.
6. Shpitzer T, Hamzany Y, Bahar, G et al. Salivary analysis of oral cancer biomarkers. Br J Cancer 2009; 101 : 1194–1198.
7. Anderson L, Lewis SEM, McClure N. The effect of coital lubricants on sperm motility in vitro. Human Reproduction 1998; 13 : 3351–3356.
8. Tulandi T, Plouffe L, McInnes RA. Effect of saliva on sperm motility and activity. Fertility and Sterility 1982; 38 : 721–723.
9. Kremer J, Jager S. The significance of antisperm antibodies for sperm-cervical mucus interaction. Human Reprod 1992; 6 : 781–784.
10. Farnaud SJ, Kosti O, Getting SJ, Renshaw D. Saliva: physiology and diagnostic potential in health and disease. Scientific World Journal 2010; 16 : 434–456.
11. Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. ČASOPIS??NÁZEV?? 2009; 55 : 1910–1913.
12. Greabu M, Battino M, Mohora M, et al. Saliva – a diagnostic window to the body, both in health and in disease. J Med Life 2009; 32 : 124–132.
13. Pesce MA, Spitalnik SL. Saliva and the clinical pathology laboratory. Ann NY Acad Sci 2007; 1098 : 192–199.
14. Pink R, Simek J, Vondrakova J, et al. Saliva as a diagnostic medium. Biomed Pap Med Fac Univ Palacky Olomouc Czech Republic 2009; 153 : 103–110.
15. Gröschl M. Current status of salivary hormone analysis. Ann Biol Clin (Paris) 2009; 67 : 493–504.
16. Gursoy UK, Könönen E, Uitto VJ, et al. Salivary: Interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J Clin Periodontol 2009; 36 : 922–927.
17. Vining RF, McGinley RA. The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem 1987; 27 : 81–94.
Štítky
Adiktologie Alergologie a imunologie Angiologie Audiologie a foniatrie Biochemie Dermatologie Dětská gastroenterologie Dětská chirurgie Dětská kardiologie Dětská neurologie Dětská otorinolaryngologie Dětská psychiatrie Dětská revmatologie Diabetologie Farmacie Chirurgie cévní Algeziologie Dentální hygienistka
Článek vyšel v časopiseČasopis lékařů českých
Nejčtenější tento týden
- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Kolorektální karcinom (část 1)
- EPMA summit
- Diabetes mellitus – oční komplikace
- Již počtrnácté plný Lékařský dům na konferenci Tabák a zdraví
- Doc. MUDr. Ivo Přerovský, DrSc. 1924–2014
- Vzpomínka na prof. MUDr. Zdeňka Reiniše, DrSc.
- Erratum
- ROSALYN SUSSMAN YALOWOVÁ
- Potenciální interakce mezi léčivy a doplňky stravy rostlinného původu*
- Plánované akce složek ČLS JEP
- Cirkulující nádorové buňky a prognóza karcinomu prostaty
- Krevní destičky v patogenezia léčbě solidních nádorů
- Plánované akce složek ČLS JEP
- Imunologické vlastnosti ženských slin a jejich vliv na pohyblivost spermií
- CREATIVE DESTRUCTION OF MEDICINE
- Vztah rizikových faktorů mezi metabolickým syndromem a nealkoholickým ztučněním jater u dětí a dospívajících
- OMLUVA
- Porodnicko-gynekologicky orientované texty v díle Jana Černého
- Časopis lékařů českých
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Krevní destičky v patogenezia léčbě solidních nádorů
- Imunologické vlastnosti ženských slin a jejich vliv na pohyblivost spermií
- Potenciální interakce mezi léčivy a doplňky stravy rostlinného původu*
- Cirkulující nádorové buňky a prognóza karcinomu prostaty
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



