-
Medical journals
- Career
INFLUENCE OF THE USE OF GRAVITY SETS IN A PRESSURE VOLUMETRIC INFUSION PUMP WITH AN IMPACT ON THE ACCURACY OF INFUSION SOLUTION FLOWS
Authors: Klara Fiedorova; Martin Augustynek; Marek Penhaker
Authors‘ workplace: Department of Cybernetics and Biomedical Engineering, Faculty of Electrical Engineering and Computer Science, VSB Technical University of Ostrava, Ostrava, Czech Republic
Published in: Lékař a technika - Clinician and Technology No. 1, 2020, 50, 23-31
Category:
doi: https://doi.org/10.14311/CTJ.2020.1.04Overview
The subject of the work was the experimental verification of the negative influence of gravity sets in a pressure volumetric infusion pump with an impact on the accuracy of infusion solution dosing. The quality criterion for gravity sets was the accuracy of the flow versus the reference set by the volumetric infusion pump. The solution consisted of 14-hour measurements with two types of gravity sets, Intrafix® Primeline sets were used as universal sets and Standardline IS 103 gravity sets. The insulin pump flow rate was set at 300 ml/h and 50 ml/h, and the actual flow rate of the infusion solution was recorded every hour using a graduated cylinder. Used gravity sets were also processed by mechanical tests, unused sets were subjected to these tests and the obtained data were compared with each other. Experiments carried out showed that at the set flow rate of 300 ml/h, the flow error with the universal set was -3% and at a set flow rate of 50 ml/h the error was +2.3%. Flow accuracy using gravity sets was worse, a flow error of -7.2% was detected for a flow rate of 50 ml/h and a flow error of -7.7% was measured for 300 ml/h. The volumetric pump used declares a tolerated inaccuracy of ± 3% when used with standard infusion sets. Based on the data, it can be concluded that the replacement of set types has an influence on the dosage of infusion solutions.
Keywords:
volumetric infusion pump – set for gravity-fed infusions – infusion therapy risks – flow accuracy – infusion set prolongation
Introduction
In healthcare, infusion therapy is the most common way of administering substances to the patient. The correct functionality of infusion therapy is based not only on the gravitational or pressure principle of the method, but also on the type of administration sets used [1–3]. The mutual compatibility of the infusion pump and the sets ensures a functional assembly that can meet the required requirements, especially in terms of flow accuracy. A breach of the compatibility condition be-tween the device and the infusion set may be more likely than is expected. Subsequent risks associated with potential confusion may also concern the safety of the treatment and the health of the patient, which is essential in healthcare [2, 4, 5].
Two types of infusion sets were the subject of experi-mental testing, the universal Intrafix® Primeline type from B | Braun (Germany). The reason for this step is that it is currently the most used set type, in some medical facilities it has even completely replaced conventional gravity sets. The other testing sets are Czech products manufactured by Gama® known as IS-103. According to publicly available offers of compa-nies dealing in medical supplies, these are the most accessible (and probably the most commonly used) exclusively gravity sets in the Czech Republic. The Forlong 600II volumetric pump from the Chinese manu-facturer Forlong Medical Instruments Ltd. will be used for measurements with a linear vane peristaltic pump.
The key point of the work is, based on the results of practical testing, to evaluate the effect of using a gravity infusion set in a volumetric pump application on dosing accuracy and risks in the safety of the treatment. Its solution will consist first of the theoretical determination of negative effects and possible consequences of con-fusion of different types of sets. The next step will then be to perform experimental measurements and analyse the data to verify the assumed inaccuracies of dosing. Finally, the data obtained will be evaluated concerning the theoretical opinions.
Pressure Infusion Therapy
In addition to gravity infusion therapy, the method of pressure infusion therapy is one of the most common [1]. The methods belonging to this group of infusions have an electrical force-generating device, the action of which generates the required pressure in the infusion set [6].
Pressure therapies include linear dispensers and infu-sion pumps. Treatment using an infusion pump is an en-hancement of the gravity method and was developed to provide more accurate dosing and stabilization over time [6]. It consists of creating pressure in the tube by cy-clically compressing it with rollers using the peristaltic movement [7, 8].
In the infusion technique, lamella type pumps are most often used. In their case, the peristaltic movement of the set is not created by the cyclical rotation of the rollers, but by the movement of the slats. These are arranged in a linear and controlled movement of the rotary shaft [9, 10].
Infusion pumps must meet the requirements of IEC 60601-2-24 [11]. The time of the infusion set replace-ment interval was not specified in the manual for the use of the infusion pump or in the instructions for use for the given infusion sets. For this reason, the standard EN 60601-2-24 : 2015 was studied, specifically article 201.12.1.102 Accuracy tests of this standard. However, other standards are applied to them, such as EN ISO 8536-8, which imposes requirements such as air perme-ability, microorganisms, and liquids out, or allowing fluid to be injected into a tube [12]. Also, the standard EN ISO 8536-4 is applicable to infusion pumps speci-fying that the infusion set should not deliver liquid at a rate greater than 100 ml/min [13].
Infusion Sets and Standard Requirements
Pressure devices for infusion therapy require special demands on the properties and structure of the sets used, thereby specializing in infusion sets for pressure therapy [1, 14]. Obviously, infusion pumps require different set properties than those required for gravity therapy [15]. Since infusion pressure sets may be confused with gravity sets, the question is whether dosing accuracy is maintained in this case, or the gravity set is destroyed by a volumetric infusion pump [16, 17].
The requirements of the legislation relate mainly to the material from which the files are made. Also important are the physical properties of the material concerning the flow, tightness and strength require-ments of the infusion sets. Infusion sets, as well as devices for infusion technology, fall within the regulated area of medical devices, governed by the European MDD 93/42/EEC [18]. According to the Directive, infusion sets are classified in Class IIa, subject to Rule 6—All surgically-invasive devices for transient use fall into Class IIa. To meet all MDD 93/42/EEC require-ments, the manufacturer is required to demonstrate the overall safety and functionality of the infusion set [18]. This is done by performing mechanical tests as defined in EN ISO 8536-4 [13]. This standard defines the re-quirements for mechanical tests, the functionality of the kit and the requirements for the material from which the kit is made. The testing of materials for biocompatibility is performed according to ISO 10993-1, where require-ments are set for the performance of the tests, including cytotoxicity and irritability, as well as the evaluation of the results of the tests performed [19]. Due to the nature of the medical device, a risk analysis according to EN ISO 14971 should be carried out and all risks arising from the use of the medical device should be minimized [20].
Determination of mechanical effects affecting the properties of infusion sets and dosing accuracy
Several factors contribute to changes in set properties. The fluctuation of the gravity infusion dosage is mainly due to the physical characteristics of the infusion set. With regard to material changes of the infusion set, the most significant influence is the pressure of the peri-staltic cylinders, slats and the safety control wheel on the tubing of the set. This applied pressure results in some adaptation of the tubing, which may result in a parasitic flow despite the initially fully enclosed push. At this point, it depends on the characteristics of the infusion sets used, assets intended for other uses will not meet the required mechanical properties [6, 10]. It has been shown that if polymeric substances are exposed to external pressure for a certain period, they can change their internal structure. This phenomenon may result in the loss of original material properties such as elasticity. Thus, the compression may deform the tubing of the set so that its inner diameter is reduced, which may affect subsequent flow. Another very important factor involved in the behaviour of the polymer tube during linear vane pump operation is the duration of the infusion treatment itself. However, the standard on in-fusion sets and conduits does not set any maximum length of use per kit [1, 10, 21].
Impact of Infusion Set Type Replacement
Also, during the course of the infusion treatment, the different types of sets, designed for different methods of application, differ from each other in their properties. Individual types must be properly and visibly marked according to standards. However, personnel may be mistaken, and the wrong infusion set may be selected for the method [1, 8]. Due to the possible occurrence of errors in the accuracy of treatment, a more serious case is the use of a gravity infusion line for drug delivery with a volumetric infusion pump. One of the risks is, for example, the parasitic free flow under the pump fins during their peristaltic movement. Gravity infusion sets are made of harder materials than infusion sets suitable for pressure equipment, which partially reduces their flexibility [6, 8]. Another cause may be a situation where the long-term compression of the blades of the pump leads to a flattening of the cross-section of the tubing, which in the peristaltic movement of the blades may not contain the volume calculated by the pump. The resulting flow rate may be lower than the set value [8, 9].
Flow measurement
Since the aforementioned phenomena and effects on the course and accuracy of infusion therapy are only assumed and theoretically determined, it is necessary to support them with real, experimentally obtained data.
Measuring Chain
The measurement chain was composed of an original 2,000 ml saline (0.9% aqueous NaCl) infusion bag from Baxter (United States), a Forlong 600II volumetric infusion pump from Forlong Medical Instruments Ltd. with a linear vane peristaltic pump and standard infusion sets of several types, available on the Czech market and thus properly approved for use in infusion therapy. A device with valid safety controls has been used and is therefore suitable for use in medical operations and with two types of infusion sets, whose properties are listed in the next chapter. The parameters specified in the Instruc-tions for Use indicate a tolerated inaccuracy of ±3% when used with standard infusion sets. The measure-ment was performed under standard conditions (tem-perature 21 °C, pressure 980 hPa, humidity 45%).
Infusion Sets in Flow Measurement
Two types of infusion sets, B | Braun's Intrafix® Primeline and IS-103 sets by Gama®, were the subject of the experimental testing. The features of Intrafix® Primeline Universal Sets include a maximum pressure of 2 kPa, a length of 150 cm and an inner diameter of 3 mm. It is made of DEHP free PVC. Standardline IS‑103 gravity sets have a length of 140 cm, an inner diameter of 3 mm, and the maximum pressure is not shown. PVC is used for production.
Calibration of the infusion pump
Calibration was performed for the recommended infusion set according to the instructions provided in the user manual for the Forlong 600II infusion pump. In addition, verification of the infusion pump and the new infusion set was performed using the drop method using a measuring cylinder with an accuracy of 0.5 ml for 60 minutes.
It should be emphasized that despite the calibration of the pump for a given type of set, the time aspect has a great influence on the mechanical degradation of that set and thus the accuracy of dosing. The aim was to determine the behaviour of infusion set materials when the recommended time is exceeded, as in practice it often happens that the infusion therapy is applied during the night and sleep for a longer period of time.
Measurement Parameters
A set of experimental measurements was performed to obtain data on development and changes in flow accu-racy during the infusion application process. Measured samples were taken every hour with a graduated cylin-der, which allows measurements with an accuracy of ±0.5 ml. The total length of one measurement cycle with one infusion set was 14 hours. The technical specifi-cation of the Forlong pump makes it possible to set the flow rate of the dosing substance within the range of 1–2,000 ml/h with an accuracy of 0.1 ml. Flow rates of 50 ml/h and 300 ml/h were used. Two flow rates allow verification of the effect of slow slat movements on changes in the infusion set properties different from the faster operation of the pump.
The numbers of measurements with individual types of infusion sets were determined according to the number of pieces obtained. Five measurements with Intrafix® Primeline infusion sets and two measurements with Gama® IS-103 sets were performed for each of the units tested.
Evaluation of Measured Data
The desired and predicted flow values set on the pump are the reference levels of the measurement to which the resulting measured and acquired values flow. Absolute differences and relative differences are calculated from the measured flow values.
The formulas used are as follows:
Absolute Difference
?? is the value of the flow being measured;
Relative Differences
Where: ?? the reference value of 50 or 300 ml/h.
Intrafix® Primeline Infusion Sets Measurement
Table 1 shows the measured flow values for a pump setting of 300 ml/h and 50 ml/h. The measured values are also given with an estimate of the measurement error ±0.5 ml, which is given by the resolution of the mea-suring cylinder. Table 2 shows the absolute and relative difference values of the flow of the individual samples of Intrafix® Primeline infusion sets for 300 ml/h. In Table 3, the absolute and relative difference values of the flow of the individual samples of Intrafix® Prime-line infusion sets for 50 ml/h are presented. Values are given in a range that includes a possible measurement error. These values should be indicators of flow accu-racy. Figure 1 and Figure 2 plot the measured flow values.
1. The measured flow values compared to reference 300 ml/h. 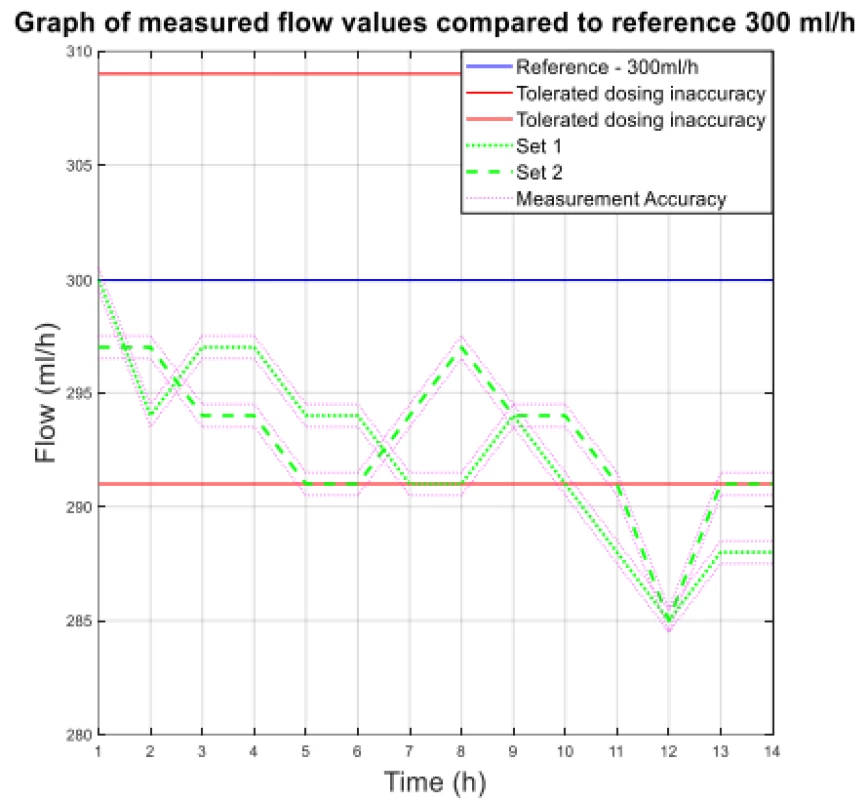
2. The measured flow values compared to reference 50 ml/h. 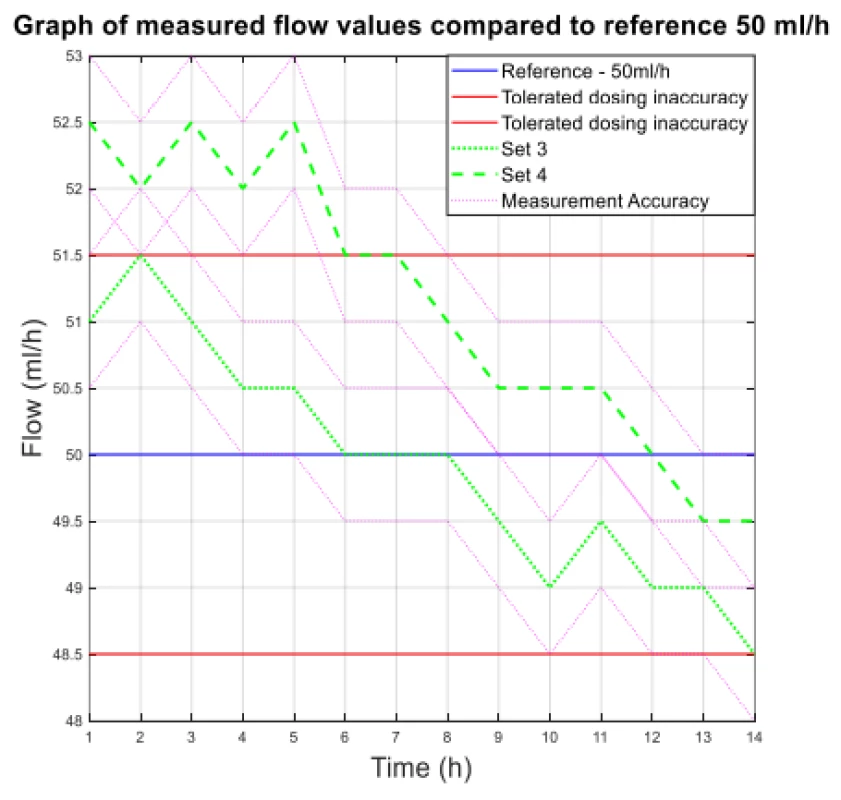
1. The measured flow values for 300 ml/h and 50 ml/h for Set 1–4. 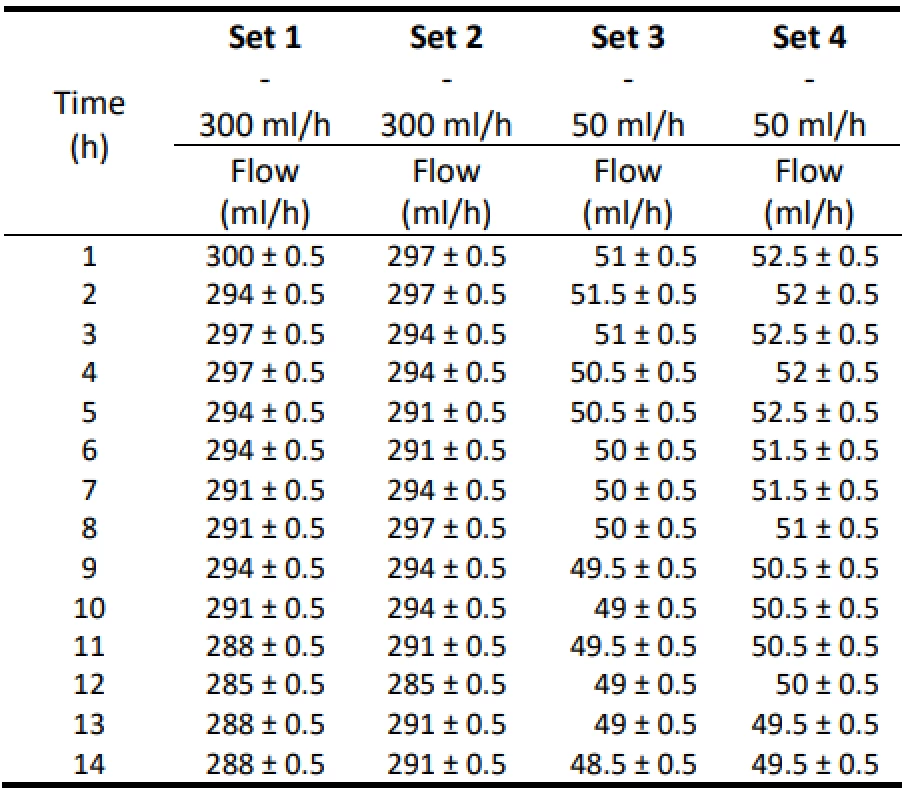
2. Absolute and relative difference values of flow for Set 1 and Set 2–300 ml/h. 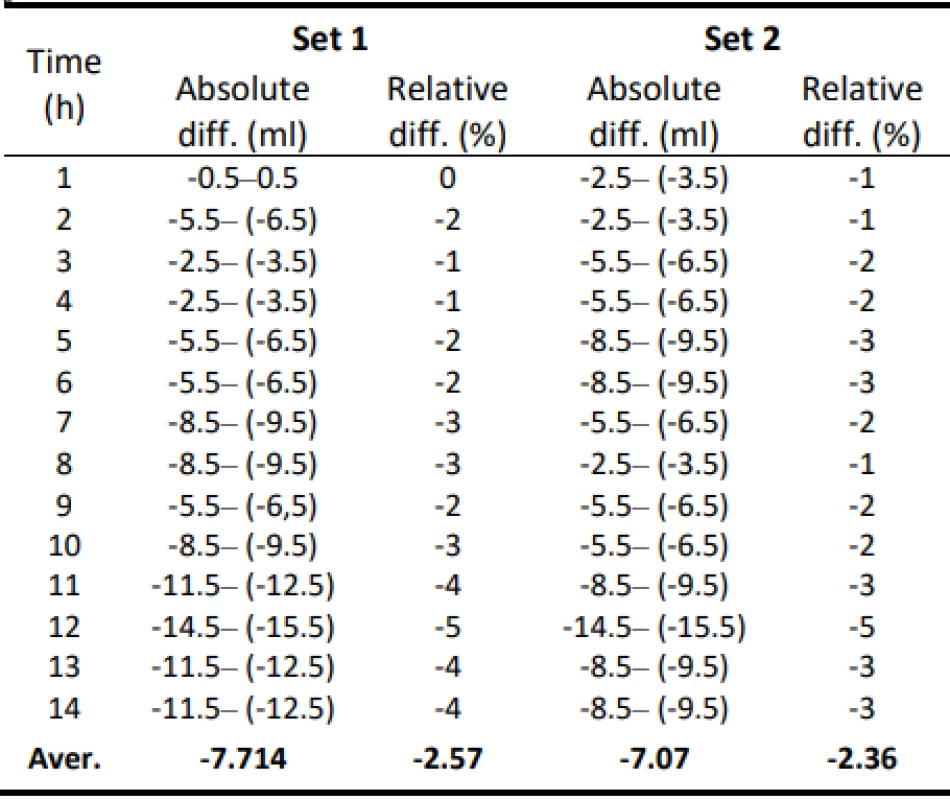
3. Absolute and relative difference values of flow for Set 3 and Set 4–50 ml/h. 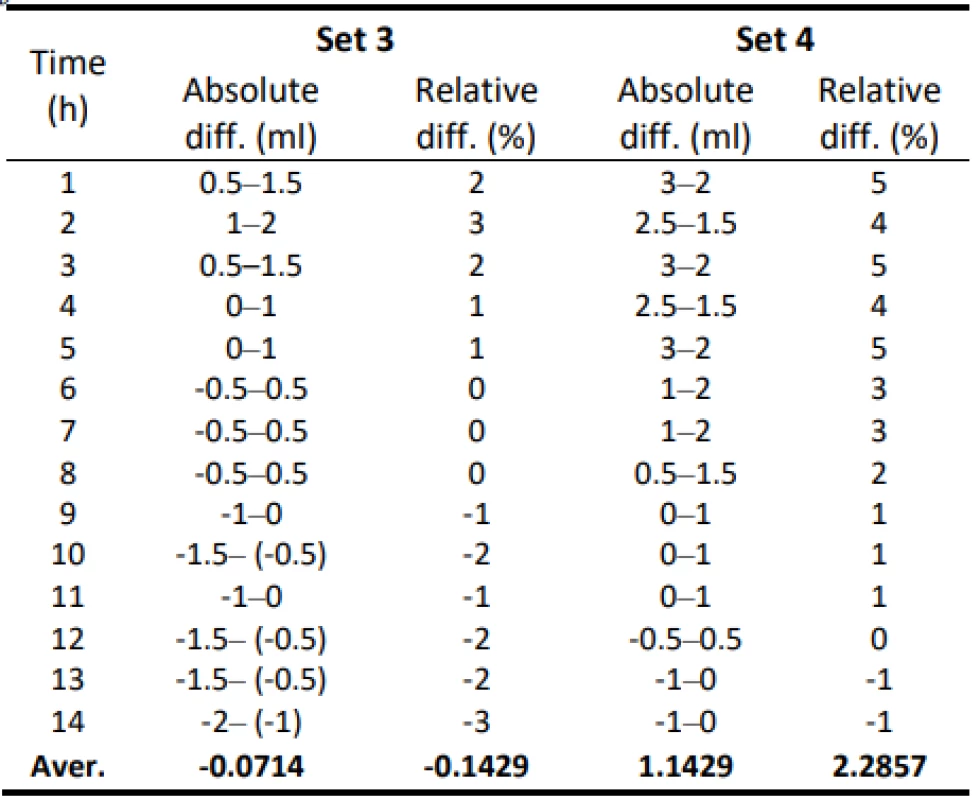
From the values given in Table 1, the decreasing trend of the measured flow as a function of the measurement time can be seen.
The values of the absolute difference and relative difference of flow confirm the decreasing flow trend. According to the relative difference, it can be determined that the inaccuracy of the average flow rate does not exceed the declared ± 3% for both measurement variants. The flow quality is not insignificant when using the tested sets, the dosing accuracy has a decreasing tendency in all measured settings. But based on the measured flow rate, the dosing accuracy in terms of therapy quality is fine.
Gama® IS-103 Infusion Sets Measurement
Table 4 shows the measurement results with a flow of 300 ml/h and 50 ml/h controlled by an infusion pump. Again, the estimated measurement error ±0.5 ml is given. The decreasing trend of the values is visible from the data.
4. The measured flow values for 300 ml/h and 50 ml/h for Set 5–6. 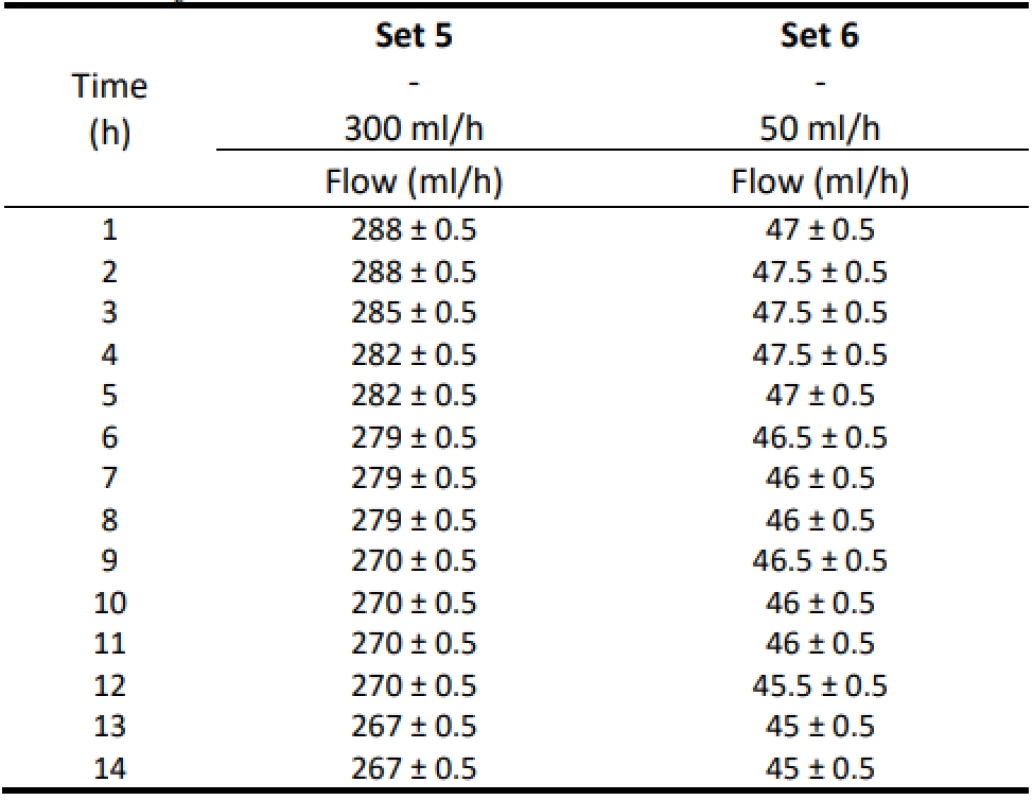
The values from Table 4 are plotted in the following graphs. Figure 3 shows the measured flow value for 300 ml/h, and Figure 4 shows the other measured flow value.
3. The measured flow values compared to reference 300 ml/h. 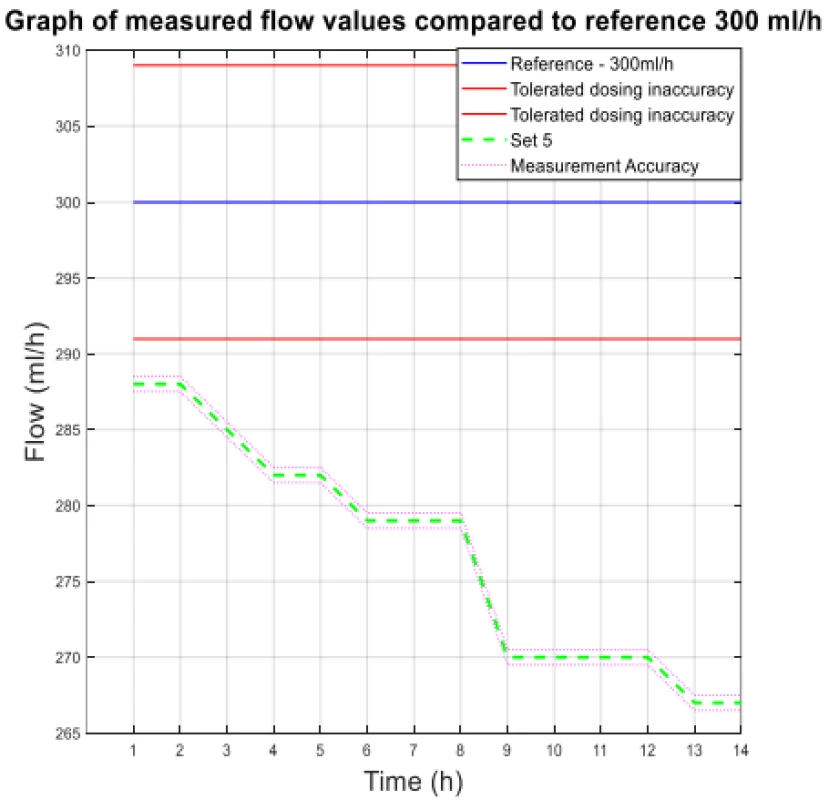
4. The measured flow values compared to reference 50 ml/h. 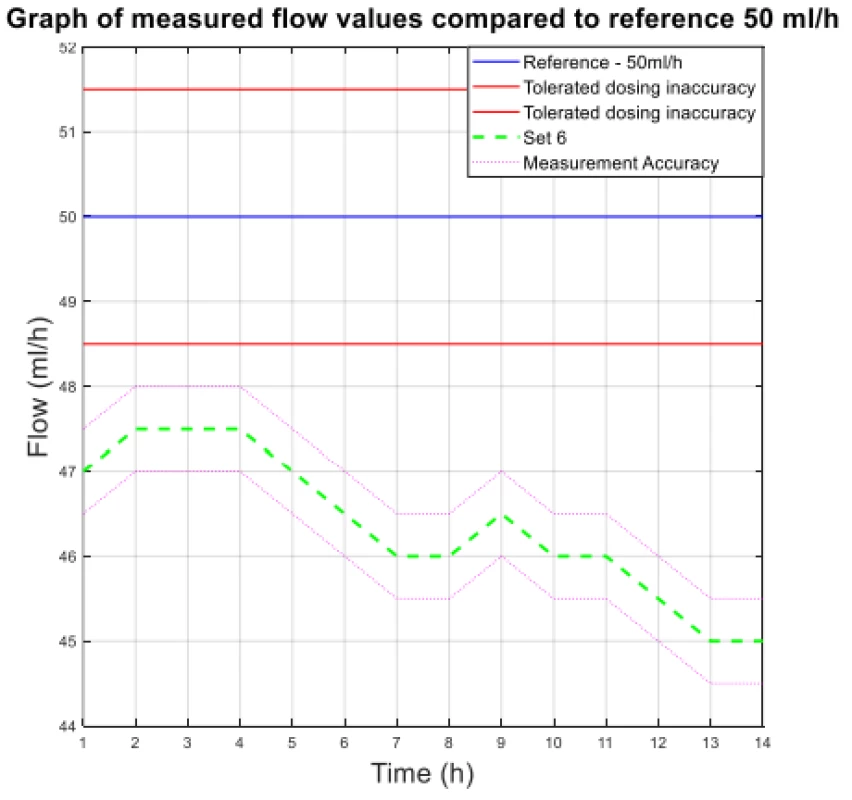
The analysis of the values and graphs showed that the use of the Gama® IS-103 gravity sets is not adequate. The combination of a volumetric pressure pump and gravity sets causes the declared flow accuracy to not be achieved. None of the measured sets ensures an inaccuracy of flow up to ±3%. Again, there is a decreasing inflow trend as in the case of the Intrafix® Primeline sets.
The following table 5 shows the absolute differential and relative differential of the flow values. Values are given in a range that includes a possible measurement error. These values confirm that the use of gravity sets together with the infusion pump causes a low flow that does not meet the declared value.
5. Absolute and relative difference values for Set 5 (300 ml/h) and Set 6 (50 ml/h). 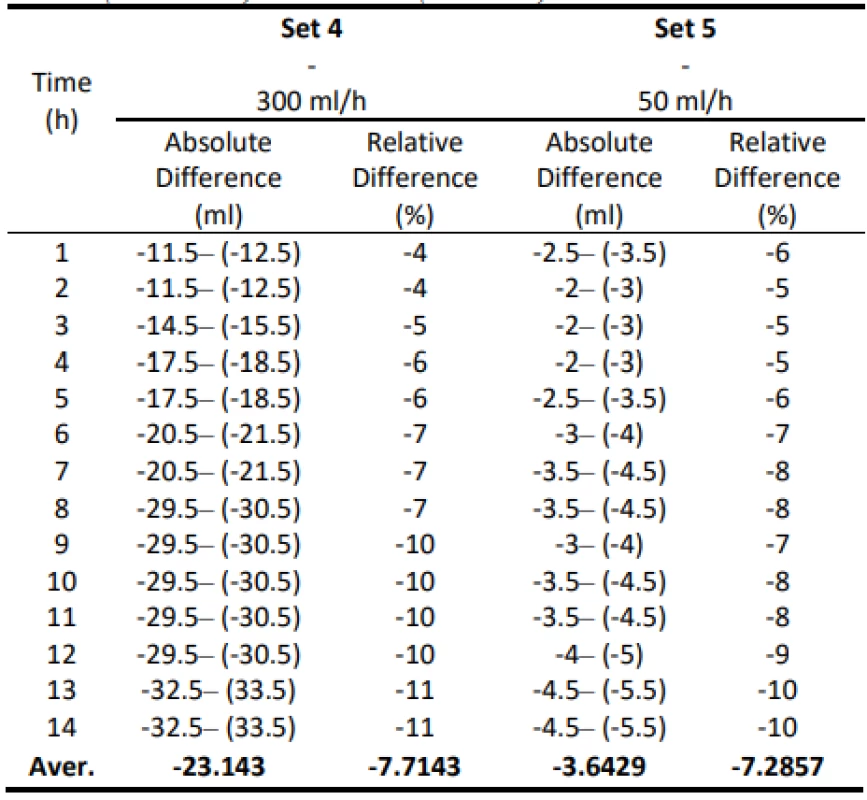
Mechanical elongation test
Mechanical tensile strength tests are used to assess changes in material properties of the infusion set. These were performed at the Department of Applied Mechanics, Faculty of Mechanical Engineering, VSB‑TUO. This laboratory is equipped with the modern Testometric M500-50CT (England) shredder.
A sample of the test material is firmly held between the jaws of the machine and stressed by stretching at a constant speed until the material ruptures. The course of the test is controlled by specialized software and recorded by the curve of the force applied to the elongation of the test sample.
In addition to this curve, the resulting test report includes accurate records of maximum force, stress, absolute elongation and tensile modulus. This quantity characterizes the strength of the material and its elastic deformation ability, as specified in Hook’s law for elastic deformation [16, 17]. For resilient polymeric materials, Hook’s law is limited to a small portion of the recorded curve. For this reason, for example, Young’s modulus is often not given for flexible PVC, but the material is characterized by a strength limit which is more significant due to the shape of the tensile curve. In this case, it is the stress exerted by the maximum force ? (?). The specific value of this limit for typical infusion set materials varies from source but is approximately in the range of 7–25 MPa [1].
Mechanical Elongation Test Parameters
The parameters of the tested sample (length, cross-section) and jaw movement speed, which was set to 50 mm/min for tests in this work, are entered in the machine. Sample control software parameters the device uses to calculate characteristic parameters include absolute elongation, strength, strength limit and modulus of elasticity. For the tubing to be tested, the original cross-section is determined as the content of the annulus enclosed by the outer (r2) and inner (r1 ) diameter of the tubing. The used tubing has a cross-section of 5.498 mm2. The stressed part was tested. The length of the test part of the infusion sets was always the same and was 10 cm. Unused sets have also been tested to compare the properties of used and unused sets.
Evaluation of Mechanical Elongation Test
The results of each tensile test are produced in the form of a protocol by a tear machine. The processing of the tensile strength test results of the infusion sets used is carried out in such a way as to make judgments about the different material properties of each type of set used, in particular whether these properties have been affected by the blades of the volumetric pump.
The values of absolute elongation, tensile strength, ultimate strength and modulus of elasticity determined by mechanical tests performed for the test sets are shown in Table 6. The mechanical test results for new, unused infusion sets are shown. Comparing the properties of the used set and the new set can serve to give a concrete idea of whether the use of the set affects its strength.
6. Overview of mean values of absolute elongation, tensile strength, strength limit and modulus of elasticity of tests according to the type of sets. Results of tests of unused sets. 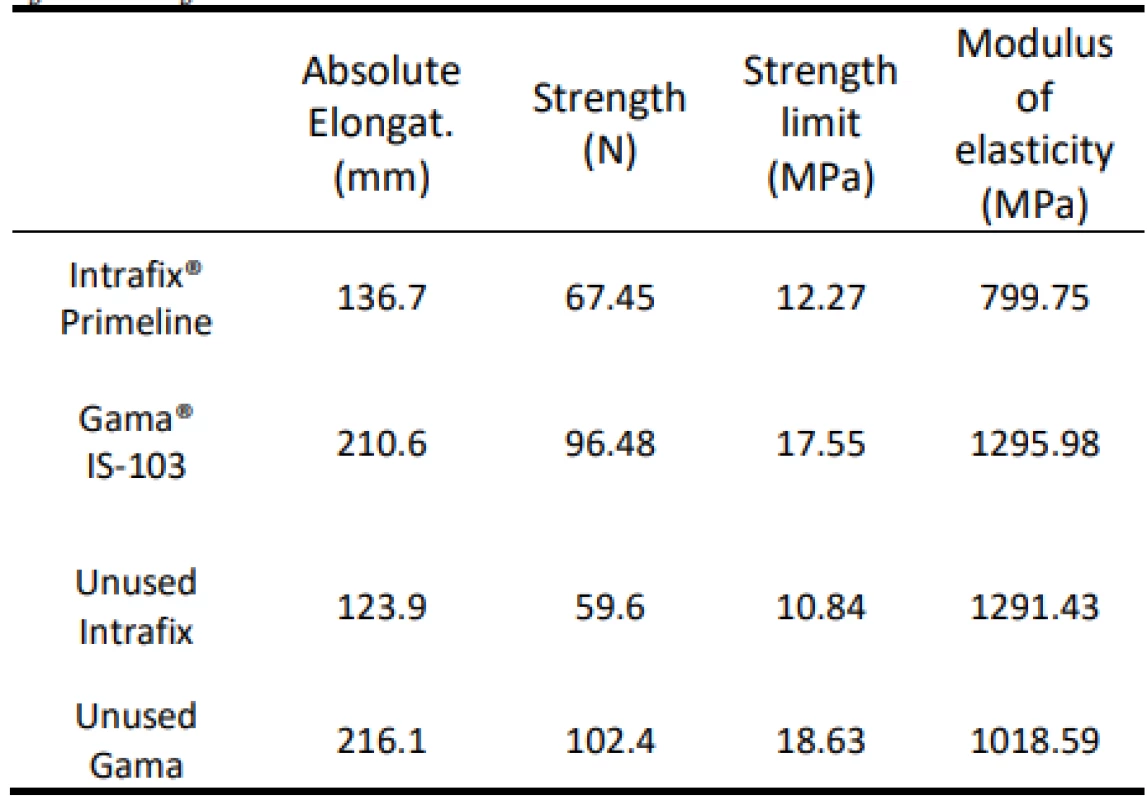
It is clear from the results that the monitored flow is not significantly influenced by the set flow rate and not affected by use. There are differences between the types of infusion sets. As a result, the Gama® IS-103 gravity sets have an 80 mm longer elongation than the Intrafix® Primeline universal sets. The elongation values are plotted in Figure 5.
5. The measured flow values compared to reference 50 ml/h. 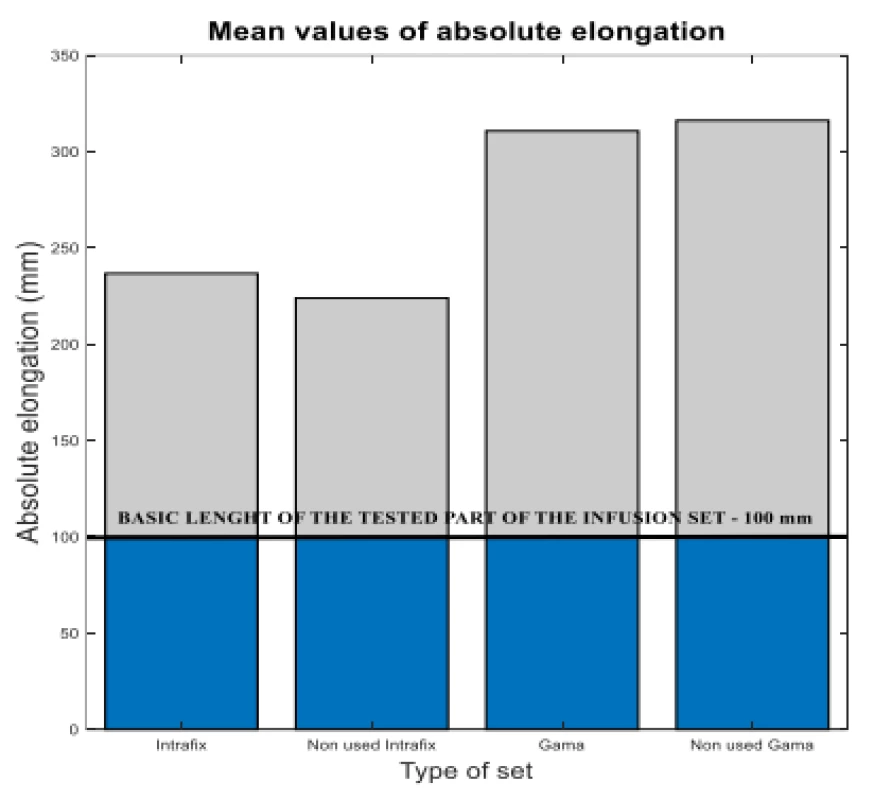
Discussion
Evaluation of flow measurement
The accuracy limit is the declared infusion accuracy in units of relative deviation. For Forlong 600II, the tolerance is ±3%.
From the above graphs, we can observe a decreasing tendency of the course of almost all measurements. The reason for reducing the flow is the compression of the tubing by the lamellar pumps to a smaller diameter and due to the less elasticity of the material, the tubing does not have the ability to return to its original state.
The best results were achieved with the Intra-fix® Primeline set, which was able to achieve correct results with a rate of ±3% over the 14 hours of the test. It was a measurement with a flow rate of 50 ml/h. However, in the other cycles, irrespective of the flow rate, it also showed values within the tolerances.
The Gama® IS-103 gravity sets have significantly lower values than universal sets. In no case was the measured value above the set flow limit, moreover, all performed measurement cycles did not reach their declared 3% limit by their average states. The average relative difference in flow was -6.57%. This is consistent with the assumption that the gravity set materials are harder than the pressure sets and exhibit less elasticity and compliance with peristaltic move-ments when operating the pump fins, resulting in a low flow rate at the start of the infusion cycle and a further continuous decrease.
The results of the measurements indicate that the type of the selected set has a greater impact on measurement accuracy. The different flow did not show much in the measured values. Intrafix® sets had greater accuracy and flow with a 50 ml/h setting with an average relative difference of flow 0.79%, while an average value of ‑1.97% at a flow rate of 300 ml/h. For the Gama® kits, in terms of the set flow rate, better results were achieved at a rate of 300 ml/h.
Evaluation of tensile strength tests
An important comparison of possible changes in the mechanical properties of infusion set materials due to stresses in the infusion pump is the relationship between the values of the used kits and the new unused pieces.
Strength and absolute elongation indicators show that even 14-hour use does not have to affect these material characteristics. However, the state when comparing the elastic moduli is surprising. While the new Intrafix set has a more than 50% increase over the average value of the pieces used, the elasticity modulus of the new Gamma set has dropped by almost 300 MPa. From the behaviour of the set, it can be concluded that the Intrafix universal set loses its elasticity by use, while the material of the Gama gravity kits needs more force to achieve the same elongation value after its use.
Influence of set destruction on quality of treatment
From all these findings, it can be concluded that the choice for pressure-based infusion therapy of the correct administration kit has a major influence on the dosing accuracy. From the safety point of view, the risk that swapping the type of infusion set can bring depends on many factors. The age, gender or physical fitness of the patient, or the nature of the active ingredient adminis-tered, its amount and concentration may play a role. It should be stressed, however, that the inaccuracies that have been achieved in the tests in this work are certainly not negligible for an intravenous treatment and that the staff or operator responsible for the infusion application should be cautious and avoid risk factors that could lead to misuse.
Conclusion
This work aimed to explain the influence of infusion set replacement on flow accuracy. The object of testing was universal infusion sets and gravity sets of two manufacturers to verify the destructive behaviour of these sets and the effect of destruction on the accuracy of flow using an infusion pump. The question was to verify the claim that the replacement of infusion pressure sets with gravity sets results in a worsening of therapy and to substantiate this claim with specific data obtained by experimental measurement. Destructive effects that may affect the quality of treatment can be manifested both by changing the set and the duration of treatment, for this reason it was measured in the work with infusion sets for 14 hours.
The obtained data showed that the infusion pump has a much smaller destructive effect on the universal sets, whose use for dosing is within the tolerated limits. On the other hand, measurements with gravity sets showed greater inaccuracies beyond the declared dosage value, which is likely to indicate that the gravity set materials are less resistant to the action of the pump. The material properties of the used sets were further verified by mechanical tensile strength tests, which showed a marked difference between the materials of the univer-sal and gravity sets and their different reactions to the action of the pump fins. Although 14-hour usage did not significantly affect the strength of both materials, their elasticity varied with the opposite trend, the decline in the universal set and the increase in gravity sets.
To further verify the conclusions of this work, it would be advisable to carry out a further flow measure-ment with multiple pieces of test kits from one manu-facturer, thereby refining the results and eliminating any measurement errors.
Acknowledgement
The work and the contributions were supported by the project SV450994 Biomedical Engineering Systems XV'. This study was also supported by the research project The Czech Science Foundation (GACR)2017 No. 17-03037S Investment evaluation of medical device development run at the Faculty of Informatics and Management, University of Hradec Kralove, Czech Republic. This study was supported by the research project The Czech Science Foundation (TACR) ETA No. TL01000302 Medical Devices development as an effective investment for public and private entities.
Ing. Klára Fiedorová
Faculty of Biomedical Engineering
VSB – Technical University of Ostrava
17. listopadu 15, Ostrava-Poruba
E-mail: klara.fiedorova@vsb.cz
Sources
- Cihak J, Augustynek M. Infuzní technika a hemodialyzační technika a technologie. VŠB-Technická univerzita Ostrava; 2013. ISBN 978-80-248-3100-8.
- Nickel B. Peripheral Intravenous Access: Applying Infusion Therapy Standards of Practice to Improve Patient Safety. Critical care nurse. 2019 Feb 1;39(1):61–71. DOI: 10.4037/ccn2019790
- Centre NC. Cost-sensitivity analysis: Monitoring and assessment strategies for intravenous fluid therapy in children. InIV Fluids in Children: Intravenous Fluid Therapy in Children and Young People in Hospital 2015 Dec. National Institute for Health and Care Excellence (UK). Bookshelf ID: NBK33814.
- Doyle GR, McCutcheon JA. Clinical procedures for safer patient care. BC Open Textbook Project; 2015.
- Magee P, Tooley M. The Physics, clinical measurement and equipment of anaesthetic practice for the FRCA. Oxford University Press; 2011 Sep 22. ISBN 9780199595150.
- Mayo M, Wolsky R, Baldini T, Vezeridis PS, Bravman JT. Gravity fluid flow more accurately reflects joint fluid pressure compared with commercial peristaltic pump Systems in a Cadav-eric Model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018 Dec 1;34(12):3132–8. DOI: 10.1016/j.arthro.2018.05.033
- Plumer AL. Plumer's principles and practice of intravenous therapy. Lippincott Williams & Wilkins; 2007. ISBN 0-7817-5944-7.
- Johnson G. Selecting and installing peristaltic pump tubing. Pharmaceutical Technology Europe. 2018;30(1):36–7. Available from: http://www.pharmtech.com/selecting-and-installing-peristaltic-pump-tubing
- Heinemann L, Waldenmaier D, Kulzer B, Ziegler R, Ginsberg B, Freckmann G. Patch Pumps: Are They All the Same?. Journal of diabetes science and technology. 2019 Jan;13(1):34–40. DOI: 10.1177/1932296818795150
- Xavier AR, Moreira CS, Neto AG, Lima AM, Neff H, Diasi FW. Real-time flow monitoring for a roller-based peristaltic mini-pump. In2018 IEEE International Instrumentation and Measure-ment Technology Conference (I2MTC) 2018 May 14 (pp. 1–5). IEEE. DOI: 10.1109/I2MTC.2018.8409790
- IEC 60601–2-24: Medical Electrical Equipment—Part2–24: Requirements for the Basic Safety and Essential Performance of Infusion Pumps and Controllers. Geneva: International Electro-technical Commission. 2012.
- ISO 8536-8 : 2015, Infusion equipment for medical use — Part 8: Infusion sets for single use with pressure infusion apparatus. 2. 5. Geneva, Switzerland, International Organization for Stan-dardization, 2015.
- ISO 8536-4 : 2019. Infusion equipment for medical use - - Part 4: Infusion sets for single use, gravity feed. 6. Geneva, Switzerland, International Organization for Standardization, 2019.
- Hochman V, Cihak J, Augustynek M. Interaction of infusion set and volumetric infusion pump and their impact on the quality of treatment. In 6th European Conference of the International Federation for Medical and Biological Engineering, Springer, Cham, 2015 (pp. 645–8). DOI: 10.1007/978-3-319-11128-5_161
- Chen J, Liu Z, Wang K, Huang J, Li K, Nie X, Jiang J. Epoxidized castor oil‐based diglycidyl‐phthalate plasticizer: Synthesis and thermal stabilizing effects on poly (vinyl chloride). Journal of Applied Polymer Science. 2019 Mar 5;136(9):47142. DOI: 10.1002/app.47142
- Taurino R, Sciancalepore C, Collini L, Bondi M, Bondioli F. Functionalization of PVC by chitosan addition: Compound stability and tensile properties. Composites Part B: Engineering. 2018 Sep 15;149 : 240–7. DOI: 10.1016/j.compositesb.2018.05.021
- Fernandez-Canal C, Pinta PG, Eljezi T, Larbre V, Kauffmann S, Camilleri L, Cosserant B, Bernard L, Pereira B, Constantin JM, Grimandi G. Patients’ exposure to PVC plasticizers from ECMO circuits. Expert review of medical devices. 2018 May 4;15(5): 377–83. DOI: 10.1080/17434440.2018.1462698
- Council E. COUNCIL DIRECTIVE 93/42/EEC concerning medical devices. Official Journal of The European Communities, Luxembourg. 1993.
- ISO 10993-1 : 2018. Biological evaluation of medical devices - - Part 1: Evaluation and testing within a risk management process. 5. Geneva, Switzerland, International Organization for Stan-dardization, 2018.
- ISO 14971 : 2007. Medical devices—Application of risk manage-ment to medical devices. 2. Geneva, Switzerland, International Organization for Standardization, 2007.
- Simon N, Décaudin B, Lannoy D, Barthélémy C, Lemdani M, Odou P. Mathematical and physical model of gravity-fed infusion outflow: application to soft-bag-packed solutions. European journal of drug metabolism and pharmacokinetics. 2011 Dec 1;36(4):197–203. DOI: 10.1007/s13318-011-0062-9
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2020 Issue 1-
All articles in this issue
- FREQUENCY AND DURATION OF OXIMETER DROP-OUTS IN THE NICU: AN OBSERVATIONAL STUDY
- FUNCTIONALIZATION OF POLYMERIC NANOFIBERS USING PLATELETS FOR MELANOCYTE CULTURE
- INFLUENCE OF THE USE OF GRAVITY SETS IN A PRESSURE VOLUMETRIC INFUSION PUMP WITH AN IMPACT ON THE ACCURACY OF INFUSION SOLUTION FLOWS
- MATERIALS SUITABLE TO SIMULATE SNOW DURING BREATHING EXPERIMENTS FOR AVALANCHE SURVIVAL RESEARCH
- VERIFICATION OF CLINICAL ACCURACY OF AUTOMATED NON-INVASIVE SPHYGMOMANOMETERS: IS IT APPROPRIATE TO USE BLOOD PRESSURE SIMULATORS?
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- VERIFICATION OF CLINICAL ACCURACY OF AUTOMATED NON-INVASIVE SPHYGMOMANOMETERS: IS IT APPROPRIATE TO USE BLOOD PRESSURE SIMULATORS?
- INFLUENCE OF THE USE OF GRAVITY SETS IN A PRESSURE VOLUMETRIC INFUSION PUMP WITH AN IMPACT ON THE ACCURACY OF INFUSION SOLUTION FLOWS
- FUNCTIONALIZATION OF POLYMERIC NANOFIBERS USING PLATELETS FOR MELANOCYTE CULTURE
- FREQUENCY AND DURATION OF OXIMETER DROP-OUTS IN THE NICU: AN OBSERVATIONAL STUDY
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



