-
Medical journals
- Career
Management after bariatric surgery
Authors: Dana Müllerová 1; Martin Fried 2
Authors‘ workplace: Department of Public Health and Preventive Medicine & 1st Medical Department, Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic 1; OB Clinic, Center for Treatment of Obesity and Metabolic Disorders & 1st Faculty of Medicine, CU, Prague, Czech Republic 2
Published in: Forum Diab 2018; 7(2): 126-131
Category: Review Article
Overview
Bariatric surgery is the only proven effective long term weight loss therapy for severe obesity and related co-morbidities. However, overall interdisciplinary support after bariatric surgery is extremely important in achieving long term success and preventing possible serious clinical disorders as well. Authors present algorithms for post-surgical patient follow-up, timings, meal progression and diet composition as well as relevant clinical, lab and nutritional checks linked to particular bariatric procedures. Recommended dosages of proteins, micronutrient supplementation after operations limiting absorption of nutrients, stomac capacity, or combined ones according contemporary guidelines are summarized. Management of post-bariatric patient in pre-gestational period and during pregnancy is also discussed.
Key words:
bariatric surgery, dietary management, nutritional deficiencies
Introduction
Bariatric surgery has become highly popular during the last 25 years. While Buchwald and Oien [1] have calculated that more than 344 thousand bariatric surgery operations were performed in 2008 worldwide, the number of procedures has risen to approximately 500 thousands in 2013 [2]. This is the result of obesity epidemic, increased proportion of severely obese and especially the fact, that bariatric and metabolic surgery is safe and highly effective therapy of clinically severe obesity and its metabolic comorbidities in long run.
Bariatric procedures can be categorised as predominantly restricting stomach capacity (restrictive), such as laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG) and greater curvature plication (GCP), combined, such as the most prevalent bariatric procedure in US, Roux-en Y gastric bypass (RYGB), and malabsorptive thus limiting intestinal absorption, such as biliopancreatic diversion (BPD) or biliopancreatic diversion with duodenal switch (BPD DS). Effect on weight loss is the greatest after malabsorptive procedures, followed by combined and finally by restrictive operations, as has been shown from several meta-analyses, i.e. by Buchwald and co-workers [3]. Inversely proportions to weight loss effectiveness, in general purely restrictive procedures lead to fewer serious adverse effects and are associated with lower risk of potential nutritional derangements postoperatively. Restrictive procedures act mostly through reducing capacity of stomach and as a result limit patient in food portion size. Some procedures, such as LSG, RYGB and potentially GCP decrease secretion of hydrochloric acid, thus compromise the oxidation of ferrous substances to ferritic ones. Depressed secretion of intrinsic factor of vitamin B12, may lead to lowered absorption of this vitamin in ileum.
RYGB, BPD and BPD-DS bypass duodenum, proximal jejunum and in BPD and BPD–DS even larger segments of proximal and mid part of small intestine. Duodenum and proximal jejunum play important role in the absorption of calcium, iron, zinc, magnesium, and copper; due to after RYGB, BPD and BPD-DS there is a limited absorption of these minerals. Moreover as biliopancreatic juices are diverted to more distal part of the small intestine (depending on the type of procedure), thereby causing maldigestion of macronutrients and limiting absorption of glucose and water soluble vitamins in the case of RYGP or in the case of BPD also the absorption of fat, vitamins A, D, E, K, vitamin B 12 and bile acids reabsorption. There is also higher risk of protein malnutrition especially after procedures that leave relatively short common limb.
Several studies focused on the risk of possible nutrient deficiencies, namely after RYGB and BPD were published [4,5]. However results of these studies differ significantly, depending on bariatric procedures modification such as the length of the alimentary limb in RYGB or the length of the common limb in BPD, the time of study after operation, algorithm of postoperative supplementation, weight loss achievement, food intolerances, diet composition, nutritional state of patients prior operation, etc. [6,7].
Surgical alterations in gastrointestinal anatomy may lead to the development of gastrointestinal maldigestive symptoms like vomiting, more prevalent after restrictive procedures, an early and late dumping syndrome after procedures bypassing pylorus, the state of hyperinsulinemic hypoglycaemia, lactose intolerance emerging in some patients after RYGB and BPD or steatorrhea, bloating and foul smelling associated mostly with BPD. These symptoms need nutritional management and have a tendency to disappear over time.
Bariatric surgery procedures improve metabolism and increase satiety through several pathways [8,9,10,11]. One of the possible mechanisms of action may be based on modulating nutrient-neuro-endocrine signalling of gastrointestinal tract to peripheral tissues by enhancing insulin sensitivity and improving beta cells function mediated through incretin changes after bariatric procedures. Changed gastrointestinal signals after bariatric surgery may influence not only satiety, but also, in the integration with other peripheral signals, may arrange a new set - up point for body weight, metabolism and nutrition intake control by brain.
Taking into account all these facts postsurgical patients need comprehensive dietary management in both short and long-term postoperative period (tab. 1).
1. Goals of dietary management in both short and long-term postoperative period 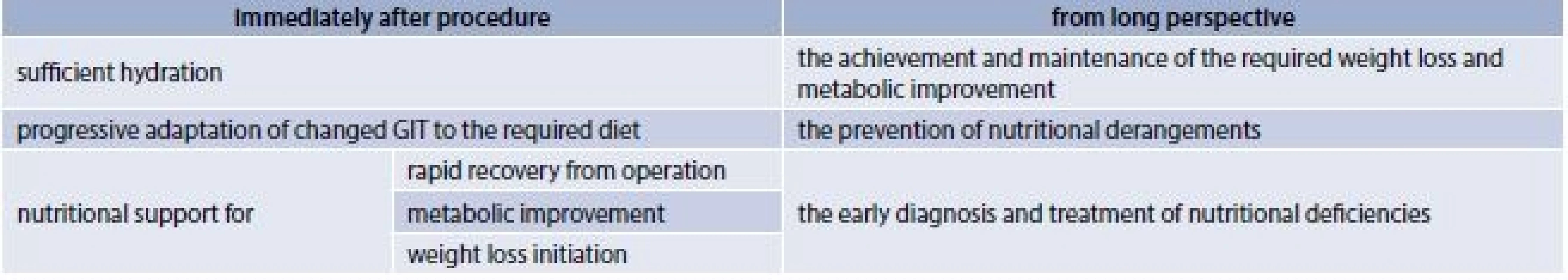
The early post-surgery diet should be focused in general on sufficient hydration, with fluid intake over 1.5 l/ per day, maintaining a urine output of more than 30mL/h. However on the other hand it´s also important to avoid volume overload by drinking smaller volumes rather frequently, and meet protein needs depending on type of bariatric procedure of minimally 60 g/day [12]. Progression of diet consistency from clear liquids to full liquids, than from pureed to soft solid and to solid diet is also very important, especially after restrictive operations. Patients should be instructed about their eating manner: from sipping to eating multiple small meals each day, chewing thoroughly and waiting at least 30 minutes between separately consuming liquids and solids [13,14,15] .
During the initial clear liquids phase usually introduced after restrictive procedures on postoperative day 1 – 2 or even later on (post-op days 2–3) following combined (hybrid) or malabsorptive procedures, patients start on sips 30–60 ml per hour of no calories, no sugar, no caffeine, no carbonated water and salty fluids (crystal light).
Next, so called full liquid phase is composed of liquid or semiliquid consistency of food and sufficient amount of protein in the form of protein-rich liquids, as chicken broth, skimmed or not fat milk or soymilk, sugar free gelatine or protein shakes (20 g of protein/serving). Patient should be instructed to sip 2.5 l of these liquids with content more than 60 g protein daily.
Pureed diet is based on soft, moist, diced, ground or pureed protein sources as tolerated, given 4–5 times daily in different volumes depending on the type of the procedure, completed by 2 l of liquids daily. Pureed chicken, turkey, tuna, but also peeled pureed fruit and vegetables, pudding, oatmeal, yogurt are gradually added into the diet with the aim to reintroduce foods from all different food groups. During pureed phase many bariatric centres recommend initiation of usage of chewable multiple vitamin and minerals supplements.
When soft solid diet is advanced special attention must be given to mindful eating and chewing. This apply especially after gastric band procedures, where stomach restriction together with presence of relatively narrow channel connecting proximal and distal parts of the stomach may increase risk of obstruction in case food is not thoroughly chewed. Soft solid diet should be focused on soft consistency and on variety of foods from each food category, however patients are instructed to avoid food sources of carbohydrate such as rice, pasta, bread, until consuming 60 g of protein, completed by peeled soft fruit and well-cooked vegetables. During this phase foods should be chopped or diced, encompassing low content of sugar and fat, given in 3–6 small meals, separately from liquids. Recommended daily dose of protein differ according to procedure, with the daily amount of 60 g or more for those with purely restrictive or RYGB or should be in the range of 80 to 120 g for patients with BPD (DS) [12].
The timing of previously described gradual reintroduction of diet generally differs according to procedures (tab. 2), but also according to bariatric centers. There is no set standard but the adjustments of diet protocol should be made according to each patient`s individual needs.
2. The minimal requirement on laboratory test after bariatric operations 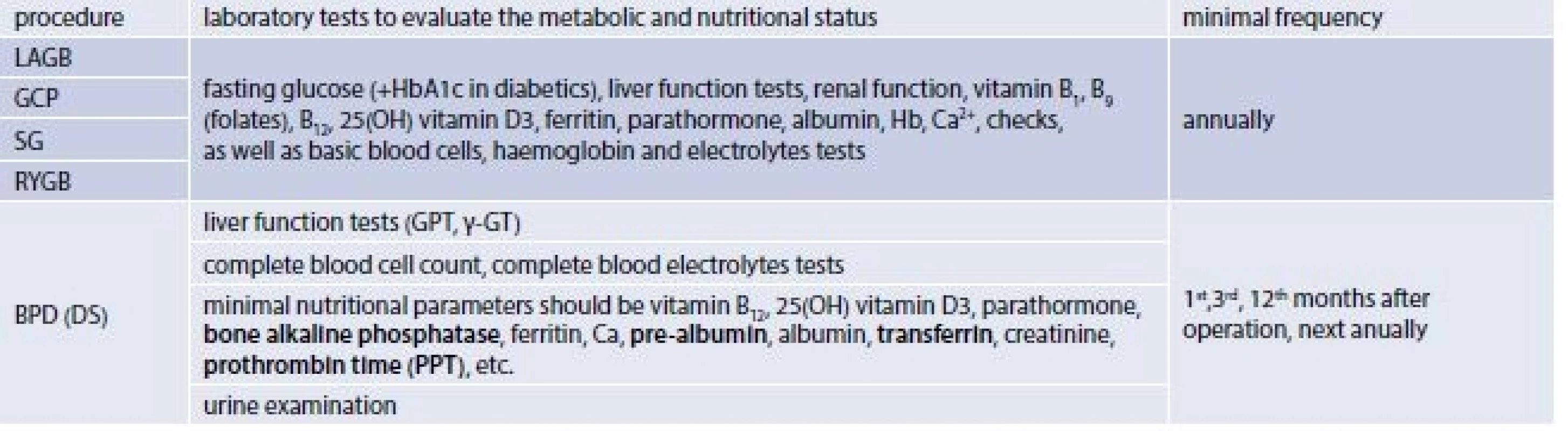
A healthy balanced diet for early postsurgical bariatric patients must be energy limited, based on protein, calcium, and iron sources, completed by vegetables and fruit and by limiting the amount of carbohydrate sources. There are foods, which generally should be avoided because of free or soft calories and the risk of stoma obstruction as snack foods (French fries, potato chips, nuts), high-calorie sweets, carbonated sweetened or alcoholic beverages, sweetened water, fibrous vegetables (dried beans, peas, celery and cabbage).
The late post-surgery diet and minimal requirements for nutritional management are defined i.e. in the Interdisciplinary European guidelines on surgery of severe obesity (IEGSSO), published in 2008 by the Bariatric scientific collaborative group [16] and in the Interdisciplinary European guidelines on bariatric and metabolic surgery, published in 2014 by International Federation for the Surgery of Obesity – European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); and European Association for the Study of Obesity Management Task Force (EASO OMTF) [17]. According to these guidelines all patients after bariatric procedures require regular lifelong qualified surveillance. The minimal Requirements for Follow-up after Bariatric Surgery recommend: a check-up after 1 month after initial surgery, a subsequent minimal follow-up every 3 months during the first postoperative year, than every 6 months during the second year and annually thereafter. In the case of LAGB band adjustments should be performed according to the individual patient weight loss and the type of the implant, during the second postoperative year the minimal requirement for follow up is only one check-up. Follow-up should be carried out by the interdisciplinary team and should include dietary change/behavioural modification/physical activity interventions and encouragement as well as pharmacology support and surgical revision if appropriate [17]. As of nutritional requirements follow-up close weight loss control is recommended at each follow-up visit as well as check of nutritional value of ingested food and supplements (diet composition from diet records, control of food tolerance and supplements usage), comprehensive management of maldigestive symptoms, and monitoring of laboratory values and an immediate correction of nutritional and metabolic changes is desirable (tab. 2).
According to the Swedish Obese Subject Study published data [18], postoperative weight development can be divided into 3 parts: weight loss phase where the vast of majority of weight loss is accomplished in about 12 to 18 months postoperatively, followed by approximately 5 year of weight regain phase, in which about one –tenth to one-third of the initial weight loss is regained and the weight stability phase lasting for 6–15 years, during which the study was conducted.
Different weight loss outcomes according to the procedure performed as well as patient related variability in weight loss can be expected. However, in case of grossly inadequate (low) weight loss it is useful to seek for technical-surgical failure, and/or look for potential presence of maladaptive eating behaviour, altered and poor compliance with dietary recommendations and behavioural changes, non-compliance with dietary plan or presence of concomitant psychological complications, which need to be treated by multidisciplinary team of professionals.
Basic nutritional recommendations according the Interdisciplinary European guidelines on surgery of severe obesity (IEGSSO).
Following restrictive (food limitation) operations metabolic and nutritional status of patients should be regularly monitored to prevent nutrients deficiency and allow appropriate supplementation. It is essential that the patients are provided with continuous educational nutrition support which should decrease the risk of vomiting. It is important to turn patient´s attention to early signs of fullness, to teach them how to eat slowly, chew food well and drink plenty of non-caloric, non-carbonated fluids, separately from solid foods. Portion size should be reduced according to the type of food limitation operation performed, and in the case of further insufficient weight loss in patients with gastric band an adjustment of the band may be considered.
After combined bariatric procedures, oral vitamin and mineral supplements should be routinely prescribed. Laboratory tests should be carried out at least annually to monitor the patients` nutritional status and deficiencies should be corrected usually by parenteral supplementation. Patients should be educated as to how to manage possible lactose intolerance, dumping syndrome or hyperinsulinemic hypoglycaemia. In case of secondary lactose intolerance, patients displaying a wide range of gut and systemic symptoms, including bloating, gut pain, diarrhoea or constipation, severe headaches, severe fatigue. Because lactose intolerance has tendency to disappear with time after operation symptoms may be temporarily ameliorated through decrease intake of lactose up to 12 g daily according to patient individual tolerance [19]. Lactose may be completed with oral lactase, and probiotics administration. Dumping syndrome may produce symptoms such as postprandial sensation of abdominal pain and distension, with vasomotor changes including flushing, palpitations, and dizziness [19], vomiting, and/or diarrhoea and hypoglycaemia. These symptoms can usually be prevented by avoiding simple carbohydrates and by including proteins to each meal or snack, eating and drinking slowly, chewing foods thoroughly, drinking liquids between meals and before, however drinking together with meals should be avoided. Reclining position for 30 minutes after meals may help to reduce the undesirable symptoms. Dumping syndrome usually spontaneously disappears after 12–18 months after operation. Some patients after RYGB experience hyperinsulinemic hypoglycaemia [21], which may be resistant to nutritional interventions such as avoiding simple sugars, or pharmacological treatment by verapamil or acarbose, the partial pancreatectomy in serious states may be necessary [22].
Recommendations for minimal follow up after operations limiting absorption of nutrients are slightly more complex because of higher risks of malnutrition. Thus laboratory tests should be performed 1, 4, 12 months after BPD or BPD-DS and then at least annually, with the aim to early diagnose and treat potential nutritional deficiency [17]. Education of patients should be targeted to their compliance with lifelong nutrient supplementation and regular follow ups. Their diet should contain sufficient amount of protein, and should be low fat. Proton pump inhibitors may be useful in case of presence of hyperacidity symptoms and in case of bloating or foul smelling stools oral treatment with neomycin or metronidazole or pancreatic enzymes is recommended.
For all bariatric surgery patients is recommended to use daily vitamin and micronutrient supplements to compensate for their possible reduced intake and absorption. A routine supplementation after SG, RYGB, BPD and BPD DS consists of one or two multivitamin with minerals tablets. It´s recommended to supplement separately calcium in a daily dose of 1g preferably in the form of citrate, completed by vitamin D 2000–4000 IU daily [23]. However during all check out is necessary to actively seek out possible macro - and micronutrients deficiencies, validate clinical symptoms by laboratory tests and correct them by appropriate nutritional management. Except protein malnutrition, occurring especially after BPD (DS) and RYGB, the most prevalent deficiencies are: iron, calcium, vitamin D, vitamin B12, folate, vitamin B1, vitamin K, vitamin A, vitamin E, zinc, copper.
Iron deficiency should be treated by daily oral dose of 60 – 180 mg of elemental iron in the form of fummarate. Cobalamin – vitamin B12 may be substituted orally in dose 100–1000 µg daily, however probably the best way of administration of vitamin B12 is intramuscular injection 1000 µg monthly or 3000 µg every six months [12,23]. Patients with preoperative or postoperative already developed biochemical deficiency need even higher doses: vitamin A (10000 -20 000 IU/d), vitamin D (400–50000 IU/d), vitamin K (1 mg/d), folate (400–1000 µg), thiamine (initially 500 mg daily intravenously, than stepwise reduce to100 mg orally [12,23]. However individual adjustment of supplementation according to laboratory values is imperative to prevent not only deficiencies but also the adverse effect from overdoses of nutrients [24]. Restrictions for simultaneous administration of certain supplements in order to prevent undesirable interactions, for example iron – calcium, iron - zinc, zinc – copper should be respected.
The most prevalent nutritional dysbalancies and disorders after bariatric surgery are anemia, caused by iron, vitamin B12, or folate deficiencies; metabolic bone disease which needs the care of a specialist and in some cases treatment with mega doses of vitamin D [13,25,26]. Peripheral neuropathy caused by thiamine and vitamin B12 is also a relatively frequent problem. Protein malnutrition is not diagnosed that frequently after bariatric surgery, however attention in this regards should be paid no only to post BPD (DS) patients, but to post RYGB patients as well [27].
Relatively rare nutritional deficiencies after bariatric surgery usually presented in literature as case reports are listed in tab. 3.
3. The timing of gradual reintroduction of diet according to particular procedures 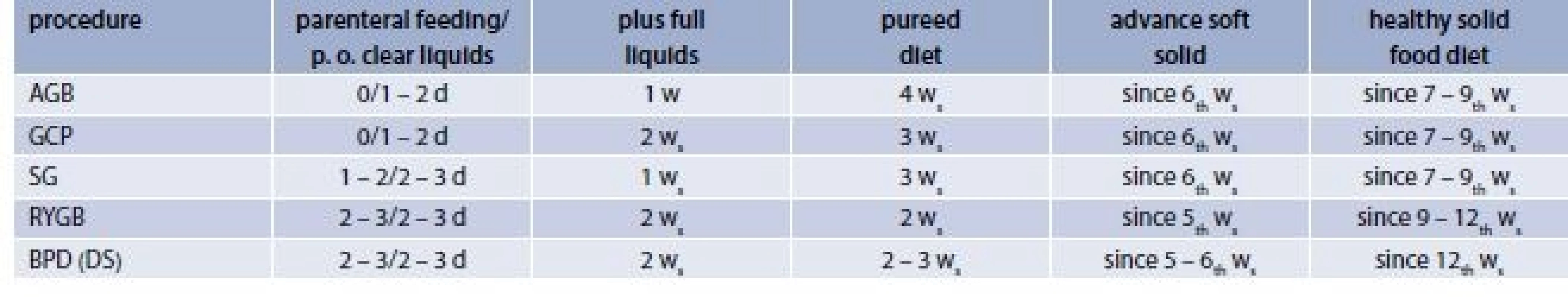
d – days w – week 4. Relatively rare nutritionally determined problems after bariatric surgery 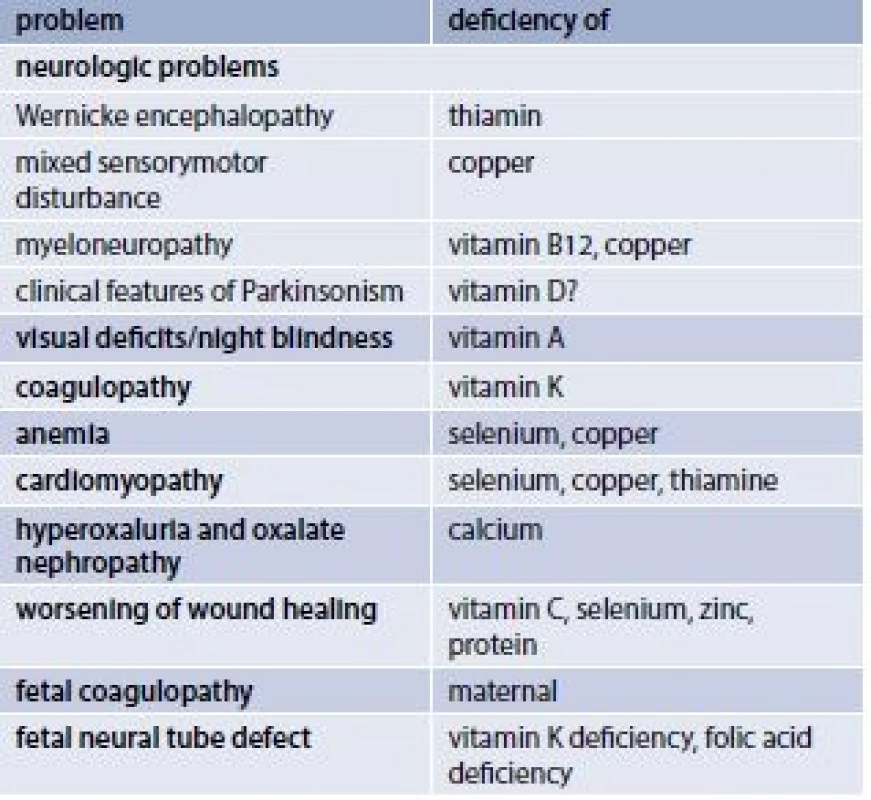
Special attention is required in patients with protracted vomiting after any bariatric procedure. They should be screened for thiamine deficiency and for mineral derangement. Which is the best diagnosed by the determination of the effect of thiamin pyrophosphate in erytrocyte thiamin transketolase, where low erytrocyte thiamin transketolase activity with greater than16% response to thiamin pyrophosphate is diagnostic of thiamine deficiency [28]. In patients displaying neurologic symptoms suggestive of thiamine deficiency, ophthalmoplegia, ataxia, and encephalopathy with unexpected mental status changes, weakness, dizziness, blurred vision, confusion, disorientation, (Wernicke encephalopathy), an aggressive parenteral supplementation of thiamine 500 mg/d should be started immediately after blood samples have been drawn.
Specific care should be provided to postbariatric women in the child bearing period. Women are generally recommended to delay pregnancy for at least 12–18 months after surgery. During this pre-gestational period it is important to correct nutritional deficiencies and supplement folic acid in a daily dose of 1 mg. A daily intake of higher than 1600 kcal is recommended during pregnancy to achieve moderate weight gain of 6 to 9 kg or 7 to 11.5 kg in case of obesity or overweight respectively [29]. Monitoring of protein, iron, calcium, vitamin D, vitamin A, and other nutrients in the diet and according to plasma nutritional laboratory levels is important.
Conclusion
Apart from limited absorption of nutrients, food restriction and change of satiety, which are considered as part of treatment strategy, beside food intolerance, which should be under the control of dieticians, and exceptionally surgical and metabolic complications, the risk of nutritional derangement rises with the degree of weight loss and with patient non - compliance to diet, supplements and follow ups. The highest risk factor is the lack of patient adherence to both supplement recommendation and follow ups. Poor compliance to the diet recommendation is modifiable by repeated patient centred education by a multidisciplinary team. This team basically consists of a medical physician, surgeon, gastroenterologist or another specialist, dieticians and psychologist. It is well known and evidence based that bariatric surgery is associated with: long term weight loss, the improvement and disappearance of metabolic co-morbidities, and decreased overall mortality. However outcomes of these procedures, and especially prevention and early treatment of potential nutritional deficiencies with potential serious health effect, depend not only on the skills of surgeons, and type of bariatric procedure, but as well on multidisciplinary support and patient-centred education prior to and after surgery, from short and long term follow ups, where the nutritionists and dieticians play an important role.
Acknowledgements: Supported by the Charles University Research Fund (project number Q 39)
Received 14. 2. 2018
Accepted 31. 3. 2018
doc. MUDr. Dana Müllerová, Ph.D.
Sources
- Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg 2009; 19(12): 1605–1611. Available on DOI: <http://dx.doi.org/10.1007/s11695–009–0014–5>.
- KhorgamiY, Shoar S, Andalib A et al. Trends in utilisation of bariatric surgerz, 2010–2014:sleeve gastrectomy dominates. Surg Obes Relat Dis 2017; 13(5): 774–778. Available on DOI: <http://dx.doi.org/10.1016/j.soard.2017.01.031>.
- Buchwald H, Avidor Y, Braunwald E et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292(14): 1724–1737. Available on DOI: <http://dx.doi.org/10.1001/jama.292.14.1724>. Erratum in JAMA 2005; 293(14): 1728.
- Skroubis G, Sakellaropoulos G, Pouggouras K et al. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg 2002; 12(4): 551–558. Available on DOI: <http://dx.doi.org/10.1381/096089202762252334>.
- Aasheim ET, Björkman S, Søvik TT et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr 2009; 90(1): 15–22. Available on DOI: <http://dx.doi.org/10.3945/ajcn.2009.27583>. Erratum in Am J Clin Nutr 2010; 91(1): 239–240.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010; 26(11–12): 1031–1037. Available on DOI: <http://dx.doi.org/10.1016/j.nut.2009.12.003>.
- Bloomberg RD, Fleishman A, Nalle JE et al. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg 2005; 15(2): 145–154. Available on DOI: <http://dx.doi.org/10.1381/0960892053268264>.
- Rubino F, R’bibo SL, del Genio F et al. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010; 6(2): 102–109. Available on DOI: <http://dx.doi.org/10.1038/nrendo.2009.268>.
- Ochner CN, Gibson C, Shanik M et al. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond) 2011; 35(2): 153–166. Available on DOI: <http://dx.doi.org/10.1038/ijo.2010.132>.
- Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia 2009; 52(11): 2270–2276. Available on DOI: <http://dx.doi.org/10.1007/s00125–009–1511–8>.
- Scopinaro N. Biliopancreatic diversion: mechanisms of action and long-term results. Obes Surg 2006; 16(6): 683–689. Available on DOI: <http://dx.doi.org/10.1381/096089206777346637>.
- Mechanick JI, Kushner RF, Sugerman HJ et al. [American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery]. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 2009; 17(Suppl 1): S1-S70. Available on DOI: <http://dx.doi.org/10.1038/oby.2009.28> Erratum in Obesity (Silver Spring) 2010; 18(3): 649.
- Snyder-Marlow G, Taylor D, Lenhard MJ. Nutrition care for patients undergoing laparoscopic sleeve gastrectomy for weight loss. J Am Diet Assoc 2010; 110(4): 600–607. Available on DOI: <http://dx.doi.org/10.1016/j.jada.2009.12.022>.
- Barth MM, Jenson CE. Postoperative nursing care of gastric bypass patients. Am J Crit Care 2006; 15(4): 378–387; quiz 388.
- Furtado LC. Nutritional management after Roux-en-Y gastric bypass. Br J Nurs 2010 ; 19(7): 428–436. Available on DOI: <http://dx.doi.org/10.12968/bjon.2010.19.7.47440>.
- Fried M, Hainer V, Basdevant A et al. Interdisciplinary European guidelines on surgery for severe obesity. Obesity Facts 2008; 1(1):52–59. Available on DOI: <http://dx.doi.org/10.1159/000113937>.
- Fried M, Yumuk V, Opert JM, et al. [International Federation for Surgery of Obesity and Metabolic Disorders –European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); European Association for the Study of Obesity Management Task Force (EASO OMTF)]. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 2014; 24(1): 42 : 55. Available on DOI: <http://dx.doi.org/10.1007/s11695–013–1079–8>.
- Sjöström L, Narbro K, Sjöström CD et al. [Swedish Obese Subjects Study]. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357(8): 741–752. Available on DOI: <http://dx.doi.org/10.1056/NEJMoa066254>.
- Wilt TJ, Shaukat A, Shamliyan T et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep) 2010; (192): 1–410.
- Salameh BS, Khoukaz MT, Bell RL. Metabolic and nutritional changes after bariatric surgery. Expert Rev Gastroenterol Hepatol 2010; 4(2): 217–223. Available on DOI: <http://dx.doi.org/10.1586/egh.09.67>. Erratum in Expert Rev Gastroenterol Hepatol 2010; 4(3): 386.
- Marsk R, Jonas E, Rasmussen F et al. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia 2010; 53(11): 2307–2311. Available on DOI: <http://dx.doi.org/10.1007/s00125–010–1798–5>.
- Ziegler O, Sirveaux MA, Brunaud L et al. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 2009; 35(6 Pt 2): 544–557. Available on DOI: <http://dx.doi.org/10.1016/S1262–3636(09)73464–0>.
- Mia MA, Mechanick JI. Nutritional and Micronutrient care of bariatric surgery patients: Current evidence update. Curr Obes Rep 2017; 6(3): 286–296. Available on DOI: <http://dx.doi.org/10.1007/s13679–017–0271-x>.
- Aarts EO, Janssen IM, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obes Surg 2011; 21(2): 207–211. Available on DOI: <http://dx.doi.org/10.1007/s11695–010–0316–7>.
- Bacci V, Silecchia G. Vitamin D status and supplementation in morbid obesity before and after bariatric surgery. Expert Rev Gastroenterol Hepatol 2010; 4(6): 781–794. Available on DOI: <http://dx.doi.org/10.1586/egh.10.69>.
- Williams SE. Metabolic bone disease in the bariatric surgery patient. J Obes 2011; 2011 : 634614. Available on DOI: <http://dx.doi.org/10.1155/2011/634614>.
- Faintuch J, Matsuda M, Cruz ME et al. Severe protein-calorie malnutrition after bariatric procedures. Obes Surg 2004; 14(2): 175–181. Available on DOI: <http://dx.doi.org/10.1381/096089204322857528>.
- Malone M. Recommended nutritional supplements for bariatric surgery patients. Ann Pharmacother 2008; 42(12): 1851–1858. Available on DOI: <http://dx.doi.org/10.1345/aph.1L321>.
- Kuehn M. Guideline for pregnancy weight gain offers targets for obese women. JAMA 2009; 302(3): 241–242. Available on DOI: <http://dx.doi.org/10.1001/jama.2009.964>.
Labels
Diabetology Endocrinology Internal medicine
Article was published inForum Diabetologicum

2018 Issue 2-
All articles in this issue
- The current situation in the management of obese patients in Slovakia: the concept of national comprehensive obesity management in the Slovak Republic
- The management of the obesity-induced hypertension
- Is obesity a real cardiovascular risk factor?
- Obesity paradox in patients with heart failure
- Nonalcoholic fatty liver disease and metabolic syndrome
- Can bariatric surgery help in the treatment of obese type 2 diabetic patients?
- Predictors of weight loss and weight regain after bariatric surgery
- Management after bariatric surgery
- Forum Diabetologicum
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Nonalcoholic fatty liver disease and metabolic syndrome
- The current situation in the management of obese patients in Slovakia: the concept of national comprehensive obesity management in the Slovak Republic
- Obesity paradox in patients with heart failure
- Can bariatric surgery help in the treatment of obese type 2 diabetic patients?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

