-
Medical journals
- Career
Marketing research of combined drugs for treatment of cardiovascular diseases
Authors: Nataliia S. Behei; Oksana V. Tryhubchak
Published in: Čes. slov. Farm., 2023; 72, 79-94
Category: Original article
doi: https://doi.org/https://doi.org/10.5817/CSF2023-2-79Overview
The work is devoted to the results of complex marketing research of all combined cardiovascular drugs. The market of combined drugs from group C according to the ATC classification in 41 countries of the world during 2019–2022 was analyzed. Segment markets of the 27 European Union countries, Albania, Belarus, Bosnia and Hercegovina, Canada, Colоmbia, Great Britain, India, Moldova, Norway, Russian Federation, Switzerland, and Ukraine, were studied. The pharmaceutical market of Australia and the United States were also studied. The structure of this group of drugs was characterized, and the most common combinations in the analyzed markets were identified. It was found that group C09 is the most filled with combined drugs, and the number of combinations is most diverse in C09 drug groups that act on the renin-angiotensin system, C10 hypolipidemic drugs, C07 beta-blockers, and C03 diuretics, which are the drugs of the first choice for arterial hypertension and coronary heart disease. There are two promising areas for expanding the range of drugs that affect the cardiovascular system.
Keywords:
arterial hypertension – cardiovascular diseases – combined cardiovascular drugs – marketing research – directions of assortment expansion
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide and one of the most common diseases affecting a person’s whole life. In 2011, the United Nations formally recognized noncommunicable diseases, including CVD, as a major global health problem1, 2). These diseases accounted for more than 17 million deaths in 2008, and the World Health Organization estimates that about 23.6 million people will die from CVD in 2030. The equivalent contribution of prevention initiatives, pharmaceutical development, and technological initiatives has significantly reduced CVD mortality in some Western countries. However, increased life expectancy, incomplete adherence to guidelines, and difficulties in persuading the public to support and adhere to prevention measures still make the burden of CVD extremely high.
Hypertension is the strongest or one of the strongest risk factors for almost all different CVD acquired during life, including coronary heart disease, left ventricular hypertrophy and valvular heart diseases, cardiac arrhythmias including atrial fibrillation, cerebral stroke, and renal failure. The continuous relationship between blood pressure and cardiovascular and renal events distinguishes between high normal blood pressure and hypertension based on arbitrary cut-off values for blood pressures3). The pathophysiology of high blood pressure suggests that the onset of changes should be due to increased cardiac output, increased peripheral vascular resistance, or a combination of both. Each of these mechanisms is regulated, in turn, by hemodynamic, nervous, humoral, and renal processes, all of which differ in their contribution from one person to another. According to updates to the European ESH / ESC (2018) guidelines, the higher the overall risk for the patient’s cardiovascular system, blood pressure should be more strictly monitored, and treatment should be initiated urgently to reduce blood pressure to less than 140/90 mmHg4).
Drug treatment of hypertension can be started with one drug or a drug combination5). In the updated joint guideline, ESC and ESH recommend that most patients take two antihypertensive drugs at the start of pharmacotherapy, preferably in combination in a single tablet6). The recommended first-line treatment consists of medicines comprising the following four groups of drugs: angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (sartans), calcium channel blockers dihydropyridine type, and thiazide-like diuretics.
Although beta-blockers are inferior to these classes of cardiovascular agents7), they are considered an appropriate component of first-line treatment in some countries. Beta-blockers are used in patients with angina who have suffered a myocardial infarction or had a failure or heart rate monitoring6).
Patients are best suited to drugs with a long half-life that can be administered once daily. Due to the circadian rhythms of blood circulation regulation, it is preferable for patients to take long-acting antihypertensives in the evening8). Still, a positive effect on CVD remains unclear.
Many drugs are used to treat CVD worldwide, and each market has unique medicines for treatment. Marketing studies of specific groups of antihypertensive drugs are common, but comprehensive analyses of all combination cardiovascular drugs have not yet been conducted, which was the aim of our study.
Experimental part
The experiment involved researching the market of combined drugs from group C according to the anatomical-therapeutic-chemical (ATC) classification in the world register of medicines in 41 countries found on the Internet and available literature from 2019–2022. While doing an experiment, a market segment of the 27 European Union countries, Albania, Belarus, Bosnia and Hercegovina, Canada, Colombia, Great Britain, India, Moldova, Norway, Russian Federation, Switzerland, and Ukraine, were studied. The pharmaceutical market of Australia and the United States were also analysed9–51).
For our research, 27 countries that are part of the European Union (EU) were selected. In addition, the USA, UK, Canada, and Switzerland were included as the world market leaders, Ukraine as the domestic market, Moldova, Russia, and Belarus as the countries geographically closest to the domestic market, and India and Colombia representing countries with emerging pharmaceutical markets. Finally, Albania, Bosnia, Herzegovina, and Norway were covered as non-EU countries.
Since the Ukrainian pharmaceutical market is a domestic market, we focused on its analysis on a broader scale. We investigated which combined medicines from group C according to the ATC classification occupy the largest market share regarding patient sales.
Comprehensive marketing research was conducted in two areas: operational and strategic. Operational analysis was aimed at assessing market conditions, and at the strategic level, an in-depth analysis was conducted to identify promising areas of development. For a more detailed study of the Ukrainian market, the Pharmxplorer program was used.
The methods of grouping, comparison, analysis, mathematical-statistical, graphic, and generalization are used in the study.
Results
According to the ATC classification, cardiovascular drugs belong to group C Medicines affecting the cardiovascular system. Their characteristics are shown in Table 1.
1. Number of combined cardiovascular drugs according to ATC classification 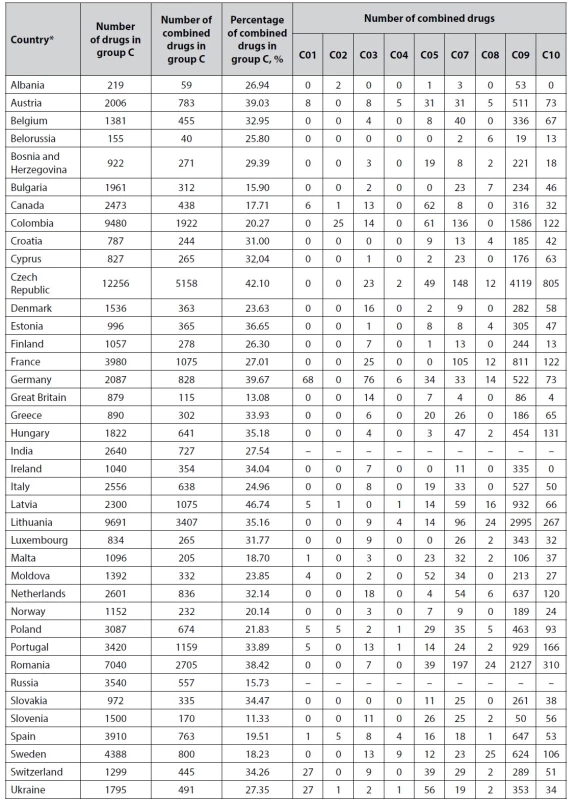
* Pharmaceutical markets of Australia and USA do not provide the division of drugs according to ATC classification in their electronic drug risters. Therefore, they are not included. C01 Cardiac drugs
Cardiac drugs enhance the contractile activity of cardiomyocytes and eliminate the phenomenon of heart failure, which can be defined as systolic and (or) diastolic myocardial dysfunction. It leads to remodelling of the heart and blood vessels and impaired hemodynamics and homeostasis.
Most combinations of this group are found in Germany (68 combinations), Ukraine (27 combinations), and Switzerland (27 combinations). In other countries, there are up to 10 combinations.
The most common combination drugs are based on plants. The most popular combinations are preparations based on Crataegus herb in combination with other herbal infusions that have a cardiac effect, such as lemon balm, valerian, peppermint, and others (Ukraine, Germany, Switzerland, Moldova, Austria).
In Ukraine, preparations based on Crataegus occupy a market share of less than 5% (realization in money). For example, preparations based on Crataegus L. + Leonurus cardiaca/quinquelobatus gilib. + taurin are 2.02 %, and Crataegus L + Valeriana officinalis + Leonurus cardiaca/quinquelobatus gilib are 1.6 %.
The C01 group also includes combinations based on potassium and magnesium asparaginate (Ukraine, Germany), combinations of ivabradine with carvedilol (Portugal), and ivabradine with metoprolol (Poland).
C02 Antihypertensive drugs
Antihypertensives are drugs of different pharmacological groups and chemical structures that can lower blood pressure.
Group C02 Antihypertensives are characterized mainly by combinations of reserpine with other substances in this group or other agents that affect the cardiovascular system. The most common are combinations of reserpine with bendroflumethiazide (Albania, Netherlands), clopamide and dihydroergocristine (Latvia, Netherlands, Poland, Russia, and Ukraine), hydrochlorothiazide (Canada, Slovenia, USA), hydrochlorothiazide and dihydralazine (Russia), hydralazine and hydrochlorothiazide (USA). The combination of hydrochlorothiazide with methyldopa was also observed in Colombia and clopamide with dihydroergocristine in Ukraine.
In Ukraine, the drug based on reserpine + clopamide and dihydroergocristine is called Normatens®, manufactured by IC Polfa Rzeszow JSC, Poland. The market share of this preparation was 9.58 % in 2019 and gradually decreased. In 2022, it was 5.97 %.
C03 Diuretics
Diuretics increase the excretory function of the kidneys and the amount of urine.
Group C03 is characterized mainly by combinations of hydrochlorothiazide with other substances in this group or other agents that affect the urinary system. Hydrochlorothiazide is combined with amiloride hydrochloride (21 countries), spironolactone (Greece, Italy, Canada, Colombia, Moldova, Portugal, USA), triamterene (Canada, Colombia, Germany, Russia, USA, Ukraine), butizide (Colombia). In Ukraine, the combination of hydrochlorothiazide + triamterene is registered under the trade name Triampur® composite, AVD Farma GmbH & Co. KG/PLIVA Hrvatska d.o.o., Germany/Croatia. The drug is not popular among diuretics, and the share of the drug is 0.36%.
The combination of furosemide with spironolactone is popular in the world. This combination can be found in 11 countries, including Ukraine. Spironolactone is also combined with butizide (Austria, Germany) and altizide (Iceland, Portugal).
Furosemide is combined with amiloride hydrochloride (UK, India, Ireland, Malta, Luxembourg, Greece), and triamterene (Iceland, Italy).
Chlortalidone is combined with metoprolol tartrate (Slovakia), amiloride hydrochloride (Czech Republic), atenolol (Russia), and azilsartan medoxomil (Russia).
The C03 group is also filled with a combination of potassium-sparing agents with altizide (Luxembourg), epitizide (Netherlands), butizide (Italy), and bendroflumethiazide (Greece, Ireland, Norway, Sweden).
C04 Peripheral vasodilators
This group includes drugs used to treat cerebrovascular or peripheral circulatory disorders.
Group C04 has a tiny number of combinations of active pharmaceutical ingredients (APIs), which enhance the contractile activity of cardiomyocytes and eliminate the phenomena of heart failure.
This group includes the following combinations: bendazole hydrochloride + papaverine (Ukraine, Russia), papaverine + platyphylline (Russia), and buflomedil + cilostazol (Colombia).
According to research data, the drug under the trade name Papazol-Darnytsia® (Ukraine), based on bendazole hydrochloride in combination with papaverine, is not the first choice for treating cerebrovascular or peripheral blood circulation disorders. This product accounted for a 3% market share in 2019–2022.
C05 Angioprotectors
Angioprotectors are a group of drugs that improve microcirculation, normalize vascular permeability, reduce tissue swelling and improve metabolic processes in the walls of blood vessels. Angioprotectors also show antispasmodic activity, cause vasodilation, normalize the rheological properties of blood and vascular permeability, and increase the resistance of capillaries.
The largest number of combinations (more than 30) between different APIs in the C05 group are found in Austria, Canada, Colombia, Moldova, Germany, Romania, Ukraine, and Switzerland.
The most common combinations among the analyzed markets are based on heparin sodium in combination with dexpanthenol (Austria, Germany), allantoin + dexpanthenol (Moldova, Switzerland) dimethylsulfoxide + dexpanthenol (Moldova, Switzerland), vennorutinol + dexpanthenol (Moldova, Ukraine), diclofenac sodium (Moldova), hydroxyethyl salicylate + menthol (Austria, Colombia), benzocaine (Moldova, Ukraine), benzocaine + benzyl nicotinate (Moldova), prednisolone acetate + lauromacrogol 400 (Moldova), prednisolone + polidocanol (Moldova, Ukraine), dexpanthenol + troxerutin (Moldova), hippocastani semen (Auszug) + arnica flower (Auszug, Germany), menthol + hydroxyethyl salicylate (Germany), escine + diethylamine salicylate (Romania), hippocastani semen with extract spissum (Romania), escine (Ukraine), hydrocortisone acetate + framycetin sulfate + esculoside + benzocaine + butamben (Ukraine), escine + epikuron (Ukraine), allii cepae extract + hyoscyami maceratum oleosum + allantoin + avobenzone + 3-(4-methyl)benzylidene-bornan - 2-on (Switzerland).
This group includes a combination that is registered in most of the analyzed countries. It is a combination of diosmin with hesperidin. It is used as a venotonizing agent.
There are many combinations with troxerutin. This API is combined with hippocastani semen (auszug), hamamelidis folium (auszug) + meliloti herba (auszug) + hippocastani semen (auszug), hippocastani semen (auszug) + crataegi folium cum flore (auszug) + hamamelis folium (auszug) + meliloti herba (auszug), menthol + hippocastani semen (auszug) + hamamelis folium (auszug) + benzocaine + zinc oxide, crataegi folium cum flore (auszug) + hippocastani semen (auszug), indomethacin, carbazochrome etc.
The C05 group also includes drugs used to treat haemorrhoids. These drugs are often combined based on prednisolone, tribenoside, local anesthetics such as сincocaine and lidocaine, and plant extracts.
In Ukraine, by the number of sales, the top three are drugs based on diosmin + hesperidin, troxerutin, and L-lysine aescinat. The market share in 2022 is 37.20%, 9.25%, and 8.50%, respectively. The sales leader is the Detralex® drug based on diosmin + hesperidin, manufactured by Les Laboratoires Servier Industrie, France.
C07 Beta-blockers
Beta-blockers are a class of pharmacological drugs designed to block the function of the beta-subtype of adrenoceptors in the body. Βeta-blockers have antianginal, antiarrhythmic and antihypertensive effects. Βeta-blockers exhibit positive effects in cardiology due to the blockade of beta-adrenoceptors. They reduce the strength of heart contractions, heart rate (negative inotropic and chronotropic effect), reduce excitability, and myocardial conduction (negative dromotropic and bathmotropic effects). Due to this depressant effect, myocardial oxygen demand is reduced. The reduction of blood pressure is cowed by a reduction in cardiac output due to a decrease in heart rate and a reduction of stroke volume of the heart. When using non-selective beta-blockers, peripheral vascular resistance is moderately increased.
Of all the analyzed combined drugs in this group, the most significant number is based on atenolol. Atenolol is combined with the following APIs: chlortalidone (19 countries), nifedipine (Austria, Luxembourg, Germany, France, India), amlodipine besylate (Belarus, Ukraine, Russia, India), chlorthalidone + nifedipine (Ukraine, Moldova), hydrochlorothiazide + amiloride hydrochloride (Belarus, Spain), bendroflumethiazide (Spain).
In Ukraine, atenolol + chlorthalidone + nifedipine preparations also lead among all combination medicines. The sales study showed that these drugs occupy the fourth place in the C07 group, and their market share in the last 3 years is 8%.
The second place in the number of combinations belongs to metoprolol. Among the countries analyzed, the following combinations can be singled out: metoprolol tartrate + ivabradine hydrochloride (15 countries), metoprolol succinate + felodipine (Belgium, Belarus, Iceland, Spain, Luxembourg, Sweden, Russia, Switzerland), metoprolol succinate + hydrochlorothiazide (Austria, Belarus, Netherlands, Germany, USA), metoprolol tartrate + chlortalidone (Switzerland, USA) and vinpocetine + indapamide + metoprolol + enalapril maleate (Russia). Metoprololbased combination products are not registered in Ukraine.
The third place went to bisoprolol and timolol. Bisoprolol is combined with hydrochlorothiazide (26 countries), amlodipine besylate (19 countries), acetylsalicylic acid (11 countries), and perindopril (Belarus, Russia). In Ukraine, preparations based on bisoprolol + amlodipine besylate are sold in small volumes, as the market share in 2022 was 3 %. Timolol maleate is combined with the following APIs: hydrochlorothiazide + amiloride (Belarus, Portugal), bendroflumethiazide (Ireland, UK), hydrochlorothiazide (Canada, Colombia), amiloride (Colombia).
Combination preparations based on propranolol hydrochloride include diazepam (India), alprazolam (India) or hydrochlorothiazide (Canada, USA).
Combining nebivolol hydrochloride with other APIs is common: hydrochlorothiazide (26 countries) and amlodipine besylate (India).
Carvedilol is combined with ivabradine hydrochloride (13 countries) and hydrochlorothiazide (Austria, Germany). In Ukraine, carvedilol is registered as a single - component drug.
In addition, there are combinations of acebutolol hydrochloride + hydrochlorothiazide (Belgium, Belarus, Luxembourg, USA, Colombia), pindolol + hydrochlorothiazide (Canada, USA), labetalol hydrochloride + hydrochlorothiazide (USA), sotalol + hydrochlorothiazide (Belarus, Netherlands), pindolol + clopamide (Belarus, Slovenia, Russia), nadolol with bendroflumethiazide (Canada, USA, Colombia).
A diagram of the combined drug ratio from group C07 is shown in Figure 1.
Fig. 1. Diagram of the ratio of combined drugs from group C07 
C08 Calcium antagonists
Calcium channel antagonists are a heterogeneous group of drugs with antianginal and antihypertensive properties. They exhibit the same mechanism of action based on blocking slow L-type calcium channels in the myocardium, conduction system, and vascular smooth muscle. They differ in chemical structure, pharmacokinetics, and pharmacodynamics, including effects on vasodilation, cerebral vessels, and conduction and contractile function of the myocardium.
Of the analyzed countries, this group includes mainly single drugs. There are only a very few combinations. Registered combined drugs are based on amlodipine besylate + indapamide (17 countries), trandolapril + verapamil hydrochloride (Australia, Belarus, Bulgaria, Latvia, Netherlands, Portugal, Poland, Croatia), amlodipine besylate + bisoprolol (Russia), amlodipine besylate + nebivolol (Russia), amlodipine besylate + celecoxib (USA), acetylsalicylic acid + nifedipine (Canada), and amlodipine besylate + hydrochlorothiazide (Colombia).
In Ukraine, drugs based on amlodipine besylate + indapamide take fourth place in sales volume; before, there were only single-component drugs. One drug based on amlodipine besylate + indapamide is registered on the Ukrainian market under the trade name Arifam®, Servier (Ireland) Industries Ltd. In 2021, this drug had sales of $ 1 million 653 thousand.
There are more combinations based on amlodipine besylate in India also. These are combinations with atenolol, enalapril, lisinopril, ramipril, valsartan, benazepril, atorvastatin, losartan, nebivalol, telmisartan + hydrochlorothiazide.
C09 Drugs acting on the renin-angiotensin system
ACE inhibitors depress the action of angiotensin-converting enzyme, which converts biologically inactive angiotensin I into angiotensin II, which has a vasoconstrictive effect. In the mechanism of their antihypertensive action, the ability to block the angiotensin-converting enzyme plays the role, followed by inhibition of angiotensin II production from angiotensin I. This, in turn, reduces the synthesis of aldosterone in the adrenal cortex, accompanied by increased excretion of sodium and water from the body. The influence of ACE inhibitors improves renal blood flow and filtration processes in the kidneys. Drugs increase the synthesis of vasodilators such as prostacyclin, nitric oxide, depressant prostaglandins, and atrial natriuretic hormone and reduce the inactivation of bradykinin. ACE inhibitors reduce the sympathetic-adrenal system’s activity and inhibit the development of hypertrophy of the myocardium and smooth muscle of the vascular wall. They are one of the most effective modern antihypertensive agents.
This group has a very large number of combined drugs. Group C09 includes combinations based on subgroups of ACE inhibitors, angiotensin II receptor antagonists, and direct renin inhibitors (Table 2).
2. Extended description of combined drugs from group C09 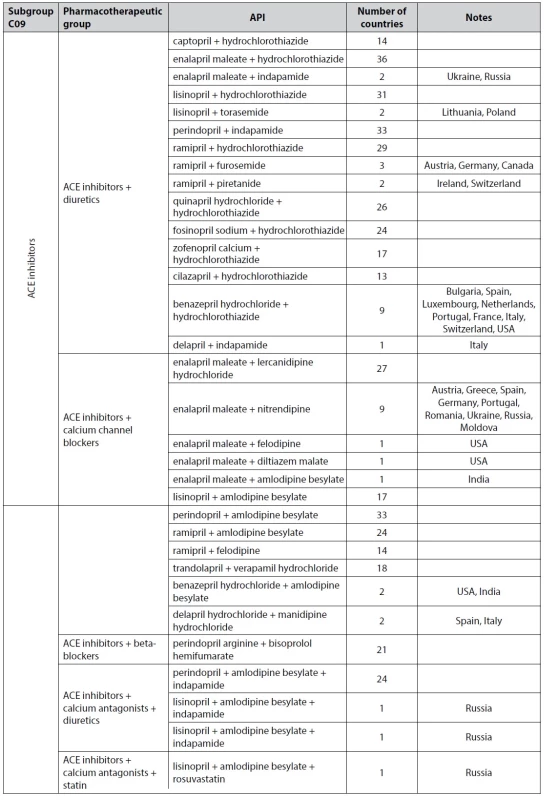

ACE inhibitors – angiotensin-converting enzyme inhibitors, API – active pharmaceutical ingredient The most popular combinations, found in 30 countries, are based on the following substances: amlodipine besylate + perindopril arginine, amlodipine besylate + valsartan, candesartan cilexetil + hydrochlorothiazide, enalapril maleate + hydrochlorothiazide, hydrochlorothiazide + irbesartan, hydrochlorothiazide + losartan, hydrochlorothiazide + telmisartan, hydrochlorothiazide + valsartan, indapamid + perindopril arginine, and sacubitril + valsartan.
Popular combinations found in 20 of the analyzed countries are based on the following APIs: aliskiren + hydrochlorothiazide, amlodipine besylat + hydrochlorothiazide + valsartan, amlodipine besylat + olmesartan medoxomil, amlodipine besylate + ramipril, amlodipine besylat + telmisartan, bisoprolol hemifumarat + perindopril arginine, enalapril maleate + lercanidipine hydrochloride, hydrochlorothiazide + amlodipine besylate + olmesartan medoxomil, hydrochlorothiazide + fosinopril sodium, hydrochlorothiazide + lisinopril, hydrochlorothiazide + olmesartan medoxomil, hydrochlorothiazide + quinapril hydrochloride, and hydrochlorothiazide + ramipril, indapamide + perindopril arginine + amlodipine besylate.
In Ukraine, there are 30 combinations with different APIs. The most popular of all drugs of the C09 group is a combination based on amlodipine besylate + indapamide + perindopril arginine. The market share of these products in 2022 was 17.7%. Indapamide + perindopril and hydrochlorothiazide + valsartan occupy the second and the fourth place (the third place belongs to enalapril maleate) with a market share of 12.3% and 5.0%, respectively.
There are also unique combinations that are registered in a particular country. Only in the USA are the following combinations available: diltiazem maleate + enalapril maleate and enalapril maleate + felodipine. In addition, the following combinations were found in individual countries: in the Czech Republic – amlodipine besylate + perindopril erbumin, in Russia - amlodipine besylate + lisinopril + rosuvastatin and amlodipine besylate + indapamide + lisinopril, hydrochlorothiazide + irbesartan, in India – amlodipine besylate + enalapril maleate, amlodipine besylate + telmisartan, hydrochlorothiazide + losartan potassium, enalapril maleate + losartan potassium, ramipril + losartan potassium and ramipril + hydrochlorothiazide, in Netherlands – azilsartan medoxomil + hydrochlorothiazide, in Spain – delapril hydrochloride + manidipine hydrochloride and valsartan + dihydrochlorothiazide, in Italy – delapril + indapamide, and in Austria – perindopril + tert-butylamine and perindopril arginine + amlodipine besylate.
C10 Hypolipidemic drugs
Hypolipidemic drugs (antihyperlipidemic, antiatherosclerotic) prevent the development or promote regression of the atheromatous process. In the basics of the development of atherosclerosis – lipid metabolism disorders, mainly cholesterol. Lipids in blood plasma form complexes with proteins – lipoproteins that can penetrate the arteries’ inner lining.
This group includes a combination of statins with APIs from other groups that affect the cardiovascular system. The most common are combinations based on atorvastatin. It is combined with amlodipine besylate (27 countries), ezetimibe (26 countries), amlodipine besylate + perindopril arginine (21 countries), perindopril arginine (16 countries), acetylsalicylic acid + ramipril (13 countries), acetylsalicylic acid (Belgium, Ukraine, Russia, India), fenofibrate (India), nicotinic acid (India), fenofibrate + ezetimibe (India), methylcobalamin + folic acid + pyridoxine (India), and amlodipine besylate + simvastatin + ezetimibe (Australia).
In Ukraine, combined drugs based on atorvastatin + acetylsalicylic acid + ramipril take fourth place in market share. Before, there were single-component drugs with significantly higher sales volumes. The market share of these drugs was 2.25% in the last three years.
Rosuvastatin-based drugs are also popular. Combinations include ezetimibe (29 countries), amlodipine besylate (20 countries), valsartan (10 countries), tert-butylamine perindopril + indapamide (Estonia, Latvia, Lithuania, Portugal, Slovenia), lisinopril + amlodipine besylate (Moldova), and acetylsalicylic acid (Russia).
Simvastatin is frequently combined with ezetimibe (27 countries), fenofibrate (16 countries), and sitagliptin phosphate (USA). Pravastatin sodium is combined with fenofibrate in 15 countries and acetylsalicylic acid only in the USA. In Canada, a combination of lovastatin and nicotinic acid can be found. A variety of nicotinic acid with laropiprant is used in Australia, Ireland, Estonia, Portugal, Romania, and Russia.
A graphic representation of the ratio of combined drugs of group C10 is shown in the form of diagrams in Figure 2.
Fig. 2. Diagram of the ratio of combined drugs of group C10 
An analysis of the pharmaceutical market for drugs affecting the cardiovascular system in 41 countries revealed that some drugs are registered in only one country (Table 3).
3. List of medicinal products registered in one country 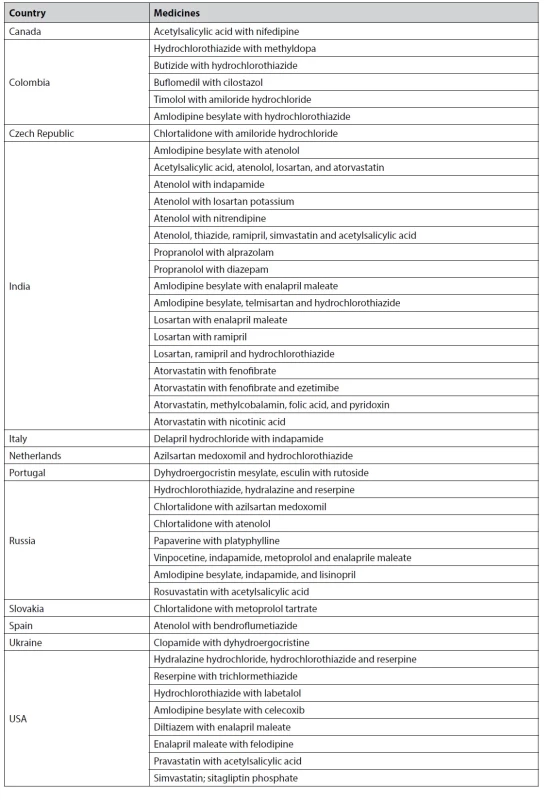
Discussion
Drugs affecting the cardiovascular system are most abundant in Lithuania, Colombia, Romania, Sweden, Spain, Portugal, and Poland markets. The highest share of combination medicines is in Latvia (46.74%), the Czech Republic (42.10%), Germany (39.67%), Austria (39.03%), Romania (38.42%), Estonia (36.65%), and Hungary (35.18%).
Among all the drugs affecting the cardiovascular system, group C09 – medicines acting on the reninangiotensin system is the most filled with combined pharmaceutical products. In the groups of C09 medications acting on the renin-angiotensin system, C10 hypolipidemic drugs, C07 beta-blockers, and C03 diuretics, the most significant number of combinations was found. These are drugs of the first choice in treating cardiovascular and renal diseases, including heart failure, acute coronary syndrome, nephrotic syndrome, diabetes, and hypertension.
Group C01 (Cardiac drugs) includes mainly preparations based on Crataegus extract. Crataegus extract contains many pharmacologically active substances, the most abundant compounds being flavonoids, triterpenic acids, and phenol carboxylic acids. Crataegus preparations are currently being marketed as an alternative treatment for hypertension, arrhythmia, and early stages of congestive heart failure by regulating the whole organism at multiple levels and targets52). Medicinal preparations based on Crataegus extracts are combined with other plantorigin materials with cardiac action to enhance the hypotensive, cardioprotective, and anticonvulsant effects53).
The most common combined drugs of the C02 group (hypotensive drugs) are drugs based on reserpine. Reserpine is an indole alkaloid extracted from Rauwolfia serpentine roots, an Indian climbing shrub. Reserpine was FDA-approved and is one of the first agents developed to treat hypertension in clinical practice. Reserpine is prescribed alone or combined with a vasodilator or thiazide diuretic, such as reserpinehydrochlorothiazide. Reserpine was initially introduced as a first-line antihypertensive therapy but is currently considered a second-line treatment. This change in status is due to newer and more favorable antihypertensive medications with reduced side effects54).
Diuretics (C03) are the first-line therapy for widespread cardiovascular and non-cardiovascular diseases. Traditional diuretics are commonly prescribed for treatment in patients with hypertension, edema, heart failure, and many kidney problems. The use of several classes of diuretics currently available for clinical use exhibits an overall favorable risk/benefit balance55). Hydrochlorothiazide remains the most popular of the thiazide diuretics, and furosemide is the loop diuretic. They are often combined with potassiumsparing diuretics to minimize side effects and improve blood pressure control. Hydrochlorothiazide is usually combined with amiloride hydrochloride, spironolactone, triamterene, and other potassiumsparing agents. Furosemide is combined with amiloride hydrochloride and spironolactone. The hydrochlorothiazide and amiloride hydrochloride combination is the most popular in the studied countries56). Non-thiazide diuretic API chlorthalidone is often combined with selective beta-receptor blockers, allowing better blood pressure control if monotherapy is ineffective57).
The C04 group is mainly filled with single-component drugs, but combinations can be found based on the myotropic antispasmodic agent papaverine. It reduces tone and smooth muscle contractile activity, resulting in a vasodilating and antispasmodic effect. Papaverine is combined with bendazole and platyphylline to enhance the antispasmodic action of smooth muscles and blood vessels58).
The results of our studies have shown that heparin (the C05 group according to the ATC classification) is included in the highest number of combinations. Heparin has an anti-edematous, anti-exudative, anti - inflammatory, and anti-coagulation effect. Heparin is combined with various drugs to expand the therapeutic effect, such as dexpanthenol (stimulates granulation and epithelization of tissues), allantoin (an anti-inflammatory, adstringent effect), diclofenac sodium, benzocaine, diethylamine salicylate, butamben (analgesics), lauromacrogol 400, polidocanol (a sclerosing agent), prednisolone acetate (a corticosteroid), or framycetin sulfate (an antibiotic for local use). There are also combinations with bioflavonoids such as vennorutinol and troxerutin. Heparin is also combined with pro-drugs (dimethyl sulfoxide and benzyl nicotinate) and biologically active substances (escin, esculin).
A combination of diosmin + hesperidin is most often used to treat chronic venous disorders. This drug is found in most countries we investigated59, 60). The study showed that troxerutin is combined mainly with herbal preparations.
The C07 group counts a sufficient number of combinations, which makes it possible to choose an effective medicine. The market analysis showed that the included top three combined drugs are atenolol, metoprolol, and bisoprolol. Most of the registered drugs were based on atenolol with chlortalidone. This combination has an antihypertensive effect due to the impact on beta-blockers and the action of a thiazidelike diuretic61). Metoprolol tartrate is most often combined with ivabradine hydrochloride. This drug is prescribed for the symptomatic treatment of chronic stable angina pectoris. The drug has a therapeutic effect due to the impact of beta-blockers and the effect on heart rate62). The combination of bisoprolol with hydrochlorothiazide is registered in many world countries. This drug is used for arterial hypertension. The therapeutic effect is achieved due to the betablockers and thiazide diuretic effects63).
Group C08 is filled with combined drugs based on amlodipine besylate. Those medicines are combined with drugs with different mechanisms of action. According to our research, these are diuretics (indapamide and hydrochlorothiazide), beta-blockers (atenolol, bisoprolol, and nebivolol), and nonsteroidal anti-inflammatory drugs (celecoxib), ACE inhibitors (benazepril, enalapril, lisinopril, ramipril), hypolipidemic drugs (atorvastatin), antiplatelet drugs (acetylsalicylic acid), angiotensin II receptor antagonists (valsartan, losartan, telmisartan).
According to our research, the most significant number of combined drugs in the C09 group is found with enalapril maleate. Given the pharmacological properties of ACE inhibitors64), combining them with diuretics, beta-blockers, and calcium channel blockers having a different mechanism of action is advisable65).
Studies have shown that the most common combinations are enalapril maleate with hydrochlorothiazide. Moreover, it has been clinically proven that this combination is one of the best for treating arterial hypertension66, 67). The most common combinations are enalapril maleate with hydrochlorothiazide, presented in such trade names, for example, in Austria Co – Renitec®, Co-Enac Hexal®, Co-Enalapril 1A Pharma ®, Co-Mepril®, Co-Renistad®9). Albania – CO-MEPRIL ®10). Ireland – Innozide®22). Poland – Enap H®, Enap HL®39). Every country on the market has its own generic drugs. Representatives of the combination of enalapril maleate with lercanidipine are Coripren®, Elernap®, Enalapril/Lercanidipine Accord®, Lercaprel®, and others. The following trade names represent ramipril with hydrochlorothiazide on the world market: Hypren plus HCT forte®, Lannaprilplus®, Lannapril plus forte®, Ramicomp Genericon mite®, Ramicomp Genericon®, Ramipril HCT Krka®, Ramipril-HCT Sandoz ®, Ramipril/HCT Actavis®, Ramipril/HCT Hexal®, and others. Indapamide with perindopril – Bipreteraxarginine ®, Preterax arginine®, Indixcombi®, Noliprel®, Co-prenessa®, Panoprist®, Perindopril + indapamide krka®, Tertensifbi-kombi®. Sacubitril with valsartan is available under the brands Entresto®, Neparvis®, Yuperio ®, and others.
In Ukraine, enalapril maleate as a single-component drug takes third place, which proves its popularity among ACE inhibitors. Its combination with hydrochlorothiazide is a leader, which coincides with studies in 41 countries.
Our studies have shown that the C10 group (hypolipidemic medicines) has many combinations. Fixed-dose combinations of hypolipidemic agents are useful and safe for managing hyperlipidemia. They combine important characteristics of each compound, such as established efficacy and a favorable safety profile. Furthermore, their complementary mechanism of action may result in enhanced hypolipidemic and pleiotropic effects. The most common drugs, such as atorvastatin and rosuvastatin, are combined with antihypertensive drugs since patients with a combination of hypertension and dyslipidemia have a triple risk of death from CVD68). Atorvastatin, rosuvastatin, and simvastatin are combined with ezetimibe. Ezetimibe is often used as an add-on therapy to statins to decrease LDL-C levels further. Fibrates can be crucial in managing dyslipidemia, especially in patients with obesity, metabolic syndrome, or diabetes who usually have residual CVD risk. Fibrates (fenofibrate) are combined with atorvastatin, simvastatin, pravastatin69).
A more extensive analysis of the Ukrainian pharmaceutical market showed that the most common drugs worldwide are not always the best sellers.
To overcome the global problem of CVD mortality, expanding the range of drugs that affect the cardiovascular system is advisable. It can take place in two promising directions. The first direction is to create new combinations that have proven themselves well in other countries70) or to develop combined drugs, including drugs often used simultaneously as single-component drugs71). It will help the individual approach prescribing pharmacotherapy and reduce the risk of adverse reactions. The second direction is implemented by registering generic drugs in the same market. It will ensure lower prices and, consequently, accessibility to all social spheres of the population and increase the likelihood of continued treatment. As a result, compliance and treatment efficiency will increase, and cardiovascular mortality will decrease72, 73).
Conclusion
A comprehensive marketing study of combined drugs of group C according to the ATС classification was conducted in 41 countries. The structure of each subgroup is analyzed, and the most common combinations are highlighted. The most common combination drugs belong to group C9. It was found that group C09 is most filled with combination drugs. The drug combinations are most diverse in groups C09 drugs that act on the renin-angiotensin system, C10 hypolipidemic drugs, C07 beta-blockers, and C03 diuretics, which are first-line drugs for the treatment of hypertension and coronary heart disease.
We conducted a more extensive analysis of the pharmaceutical market of Ukraine. The results showed which combinations from group C are the leaders and have the highest sales.
We selected drugs found in unique combinations and registered only in one country. There are two promising areas for expanding the range of drugs that affect the cardiovascular system.
Conflict of interests: none.
Received August 1, 2022 / Accepted January 10, 2023
Nataliia S. Behei • Oksana V. Tryhubchak
Farmak JSC
Kyrylivska street 74, Kyiv, Ukraine
e-mail: beheinatalia@ukr.net
Slovak Medical University in Bratislava, Slovak Republic
Sources
1. Islam S. M. S., Purnat T. D., Phuong N. T. A., Mwingira U., Schacht K., Fröschl G. Non‐Communicable Diseases (NCDs). In developing countries: a symposium report. Glob. Health 2014; 10(1), 1–8.
2. van Camp G. Cardiovascular disease prevention. Acta Clinica Belgica: Cardiovascular disease prevention. Acta Clin. Belg: JCLA 2014; 69, 407–411.
3. Kjeldsen S. E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018; 129, 95–99.
4. Mancia G., de Backer G., Dominiczak A. ESH-ESC practice guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J. Hypertens. 2007; 25, 1751–1762.
5. James P. A., Oparil S., Carter B. L., Cushman W. C., Dennison-Himmelfarb C., Handler J., Lackland D. T., LeFevre M., MacKenzie T. D.,Ogedegbe O., Smith S. C., Svetkey L. P., Taler S. J., Townsend R. R., Wright J. T., Narva A. S., Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA 2014; 311(5), 507–520.
6. Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D. L., Coca A., Simone G., Dominiczak A., Kahan Th., Mahfoud F., Redon J., Ruilope L., Zanchetti A., Kerins M., Kjeldsen S. E., Kreutz r., Leurent S., Lip G. Y. H., McManus R., Narkiewicz K., Ruschitzka F., Schmieder R. E., Shlyakhto E., Tsioufis C., Aboyans V., Desormais I. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018; 39(33), 3021–3104.
7. Wright J. M., Musini V. M., Gill R. First‐line drugs for hypertension. CDSR 2018; 4(4), CD001841.
8. Hermida R. C., Ayala D. E., Fernández J. R., Portaluppi F., Fabbian F., Smolensky M. H. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. AJH 2011; 24(4), 383–391.
9. Austrian Federal Office for Safety in Health Care. Austrian Medicines and Medical Devices Agency: Austrian medicinal product index; 2020. Available from: https://aspregister.basg.gv.at/aspregister/faces/aspregister. jspx?_afrLoop=3064459845228608&_afrWindow-Mode=0&_adf.ctrl-state=15s1umdsu_4 (last accessed 25.04.2020).
10. Agjencia Kombëtere e Bornave dhe Pajisjeve Mjekësore (AKBPM): Regjistri i barnave; 2020. Available from: http://www.akbpm.gov.al (last accessed 12.04.2020).
11. Federal agency for medicines and health products: Medicinal Product Database. Medicinal Products; 2021. Available from: https://banquededonneesmedicaments. fagg-afmps.be/#/query/human/ (last accessed 17.01.2021).
12. Ministry of health of the republic of Belarus. “Center for examinations and tests in health service”. Republican unitary enterprise: Registers of the Unitary Enterprise “Center for Expertise and Testing in Healthcare”; 2021. Available from: https://www.rceth.by/Refbank/reestr_lekarstvennih_sredstvї (last accessed 03.08.2021).
13. Bulgarian Drug Agency (BDA): Registration of medicinal products; 2019. Available from: https://ma.bda.bg/ial_register/Sites/PublicRegister/Default.aspx (last accessed 20.10.2019).
14. Bosna i Hercegovina. Agencija za lijekove I medicinska sredstva: Spisak Lijekova; 2022. Available from: http://lijekovi.almbih.gov.ba:8090/SpisakLijekova.aspx (last accessed 12.02.2022).
15. Danish Medicines Agency: Search results; 2022. Available from: https://laegemiddelstyrelsen.dk (last accessed 05.10.2022).
16. Electronic medical compendium (emc): Search results; 2022. Available from: https://www.medicines.org.uk/emc/browse-medicines (last accessed 25.05.2022).
17. EMC: Search results; 2022. Available from: https://www.medicines.org.uk (last accessed 05.10.2022).
18. EOF: medicinal product search; 2022. Available from: http://www.eof.gr/web/guest/search (last accessed 25.05.2021).
19. Finnish Medicines Agency Fimea: Search for a Medicine; 2022. Available from: https://www.fimea.fi (last accessed 05.10.2022).
20. Le Figaro fr sante: Search for a Medicine; 2022. Available from: https://sante.lefigaro.fr (last accessed 05.10.2022).
21. RX.ee. Еesti meditsiini portaal: ATC Kood; 2022. Available from: https://rx.ee/a (last accessed 25.05.2021).
22. Health Products Regulatory Authority (HPRA): Search for a Medicine; 2022. Available from: http://www.hpra.ie/homepage/medicines/medicines-information/find--a-medicine/results?showadv=true&list=HM (last accessed 05.04.2021).
23. Medline India: Pharmacological Index. Cardiovascular System; 2021. Available from: http://www.medlineindia.com/index.htm (last accessed 13.12.2021).
24. Medicine Online Information Center of AEMPS – CIMA. Agencia Española de Medicamentos y Productos Sanitarios: Browser medicines; 2020. Available from: https://cima.aemps.es/cima/publico/buscadoravanzado.html (last accessed 10.03.2020).
25. Torrinomedica: Farmaci. Codice ATC; 2021. Available from: https://www.torrinomedica.it (last accessed 12.12.2021).
26. Gaverment of Canada: Drug Product Database; 2022. Available from: https://health-products.canada.ca/dpd--bdpp/index-eng.jsp (last accessed 23.01.2021).
27. GOV.CO. Portal Único del Estado Colombiano: Oficina Virtual Invima; 2021. Available from: https://www.invima.gov.co/component/content/article/213-tramites-y-servicios/consultas-registros-y-documentos-asociados/806-listado-codigo-unico-de-medicamentos.html (last accessed 10.10.2021).
28. Republic of Cyprus – Pharmaceutical Services, Ministry of Health: Medicines search; 2022. Available from: https://www.phs.moh.gov.cy (last accessed 05.10.2022).
29. State Agency of Medicines of the Republic of Latvia: Medicinal Product Register of Latvia; 2021. Available from: https://www.zva.gov.lv/zvais/zalu-registrs/?lang= -en (last accessed 20.10.2021).
30. Ministry of Health of The Republic of Lithuania: Medicines search; 2021. Available from: https://vapris.vvkt.lt/vvkt-web/public/medications?lang=en (last accessed 15.10.2021).
31. CNS. Le Gouvernement du Grand-Duché de Luxembourg: Liste des médicaments commercialisés.Triée par code ATC; 2021. Available from: https://cns.public.lu/fr/legislations/textes-coordonnes/liste-positive.html (last accessed 06.11.2021).
32. Malta Medicine Authority: National Medecines; 2020. Available from: http://www.medicinesauthority.gov.mt/advanced-search (last accessed 10.09.2020).
33. AMED: Clasificator medicamente; 2021. Available from: http://www.amed.md/ro/clasificator-medicamente (last accessed 14.07.2021).
34. Medicines Evaluation Board: Medicines Information Bank; 2020. Available from: https://www.geneesmiddeleninformatiebank.nl/ords/f?p=111 : 1:0:::RP,1:P0_DOMAIN, P0_LANG:H,EN (last accessed 25.09.2020).
35. BCFi: Repertoriun; 2020. Available from: http://www.bcfi.be/nl/start (Last accessed 02.11.2021).
36. Rote Liste: Arzneimittelinformationen für Deutschland; 2021. Available from: https://www.rote-liste.de (last accessed 18.11.2021).
37. States legemiddelverk: Legemiddelsøk; 2022. Available from: https://www.legemiddelsok.no/sider/default.aspx?searchquery=&f=Han;MtI;Vir;ATC;Var;for;Mar;-Mid;Avr;gen;par;&pane=0 (last accessed 10.03.2022).
38. Informed: Medicines database; 2021. Available from: http://app7.infarmed.pt/infomed/pesquisa.php (last accessed 23.07.2021).
39. Centrum e-Zdrowia: Rejestry mediczne; 2021. Available from: https://pub.rejestrymedyczne.csioz.gov.pl (last accessed 24.12.2021).
40. State Register of Medicines: Pharmacotherapeutic group; 2022. Available from: https://grls.rosminzdrav.ru/grls.aspx (last accessed 09.03.2022).
41. Agenția Națională a Medicamentului și a Dispozitivelor Medicale România (ANMDMR) – Nomenclatorul medicamentelor pentru uz uman: Lista medicamen-telor din NOMENCLATOR; 2021. Available from: https://www.anm.ro/nomenclator/medicamente (last accessed 10.11.2021).
42. ADC: Humánne lieky; 2021. Available from: https://www.adc.sk/databazy/humanne-lieky?n=&nai=&cla=&frm=&hol=&sup=&code=&mf=&cat=&dr=&ord=a1 (last accessed 27.06.2021).
43. Republika Slovenija Ministrstvo za zdravje: Centralna baza zdravil; 2021. Available from: http://www.cbz.si/cbz/bazazdr2.nsf/Search/$searchForm?SearchView (last accessed 25.05.2021).
44. U.S. Food & Drug Administration: Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations; 2021. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (last accessed 12.12.2021).
45. Ministry of Health of Ukraine: State Register of Medicinal Products of Ukraine; 2022. Available from: http://www.drlz.com.ua (last accessed 25.03.2022).
46. L’Agence nationale de sécurité du médicament (ANSM): Domaine medical. produit de santé; 2021. Available from: https://www.ansm.sante.fr/var/ansm_site/storage/original/application/07789b90857c5c64930a-53ec208907c9 (last accessed 20.09.2021).
47. ALMP: Basa lijekova; 2021. Available from: http://www.almp.hr/en/Lijekovi/Baza-lijekova/#rezultati (last accessed 01.12.2021).
48. Státní ústav pro kontrolu léčiv (SUKL): Databáze léků 2021. Available from: http://www.sukl.cz (last accessed 03.10.2021).
49. Swiss Medic. Swiss Agency for Therapeutic Products: Lists and directories; 2021. Available from: https://www.swissmedic.ch/swissmedic/de/home/services/listen_neu.html#-257211596 (last accessed 10.04.2021).
50. Läkemedelsverket. Swedish Medical Products Agency: Drug; 2021. Available from: https://lakemedelsverket.se/LMF/?q=acetylsalicylsyra (last accessed 12.11.2021).
51. National Institute of Pharmacy and Nutrition - institute of Hungary: Lists and directories 2022. Available from: https://ogyei.gov.hu (last accessed 05.10.2022).
52. Wang J., Xiong X., Feng B. Effect of crataegus usage in cardiovascular disease prevention: an evidence-based approach. Evid. Based Complement. Alternat. Med. 2013; 2013, ID 149363, 16.
53. Domuschiev I. How the Natural Sedatives Hawthorn, Mint and Valerian Affect The Orthostatic Test by Measuring Pulse Rate Variability (PRV) in A 60-Year-Old Man. BJSTR 2021; 35(4), 27941–27942.
54. Cheung M, Parmar M. Reserpine. In: StatPearls. Treasure Island (FL): StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK557767/ (20.07.2022).
55. Titko T., Perekhoda L., Drapak I., Tsapko Y. Modern trends in diuretics development. Eur. J. Med. Chem. 2020; 208, 112855.
56. Mullens W., Damman K., Harjola V. P., Mebazaa A., Brunner‐La Rocca H. P., Martens P., Testani J. M., Tang W. H. W., Orso F., Rossignol P., Metra M., Filippatos G., Seferovic P. M., Ruschchitzka F., Coats A. J. The use of diuretics in heart failure with congestion–a position statement from the Heart Failure Association of the European Society of Cardiology. EJHF 2019; 21(2), 137–155.
57. Sarkar G., Gaikwad V. B., Sharma A., Halder S. K., Kumar D. A., Anand J., Mehta S. Fixed-dose Combination of Metoprolol, Telmisartan, and Chlorthalidone for Essential Hypertension in Adults with Stable Coronary Artery Disease: Phase III Study. Adv. Ther. 2022; 39(2), 923–942.
58. Hocking K. M., Putumbaka G., Wise E. S., Cheung-Flynn J., Brophy C. M., Komalavilas P. Papaverine prevents vasospasm by regulation of myosin light chain phosphorylation and actin polymerization in human saphenous vein. PloS One 2016; 11(5), e0154460. doi:10.1371/journal.pone.0154460.
59. Hnátek L. Therapeutic potential of micronized purified flavonoid fraction (MPFF) of diosmin and hesperidin in treatment chronic venous disorder. Vnitř. Lek. 2015; 61(9), 807–814.
60. Gerges S. H., Wahdan S. A., Elsherbiny D. A., El-Demerdash E. Pharmacology of diosmin, a citrus flavone glycoside: an updated review. European Journal of Drug Metabolism and Pharmacokinetics 2021; 47(1), 1–18.
61. Pareek A., Karnik N., Salagre S. B., Zawar S. D., Joglekar V. K., Chandurkar N., Naik G. S. Clinical effectiveness of low-dose chlorthalidone (6.25 mg) + atenolol combination in stage I hypertensive patients: a multicenter, randomized, controlled study. CMRO 2008; 24(6), 1771–1779.
62. Gilarevskii S. R., Lantsova E. V., Akimov A. A. Efficacy and Safety of Combined Treament with Ivabradine and Metoprolol in Patients with Stable Angina Pectoris-a Systematic Review. Kardiologiia 2020; 60(11), 1357–1357.
63. Leal U., Rincón D. Comparison of two products containing bisoprolol-hydrochlorothiazide therapeutic equivalence in patients with arterial hypertension. Revista Colombiana de Cardiología 2020; 27(4), 262–269.
64. Regulski M., Regulska K., J Stanisz B., Murias M., Gieremek P., Wzgarda A., Niznik B. Chemistry and pharmacology of Angiotensin-converting enzyme inhibitors. Current Pharmaceutical Design 2015; 21(13), 1764–1775.
65. Taddei S. Combination therapy in hypertension: what are the best options according to clinical pharmacology principles and controlled clinical trial evidence? Am. J. Cardiovasc. 2015; 15(3), 185–194.
66. Reboldi G, Gentile G, Angeli F, Verdecchia P. Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: update after recent clinical trials. Vasc. Health Risk Manag. 2009; 5(1): 411–427. doi: 10.2147/vhrm.s4235. PMID: 19475778; PMCID: PMC2686259.
67. Borghi C., Soldati M., Bragagni A., Cicero A. F. Safety implications of combining ACE inhibitors with thiazides for the treatment of hypertensive patients. Expert Opin. Drug Saf. 2020; 19(12), 1577–1583.
68. Molvanov N. H., Tursunova D. E. Combined Antihypertensive and Hypolipidemic Therapy for Arterial Hypertension Hemodynamic Changes and Lipid Spectrum. IJHMS 2022; 1(1), 57–59.
69. Pappa E., Rizos C. V., Filippatos T. D., Elisaf M. S. Emerging fixed-dose combination treatments for hyperlipidemia. JCPT 2019; 24(4), 315–322.
70. Smith M. C. Principles of pharmaceutical marketing. New York: Routledge 2014.
71. Kim D. W., Weon K. Y. Pharmaceutical application and development of fixed-dose combination: Dosage form review. J. Pharm. Investig. 2021; 51(5), 555–570.
72. Ferrario A., Dedet G., Humbert T., Vogler S., Suleman F., Pedersen H. B. Strategies to achieve fairer prices for generic and biosimilar medicines. BMJ 2020; 368, 5444.
73. Xanthopoulou S. S., Katsaliaki K. Policies and perceptions on generic drugs: The case of Greece. HSMR 2019; 32(1), 49–56.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2023 Issue 2-
All articles in this issue
- Colour and content of some metals in honey
- Selective inhibitors of cyclooxygenase 2 – their past, present and future
- Mezinárodní spolupráce tří uznávaných Farmaceutických fakult – sdílení výzkumných zkušeností a vzdělávacích technik v oblasti drug-designu
- NOVÉ KNIHY
- Role of pharmaceutical care in therapeutic regimens within the community pharmacy
- Marketing research of combined drugs for treatment of cardiovascular diseases
- Antimicrobial properties of the new combined dental gel
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Selective inhibitors of cyclooxygenase 2 – their past, present and future
- Role of pharmaceutical care in therapeutic regimens within the community pharmacy
- Marketing research of combined drugs for treatment of cardiovascular diseases
- Colour and content of some metals in honey
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

