-
Medical journals
- Career
Detection of fetal major structural anomalies at the 11-14 ultrasound scan in an unselected population
Authors: M. Novotná; L. Hašlík; K. Švabík; Z. Žižka; H. Belošovičová; M. Břešťák; P. Calda
Authors‘ workplace: Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague, Czech Republic. Chairman Prof. MUDr. A. Martan, DrSc.
Published in: Ceska Gynekol 2012; 77(4): 330-335
Overview
Objective:
The aim of the study was to determine the efficacy of the 11-14 week scan in detecting fetuses with structural anomalies.Study design and methods:
Prospective interventional study in an unselected population of pregnant women in a 5-year period (2003-2008) in a single ultrasound unit. 8889 fetuses with median CRL 65mm (45-84mm) were examined. Continuing pregnancies were rescanned at 20-22 weeks. Actual structural anomalies among newborns from the studied group were obtained from our computerized database.Results:
The median maternal age was 30 years (14-50 years). The incidence of anomalies was 16.08 per 1000 (143/8889). Of these, 99 of the 143 were detected with prenatal sonography. 46.9% (67/143) of all anomalies were detected at the 11-14 week scan. Later in pregnancy, another 22.3% (32/143) of structural anomalies were detected.Conclusions:
67.7% of all antenatally detected malformations by ultrasound were recognized in the 11 14 week scan. Obviously, the second trimester scan cannot be abandoned, as it provides effective detection of other anomalies.Key words:
fetal structural abnormalities, first trimester, prenatal diagnosis, ultrasound.INTRODUCTION
Prenatal ultrasonography has a crucial role in detection fetal malformations. When serious anomalies are recognized in the first trimester, termination of the pregnancy is possible with fewer complications and/or there is time for planning for further follow-up and interventions.
There is increasing evidence that early ultrasonography is a useful tool in screening for fetal structural abnormalities in low-risk populations. Reported detection rates of fetal structural abnormalities vary between 22.3% and 64.7% [2, 12, 13, 14, 17]. More than 80% of the most common fetal malformations develop before 12 weeks‘gestation, therefore with good visualization of the fetus at this stage, these should be detectable [12].
The aim of this study was to determine the efficacy of the 11–14 week routine scan in detecting fetuses with fetal structural anomalies in an unselected low risk population attending our clinic.
MATERIAL AND METHODS
We carried out a prospective interventional study in the unselected population of pregnant women in a 5-year period (2003–2008) in a single ultrasound unit.
Pregnant women are referred by their physicians for first trimester serum screening of free beta hCG (human chorionic gonadotropin) and PAPP-A (pregnancy-associated plasma protein A) and thereafter for ultrasound screening in our unit in the Prague metropolitan area. Costs generally are covered by health insurance. The maternal serum free beta hCG and PAPP-A are preferably obtained in the 10+0 weeks of pregnancy (+/ - 7 days) [17]. The results of biochemical testing are stored in the database. The ultrasound scan is ideally scheduled later in the 12th week of pregnancy. If the discrepancy between menstrual (LMP – last menstrual period) and ultrasound gestational was > 7 days, re-dating per the ultrasound was accepted. The LMP was useful only to guide the timing of the “dating” scan [2].
In that 12th week, we perform the malformation scan and establish the risks for trisomy 21, 18 and 13. The pregnant women are immediately given the status of the morphology of the fetus (or fetuses) and the results of trisomy 21, 18, 13 individual risk estimations. The women with continuing pregnancies are scheduled for the 20–22 week scan and then for a 30–32 week scan. This study focused on the detection of morphological malformations only. We use the Astraia TM database for perinatal studies for all patients delivering in our hospital. The information about postpartum detected malformations was obtained from the neonatal database (Medea, Stapro). The 11–14 week scan is a routine policy in our center since the year 2002. Each patient signed a consent for the ultrasound examination. The data were compiled anonymously.
Inclusion criteria were 1. live pregnancy at the time of examination and a CRL (crown-rump length) 45–84 mm at the first scan (11+1 – 14+0 weeks); 2. at least one of the examinations in the second or third trimester. Recorded during the first trimester scan for purposes of this study were: the number of fetuses, chorionicity of twin pregnancies, CRL, nuchal translucency thickness, and fetal structural defects. Fetal evaluation consisted of a detailed examination of the skull, brain, spine, abdominal wall, extremities, and visualization of stomach and bladder. Transvaginal sonography was not performed as part of the routine 11–14 week scans, but was undertaken when the visualization was suboptimal or a structural abnormality was suspected by trans-abdominal scan.
Patients undergoing first trimester scan were appointed for second trimester scan in the 20–22 weeks range and again between 30–32 weeks. The ultrasound examinations were performed by 3 supervisors and 20 operators with at least two years of experience in ultrasound scanning. The operators were trained according to the standards of the Fetal Medicine Foundation of London [13]. The equipment used during the study was either a LOGIQ 9 GE with trans-abdominal M7C and trans-vaginal E8C probes, or Antares Acuson with trans-abdominal CH6-2 and EV9F4 trans-vaginal probes. Minor structural defects such as isolated borderline ventriculomegaly (atrial width of 10–15 mm) [14], mild hydronephrosis (anteroposterior diameter of the renal pelvis of less than 6 mm in the second trimester and less than 10 mm in the third trimester) [1, 8] or choroid plexus cysts were not included in the calculations.
Increased nuchal translucency not accompanied by structural defects was not considered as an abnormality. Pregnancies with major anomalies were offered termination in agreement with local law.
RESULTS
The median gestational age at the first trimester scan was 12 weeks + 6 days (median CRL 65 mm; min 45 mm – max 84 mm). The median maternal age of the studied population was 30.0 years (14–50 years).
A total of 9150 live fetuses in the first trimester were examined. 170 morphologically normal fetuses (at the first ultrasound scan) were lost from the follow-up. 91 pregnancies (66 fetuses with chromosomal aberations) were terminated. A total of 8889 fetuses underwent both 11–14 week scan and a routine second or third trimester scan (346 twin pregnancies, one set of triplets and 8194 singletons)(Figure 1). 568 pregnancies (568/8889 =6.39%) were from the IVF program (in vitro fertilization program), (119 twins, 449 singletons).
Figure 1. In the first trimester, 67.7% of prenatally diagnosed malformations (67/99) were detected. 69.2% (99/143) of malformations were found prenatally. 
143 fetuses had major structural abnormalities, 99 of which were found prenatally (69.2%) and 44 (30.8%) postpartum. The overall prevalence of fetuses with major structural abnormalities was 16.08 per 1000 (143/8889).
46.9% of all malformations (67/143) were diagnosed at the 11–14 week scan (Table 1). Later in pregnancy, another 32 structural anomalies were found (32/143 = 22.3%) (Table 2). The most common abnormalities in the first trimester were cystic hygroma (30/67), followed by omphalocele (13/67) and megacystis (7/67). The detection rates for fetal abnormalities in the first trimester were: central nervous system defects 50% (4/8), neck anomalies 100% (30/30), neural tube defects 0% (0/2), heart anomalies 7% (1/13) and limb defects 23% (3/13). Only 14% (1/7) of fetuses with a major cardiac defect had a nuchal translucency above the 95th percentile. In 3 of these cases, the fetuses had also other minor anomalies, and these pregnancies were terminated due to genetic defects. A total of 99/143(69.2%) structural abnormalities were found prenatally and of these 67.7% (67/99) were detected in the first trimester.
1. Structural abnormalities detected in the first trimester 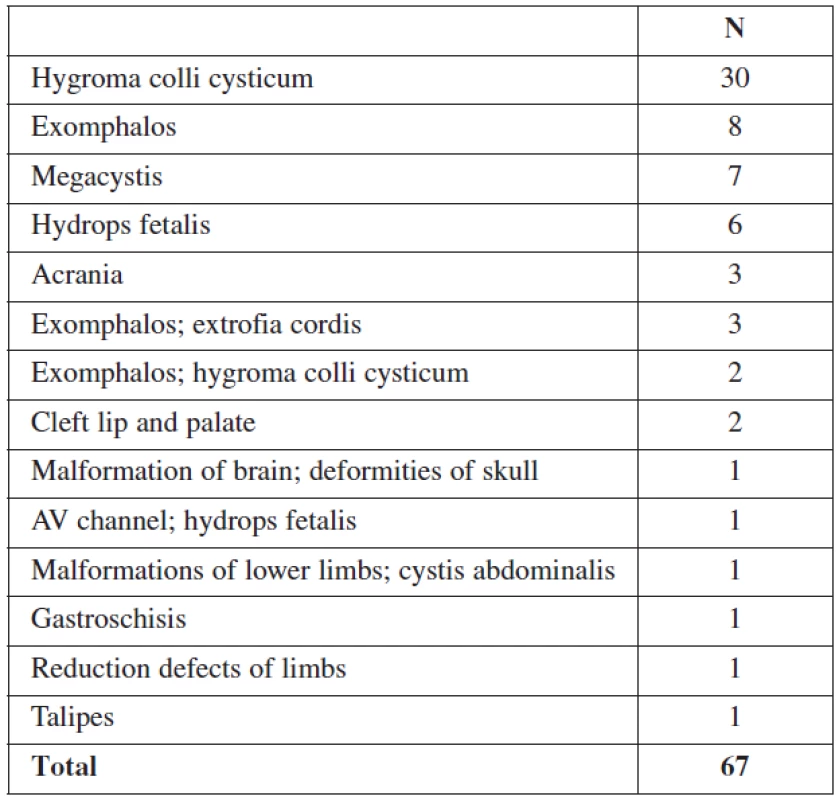
2. Structural abnormalities detected by ultrasound after the first trimester 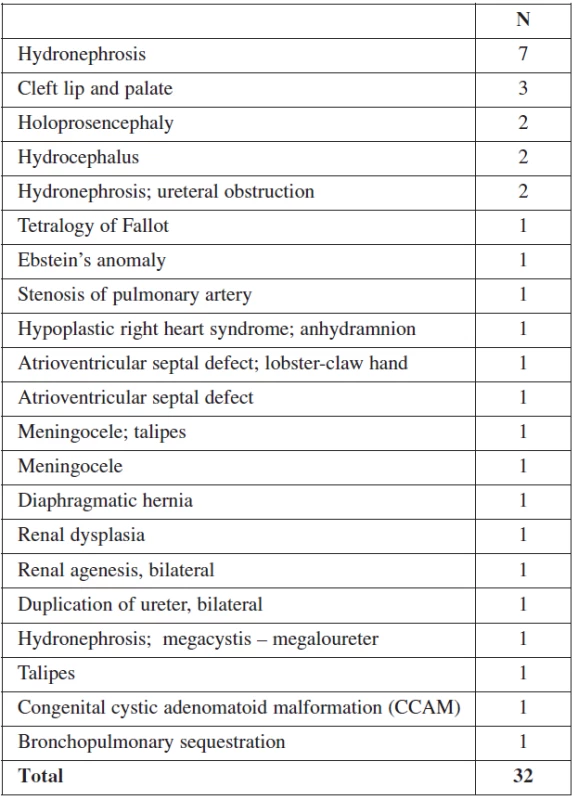
COMMENT
Our patients were examined preferably at the end of the first trimester (median gestational age 12 weeks + 6 days), which allows better visualization of the fetus. This gestational age is suboptimal for obtaining the maternal serum biochemistry, as PAPP-A delivers best results in earlier [17]. This is why we ask the mothers to have their blood test done at about at 10th week of pregnancy.
6.38% of our population recruits from the IVF program. This number is higher than in the average population, but IVF patients generally seek optimal prenatal surveillance and predispose them to seek us out for first trimester genetic screening and the follow-up scans.
This study shows that detailed examination of fetal anatomy during the routine 11–14 week scan can detect 46.9% of major structural defects and 67.7% of those, detected antenatally. Similar results have been reported from other authors, with detection rates between 38% and 65% [3, 4, 5, 6, 7, 9, 10, 11, 15, 16, 18]. However, the comparison of results from other studies is imprecise, due to different gestational age at the time of the first scan and varying definitions of ”sonographically detectable malformations”. There are also local differences in the prevalence of pathologic conditions. The more detailed the presentation of the results in the published studies, the better is the possibility to compare the results (Table 4).
3. Anomalies found postnatally 
4. Overview of the literature 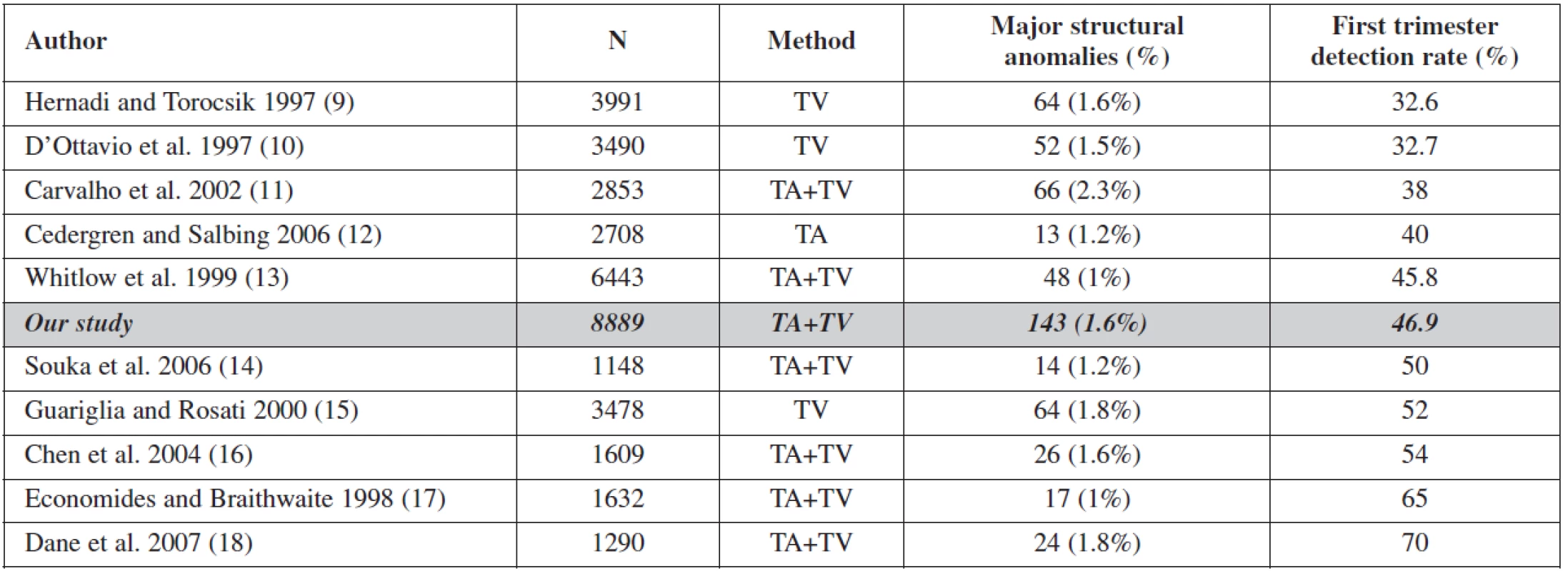
TA – transabdominal; TV – transvaginal Hygroma colli cysticum was the most common structural fetal abnormality in first trimester. Cystic hygroma was defined as a sonolucency in the soft of tissue of the occipital region, consisting of 2 cavities separated by a midline septum [16].
The detection rate of cardiac abnormalities in the first trimester was 7%. The reason for this relatively low detection rate is not only limited resolution of the heart images in the late first trimester, but also that defects, like a hypoplastic cardiac chamber, or myocardial hypertrophy manifest later.
Certain anomalies are more easily detectable in the first trimester: central nervous system defects, neck anomalies, gastrointestinal and renal defects. Others are more difficult to detect: spina bifida and cardiac defects. Comparing with other published studies, our study has the highest number of patients (8889), a similar incidence of malformations 1.6 % (min 1.0 – max 2.3%), and an overall detection rate of 46.9 % being in the middle of the range (min 32.6 – max 70).
Differences in detection rates also depend on the aim of the screening program, the experience of the examiner, the ultrasound equipment and overall working conditions. To perform examinations daily, year after year, with the same enthusiasm, rigor, and quality is challenging. The chance to diagnose very rare conditions in a low risk population depends on the nature and the sonographic appearance of the malformation.
We analyzed the spectrum of anomalies found postnatally. We failed in diagnosing several major anomalies, mostly heart defects. This reflects the fact, that special training and skill is necessary for heart defect detection in low risk population. Reduction defects of upper extremities were also problematic, while it is not complicated to make this diagnosis, detailed visualization of all extremities is necessary. We have included all morphologic malformations, even those where prenatal sonographic diagnosis usually fails. Included are esophageal atresias, craniosynostosis, isolated cleft palate, branchial cleft cyst, pulmonary valve insufficiency, larynx malformation, ventricular septum defects, and hypospadia. Excluding these anomalies would cause inaccuracies and artificially improve results. In our study, 8889 patients were included and defects with very low incidence were either not present or diagnosed as single cases.
CONCLUSIONS
67.7% of all antenatally detected malformations by ultrasound were recognized in the 11+1 – 14+0 week scan. With experience and progress in first trimester screening especially focused on detection of fetal heart malformations, the sensitivity could get even better. However, at this juncture in our center, first trimester screening has become a malformation scan, and Down syndrome screening is considered as “side effect”.
Acknowledgments
We would like to thank Helena Valtrova, Silvie Manasova, Andrea Paskova MD, Olena Svyatkina MD, Katerina Nekovarova and Alice Baxova MD PhD for their contribution to the study.
MUDr. Michaela Novotná
Gynekologicko-porodnická klinika
l. LF UK a VFN
Apolinářská 18
128 51 Praha 2
Corresponding author:
Prof. MUDr. Pavel Calda, CSc.
Apolinarska 18
128 51 Prague 2
Czech Republic
e-mail: calda@gynstart.cz
Sources
1. Berrocal, T., Pinilla, I., Gutiérrez, J., et al. Mild hydronephrosis in newborns and infants: can ultrasound predict the presence of vesicoureteral reflux. Pediatr Nephrol, 2007, 22(1), p. 91–96.
2. Bottomley, C., Bourne, T. Dating and growth in the first trimester. Best Practice & Research Clinical Obstetrics and Gynaecology (2009), doi:10.1016/j.bpobgyn.2009.01.011.
3. Carvalho, MH., Brizot, ML., Lopes, LM., et al. Detection of fetal structural abnormalities at the 11–14 week ultrasound scan. Prenat Dian, 2002, 22(1), p. 1–4.
4. Cedergren, M., Selbing, A. Detection of fetal structural abnormalities by an 11–14week ultrasound dating scan in an unselected Swedish population. Acta Obstet Gynecol, 2006, 85, p. 912–915.
5. Dane, B., Dane, C., Sivri, D., et al. Ultrasound screening for fetal major abnormalities at 11–14 weeks. Acta Obstet Gyneacol, 2007, 86, p. 666–670.
6. D’Ottavio, G., Mandruzzato, G., Meir, YJ., et al. Comparison of first trimester and second trimester screening for fetal anomalies. Ann NY Acad Sci, 1998, 847, p. 200–209.
7. Economides, DL., Braithwaite, JM. First trimester ultrasonographic diagnosis of fetal structural abnormalities in a low risk population. Br J Obstet Gynaecol, 1998, 105(1), p. 53–57.
8. Gramellini, D., Fieni, S., Caforio, E., et al. Diagnostic accuracy of fetal renal pelvis anteroposterior diameter as a predictor of significant postnatal nephrouropathy: Second versus third trimester of pregnancy. Am J Obstet Gynecol, 2006, 194(1), p. 167–173.
9. Guariglia, L., Rosati, P. Transvaginal sonographic detection of embryonic-fetal abnormalities in early pregnancy. Obstet Gynecol, 2000, 96(3), p. 328–332.
10. Hernadi, L., Torocsik, M. Screening for fetal anomalies in the 12th week of pregnancy by transvaginal sonography in an unselected population. Prenat Dian, 1997, 17(8), p. 753–759.
11. Chen, M., Lam, YH., Lee, CP., Tang, MH. Ultrasound screening of fetal structural abnormalities at 12 to 14 weeks in Hong Kong. Prenat Dian, 2004, 24, p. 92–97.
12. Jones, KL. Morphogenesis and dysmorphogenesis. In Recognizable Patterns of Human Malformation, Smith, DW. (ed). Philadelphia: W.B.Saunders, 1997, p. 695–705.
13. Nicolaides, KH., Sebire, J., Snijders, R. The 11–14 week scan. London: The Parthenon Publishing Group,1999.
14. Pilu, G., Perolo, A., Falco, P., et al. Ultrasound of the fetal central nervous system. Curr Opin Obstet Gynecol, 2002, 12(2), p. 93–103.
15. Souka, PA., Kavalakis, I., Antsaklis, P., et al. Screening for major structural abnormalities at the 11-to 14-week ultrasound scan. Am J Obstet Gynecol, 2006,194, p. 393–396.
16. Varma, TR. Cystic hygroma, colli http//www.thefetus.net/page. php?id=202 , 1992-12-07-21
17. Wenstrom, KD. First-trimester Down syndrome screening component analytes and timing for optimal performance. Semin Perinatol, 2005, 29(4), p. 195–202.
18. Whitlow, BJ., Chatzipapas, IK., Lazanakis, ML., et al. The value of sonography in early pregnancy for the detection of fetal abnormalities in an unseleced population. Br J Obstet Gynaecol, 1999, 106(9), p. 929–936.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2012 Issue 4-
All articles in this issue
- The rational preoperative diagnosis of ovarian tumors – imaging techniques and tumor biomarkers (review)
- Review of current classification and terminology of vulvar disorders
- Ultrasound in urogynecology
- The vaginal tension-free tape procedure solving stress urinary incontinence in woman
- Vaginal prolapse and levator ani avulsion injury
- Office hysteroscopy – management and results
- Endometriosis
- Laparoscopic lymph-node dissection in gynecological surgery
- Laparoscopic uterus sparing treatment of uterine fibroids
- In vitro fertilization – analysis of data in Czech National Assisted Reproduction Register from years 2007-2011
- Acute or expectant management in premature labour with preterm premature rupture of the membranes?
- Childbirth analgesia and anesthesia in the Czech Republic in 2012 The 20th anniversary of post-gradual education
- Comparison of short term results of TVT-O and TVT-S in the surgical treatment of stress urinary incontinence
- Atypical pain in the uterine rupture
- Epidemiology of genital warts in female population of Czech Republic
- Validity of the oncological cytodiagnostics and colposcopy examination versus biopsy in cervical carcinoma prevention
- Detection of fetal major structural anomalies at the 11-14 ultrasound scan in an unselected population
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Endometriosis
- Vaginal prolapse and levator ani avulsion injury
- The rational preoperative diagnosis of ovarian tumors – imaging techniques and tumor biomarkers (review)
- Ultrasound in urogynecology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

