-
Medical journals
- Career
The influence of «Saprogel» in the wound healing process on rats with a full-thickness wound model
Authors: Oksana Strus 1; Natalia Polovko 2
Authors‘ workplace: Department of Drug Technology and Biopharmaceutics, Danylo Halytsky Lviv National Medical University 1; Department of Drug Technology, National University of Pharmacy, Kharkiv, Ukraine 2
Published in: Čes. slov. Farm., 2020; 69, 75-82
Category: Original article
Overview
The article presents the study results of the effectiveness and the mechanism of the «Saprogel» action on the wound healing process and pro-/antioxidant equilibrium in serum of rats with a full-thickness wound model. The wound healing effect of tested gel samples was evaluated by the dynamics of area change (S) and the percentage reduction of the area (PRA) of wounds on the 3rd, 7th, and 14th days of the treatment. The content of lipid peroxide oxidation (LPO) products and the activity of antioxidant protection enzymes in the serum of rats were determined in the dynamics in different phases of the wound healing process in rats. «Saprogel» treatment of full-thickness wounds in rats reduced their healing time by 24.5% (p < 0.05), but there was no statistically significant difference between the effect of «Saprogel» and the comparator product on the duration of wound healing. The content of LPO products in the rat serum when using «Saprogel» was reduced and the enzymes activity of the antioxidant protection SOD and CAT was normalized, indicating that one of the mechanisms of wound healing action of «Saprogel» is its antioxidant properties.
Keywords:
sapropel extract – dermatotropic gel product – wound healing process – pro-/antioxidant equilibrium
Introduction
Nowadays, the treatment of wounds is not only a medical problem. It has a significant economic impact on the health care systems of all countries, and the cost of treatment increases dramatically1). In this regard, one of the topical directions in modern pharmacy is to find effective agents based on natural biologically active substances, which due to their multicomponent composition have a wide range of pharmacological action and low toxicity, which is advantageously different from chemical synthesis products. The resource factor exerts influence on the relevance of the search for new highly effective means of natural origin, since in today’s market conditions the availability and cheapness of raw materials determines the demand for the proposed preparations. Such substances include sapropel, which is a unique organic substance, and its reserves in Ukraine exceed 74.5 million tonnes2).
There are several classifications of wound healing phases. However, the most recognized is the classification according to which the healing process is divided into three main phases: the inflammation phase, lasting 5 days from the moment of wound formation; the regeneration phase (proliferation, granulation) which lasts from the 6th to the 14th day after the formation of the injury; the remodeling phase (epithelialization, scar formation, and reorganization) that begins from the 15th day and lasts until the complete closure of the wound bed3).
For therapy, use of dermatotropic drugs aimed at the elimination of a specific disease, as well as increased protection against the effects of environmental factors and the prevention of loss of proteins, electrolytes and water4).
Wound healing agents can be taken externally, systemically, or injected directly into the inflammation center. Drugs for external use have several advantages, including: ease of use and monitoring of treatment. In addition, most of these drugs do not get into systemic blood flow and, accordingly, do not cause complications in the body. Also rarely interact with other drugs5).
The medicines present on the Ukrainian market affect only one of the wound healing phases. Some authors consider that the use of highly effective preparations affect more than one wound healing phase, can improve the process of local treatment and expand the range of national wound healing products6, 7). According to the literature, as of February 1, 2013, in Ukraine only 25 wound healing preparations were registered for use in the II-nd phase of the wound process, of which 15 preparations were available to the consumer at the time of the study, as highlighted in the article by Rudenko8). Today, the market of registered dermatotropic preparations has changed insignificantly. Therefore, the relevance of the planned study is not in doubt.
It is noted that among the D03 group of dermatological drugs for the treatment of wounds and ulcerative lesions, according to the State Register of Medicinal Products of Ukraine9), as of January 2020, the ointments dominate. Gels make up 5% of dermatological preparations for wound treatment10). Gels have several advantages, compared to other semi-solid dosage forms11) and are relatively easier to prepare compare to ointments and creams12, 13).
А new dermatotropic gel product containing sapropel extract was developed. Its main group of biologically active substances are humic substances, which according to the research data have low toxicity, an expressed antiviral, wound healing and anti-inflammatory activities suppress, the development of malignant tumors, etc.14, 15).
Humic substances (HS) are a complex mixture of many different acids containing carboxyl and phenolate16). The presence of phenols, carboxylic acids and quinones in the structure of HSs is related to their antioxidant, antimutagenic/desmutagenic and fungicidal/ bactericidal activities17). Humic acids (HA) can have a positive effect on wound healing and cancer therapy18–23).
The biological activity of HA is associated with their effect on redox processes and with the activation of enzyme systems24, 25).
The mechanism of action of HA can also contribute to the inhibition of both the classical and alternative pathways of complement activation, as well as the degranulation of phagocytes and the production of inflammation-related cytokines such as IL-1β, IL-6, IL-10 and TNF-α26–28). HS have also been reported to stimulate the release of pro-inflammatory cytokines such as TNF-α in vitro, but only in the presence of exogenous lipopolysaccharides29), indicating that these substances should not cause inflammation under normal conditions.
Microbial contamination substantially changes the course of the wound process. Together with mechanical damage to tissues, products of bacterial life can slow down the primary healing phases, increase osmotic pressure and acidosis in the tissues, disturb the microcirculation, which leads to the development of secondary necrosis30).
It has been experimentally confirmed that even weakly bactericidal peloids inhibit the ability of pathogenic bacteria to inactivate the complement, lysozyme and bactericidal component of the interferon drug, as well as reduce the hydrophobicity of bacterial cells31). The presence of bactericidal effect in peloids provides the possibility of their use as natural antifungal agents in fungal skin ulcers caused by a number of dermatophytes31, 32).
The research of antibacterial characteristics of the aqueous extract of sapropel from Prybych natural deposits indicates its antibacterial effectiveness against Staphylococcus aureus, Escherichia coli, Basillus subtilis, Candida albicans; and minor antibacterial effect against Proteus vulgaris and Pseudomonas aeruginosa33).
In view of the above, the aim of the study was to investigate the effect of the gel with a sapropel extract, conventionally called «Saprogel», on the duration of healing and the percentage reduction in the average area of a full-thickness wound in rats and on the pro-/antioxidant equilibrium in serum.
Experimental part
Materials
Materials of the study were the gel under the conditional name «Saprogel», which contained an aqueous extract of sapropel (SE) in the amount of 15%, carbopol Ultrez 10, potassium sorbate, glycerol, purified water; carbopol-based gel containing carbopol Ultrez 10, trometamol, potassium sorbate, glycerol, purified water and «Pantestin-Darnitsa» gel as the comparator product.
The SE was obtained from the sapropel of Prybych deposit, located at Volyn region, Ukraine. The sapropel was treated with 0.1 N alkali solution. Cavitation was used at a temperature of 50–60 °C and a speed of 3000 rot/min for 60 minutes for obtaining a homogeneous mixture. The obtained extract was evaporated to 1 : 10 of basal volume33, 34). The usage of the cavitation method provides the content of HA in SE of more than 20%33, 34).
«Saprogel» – tested gel with aqueous SE was prepared according to the following technology: potassium sorbate (preservative) was dissolved in purified water, the pre-weighed carbomer Ultrez 10 was added and left to swell for 30–60 minutes. During the swelling, the mixer was periodically switched on and mixed at a speed of 60–90 rot/min. Gradually the SE and glycerol were added and homogenized at a speed of 60–90 rot/min during 5–10 minutes until obtaining a homogeneous gel10).
For preparing the carbopol-based gel the technology of «Saprogel» was applied, using trometamol as a neutralizing agent.
The comparator product, «Pantestin-Darnitsia» gel produced by «Darnitsa» (Ukraine), contains such active pharmaceutical ingredients as dexpanthenol and miramistin. According to the manufacturer’s annotation, it is used for the treatment of wounds of different localization and genesis in the II-nd phase of wound healing process9).
Methods
Animal studies
Studies of anti-inflammatory and reparative activity of «Saprogel» were performed on white laboratory rat-males with weigh of 200–250 g, n = 119. Animals were kept in the vivarium of Danylo Halytsky National Medical University under standard conditions of temperature (21 °C), lighting (12/12 h), humidity and diet (complete feed for laboratory animals K – 12-4, «Rizan-1», Ukraine), according to the Standard Rules for Organizing, Equipping and Maintaining Experimental Biological Clinics (Vivarium).
The experiments were conducted in accordance with ethical principles adopted by the First National Congress of Ukraine on Bioethics, international agreements, and national legislation in this field35–39).
The animals were treated humanely throughout the study period adhering to the guideline for use and care of animals in Declaration of Helsinki (National Research Council, 2011). The experiment design and study protocol were approved by the Animal Ethics Committee of the Danylo Halytsky Lviv National Medical University, protocol No. 3, December 10, 2019.
Before the experiment, the rats were quarantined, after which the laboratory animals were examined and weighed. Animals were individually noticed by bringing on cuts on the ear lobes. All pain procedures and surgeries were performed under general anesthesia with thiopental sodium (Thiopental sodium, Biochemie GmbH/Austria) at a dosage of 60 mg/kg animal weight. Animals were kept in individual cages under standard vivarium conditions throughout the whole experiment.
Animals were divided into 5 groups. The rats of groups 2–5 had modeled full-thickness excised plane wounds40). For this purpose, on the pre-depilated skin of anesthetized rats the skin of 1 × 1 cm2 size was excised with a surgical scalpel and forceps. The bleeding was stopped using sterile gauze swabs and 3% hydrogen peroxide solution. The treatment began as soon as the wound is designed and until complete healing40).
Experimental groups were as follows:
- I – a group of intact rats in which the physiological level of the studied parameters was measured (n = 7);
- II – a group of control rats, in which an excised plane wound was simulated and the healing occurred independently without treatment (control pathology) (n = 28);
- III – an experimental group of rats in which a full-thickness wound was simulated, which was treated with a gel base (GB) (n = 28);
- IV – an experimental group of animals with experimental full-thickness wounds, in which the developed gel, called «Saprogel», containing 15% SE (n = 28) was applied on the affected area.
- V – an experimental group of animals with experimental full-thickness wounds, in which the comparator product «Pantestin-Darnitsa» gel (n = 28) was applied on the affected area.
From II – V groups of rats on the 3rd, 7th, 14th day of the wound process, 7 animals were selected for the study of pro-/antioxidant equilibrium in serum. There were 7 animals left in these groups. They were extracted from the experiment by decapitation on the day of the complete scarring of the wound bed. The material for hematological studies was taken on the 3rd, 7th, 14th day and on the day of complete healing of the wound after starting treatment.
The wound healing effect of GB, «Saprogel» and «Pantestin-Darnitsa» gel was evaluated by the dynamics of area change (S) and the percentage reduction of the area (PRA) of wounds on the 3rd, 7th, and 14th days of treatment. The PRA was calculated according to the common formula:
PRA = ((So – S)/So) × 100,
where PRA – the percentage reduction of wound area, So – the average area of the wound before treatment, S – the average wound area at the time of measurement.
In the study groups daily the test gel samples were applied on the affected areas with using a sterile metal spatula, which was fluted before each use. Also every 24 hours the general animal condition and the condition of the affected surface were assessed visually, the size of the wound surface was also determined.
Hematological indices
Hematological studies were conducted in the Laboratory of Molecular Biology and Clinical Biochemistry of the Institute of Animal Biology of the National Academy of Agrarian Sciences, Lviv, Ukraine.
Blood serum collection
The serum of experimental rat blood was obtained from whole blood. Blood was left at 37 °C for 4 hours to remove fibrinogen and concomitant proteins. At the next stage, a clean, dry glass rod was used to carefully separate the blood clot from the test tube walls to speed up the production of serum and centrifuged for 40 minutes at 2000 rpm. The obtained serum was quickly separated from the formed blood elements, transferred the Eppendorf tube and frozen at –20 °C until further use.
In the serum of rats we determined the content of diene conjugates (DC) in heptane-isopropanol extract by the spectrophotometric method41), schiff bases (SchB) – by the fluorimetric method42); the content of thiobarbituric acid reactive substances (TBARS) was determined by the reaction with thiobarbituric acid43). The activity of superoxide dismutase (SOD) was determined by Chevari44), the activity of catalase (CAT) by Koroliuk45).
Statistical methods
The hypothesis testing of the normal data distribution was conducted via the Shapiro-Wilk test using the software package GraphPad Prism 5.04 (GraphPad Software Inc., USA). Further calculation of the results was performed using a two-way ANOVA with Bonferroni post hoc test. For each obtained result the arithmetic mean (M) and the standard error of the arithmetic mean (m) were determined. Difference between means was considered as statistically significant if p < 0.05.
Results and discussion
The study results of the change dynamics in wound area and the percentage reduction of wound bed area in the healing process of full-thickness wounds in rats, which are presented in Table 1, show that in all experimental groups the wound area gradually decreased, but the duration of complete wound closure was different. The complete closure of the wound bed in animals without treatment occurred within the 24.1 ± 0.8 day.
1. Dynamics of area change (S) and the percentage reduction of area (PRA) of wounds in wound process treatment in different phases 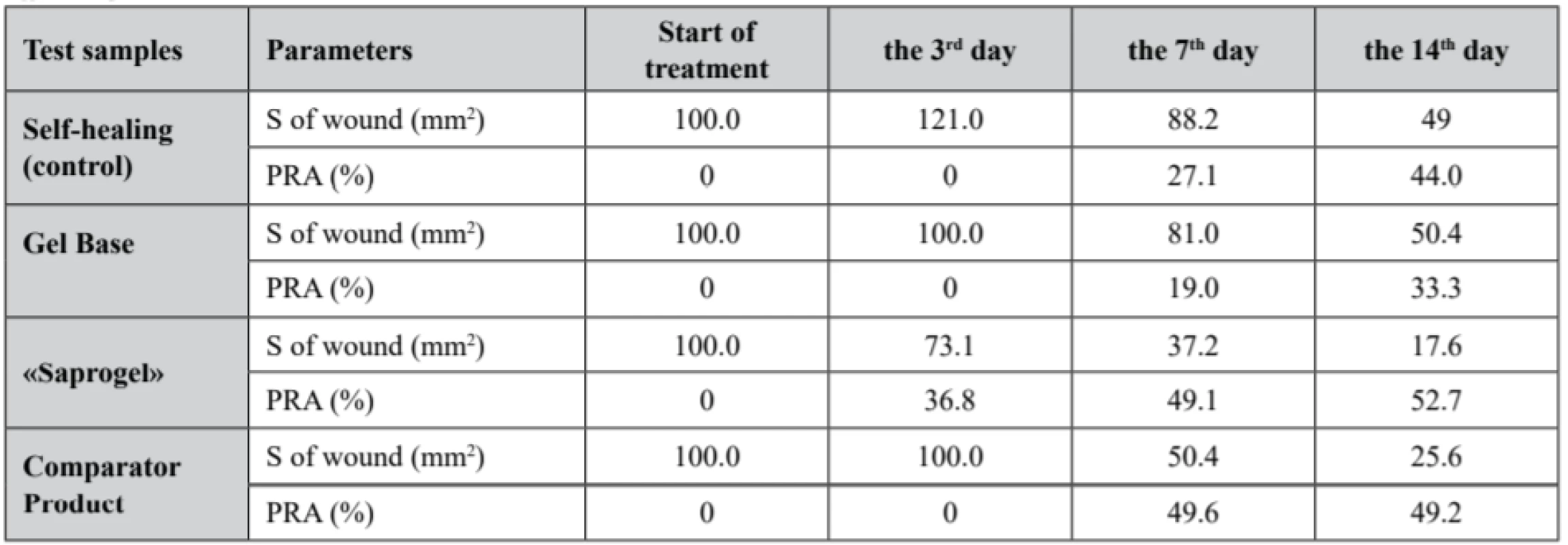
Wound bed treatment by GB did not affect the duration of wound surface healing, which lasted for 23.8 ± 1.2 day. The use of «Saprogel» reduced the healing time of the wound process to 18.2 ± 1.3 day, or by 24.5% (p < 0.05). «Saprogel» showed wound healing effect in all phases of the healing process with the most pronounced effect in the II-nd and III-rd phases of wound healing. Unlike «Saprogel», the use of «Pantestin-Darnitsa» gel in the first four days (the I-st phase of wound healing) did not have a positive effect. Comparator product was most active in phase II of wound healing and reduced the duration of wound healing to 17.9 ± 1.6 day, or by 24.8% (p < 0.05).
A macroscopic examination of the wound surface showed that in the rats with self-healing (control group) the third day was dominated by post-traumatic inflammation, the wound edges were roller-like, swollen, the wound was covered with thick brown crusts, and the bottom was hyperemic. Obviously, due to this, the area of the wound surface was larger than on the day of modeling. The obtained data corresponds to the literature, in which the area of full-thickness skin wounds in the rats on the 3rd day is larger than on the 1st day46).
The rats in the control group on the 3rd and the 4th days had more pronounced signs of inflammation than the rats treated with «Saprogel». Practically the same picture was in the first 6 days in the rats, whose wounds were treated with GB and comparator product. In animals treated with «Saprogel», the crust over the wound bed was thin and no pus was observed.
Particularly large was the difference between the control group and the groups of the rats in which wounds were treated with «Saprogel» or «Pantestin-Darnitsa» gel in the II-nd phase of healing. In this case, GB did not change both the nature of the healing process and the speed indicators of wound bed closure. On the 7th day, approximately the same changes were observed in the animals treated with «Saprogel» or «Pantestin-Darnitsa» gel. They consisted in the absence of a roller around the wound (in control it still present), the wound edges were actually in the wound bed where the beginning of granulation was visible. On the 14th day in the control group, we saw the signs of epithelialization at the edges of the wound defect and the presence of granulation tissue at its bottom.
On the 14th day, the epithelial tissue was covered by nearly 80% of the area in the animals whose wounds were treated with «Saprogel», and occupied up to 50% of the area in the animals whose wounds were treated with «Pantestin-Darnitsa» gel. Considering that the completion of the II-nd phase indicates the filling of the wound bed with granulation tissue and the beginning of epithelialization and scar formation, we can conclude that both «Saprogel» and «Pantestin-Darnitsa» gel have wound healing effect in the II-nd phase of wound healing. The III-rd phase for these preparations was shortened, but «Saprogel» was more effective in this phase.
It is proved that oxidative stress, which suppresses reparative processes in the wound skin damage, develops at violation of pro-/antioxidant equilibrium47). The violation of this equilibrium in skin damage is systemic. In this regard, we determined the content of lipid peroxide oxidation (LPO) products and the activity of antioxidant protection enzymes in the rat serum in the dynamics in different phases of the wound process.
As a result of the studies, it was found that the content of primary LPO of DC in the rat serum of the control group with wounds without treatment on the 3rd day increased by 84.4% (p < 0.01), on the 7th day (the beginning of the II-nd phase) – by 41.1% (p < 0.05) compared with the intact control (Table 2). On the 14th day (the end of the II-nd phase), the content of DC returned to the level of the intact control. The use of GB did not statistically significantly affect the content of DC in serum compared to the control.
2. The content of lipid peroxidation products in the serum of rats with full- thickness wound (M ± m, n = 7) 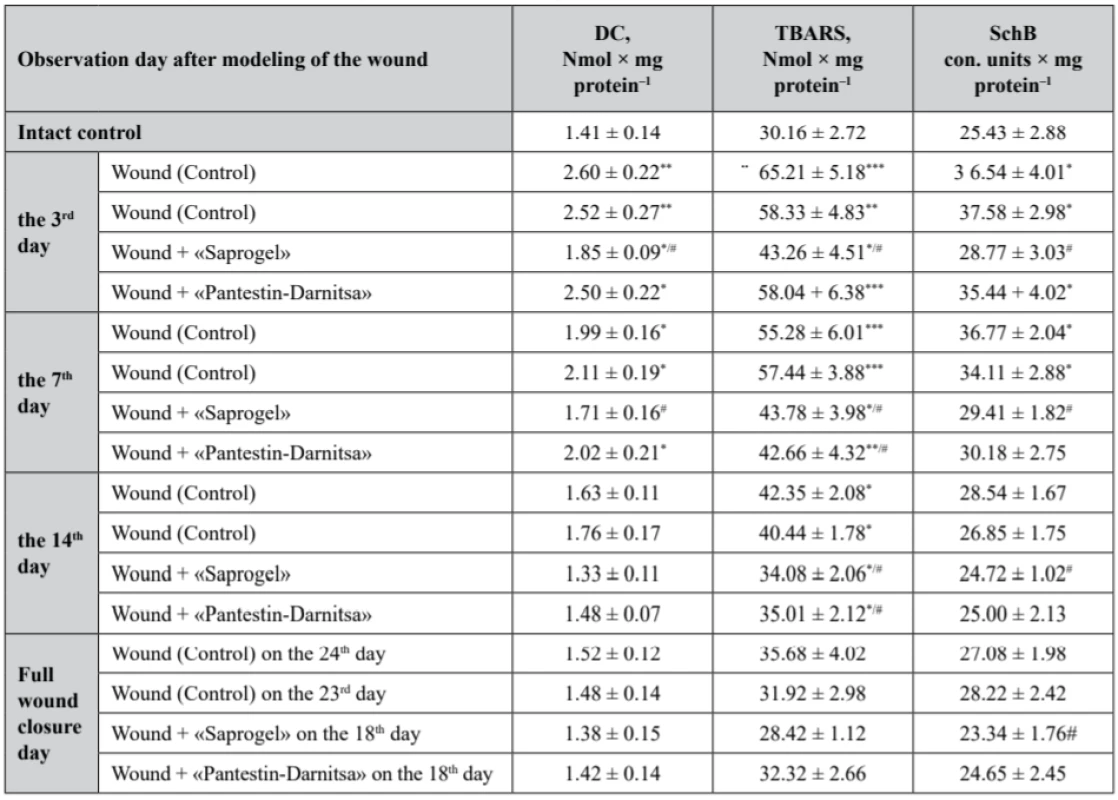
*р < 0.05, **р < 0.01, ***р < 0.001 relatively intact control, #p < 0.05 relatively control (wounds without treatment) The use of «Saprogel» reduced the DC content in the serum by 28.8% (p < 0.05) on the 3rd day compared to the control. On the 7th day the content of DC in the serum was reduced to the level of the control. The action of «Pantestin-Darnitsa» gel on the content of DC on the 3rd and the 7th day of the wound process was close to that of «Saprogel».
The content of TBARS was restored later to the content of DC, which is obviously the result of the depletion of the antioxidant system (Table 2).
2. The content of lipid peroxidation products in the serum of rats with full- thickness wound (M ± m, n = 7) 
*р < 0.05, **р < 0.01, ***р < 0.001 relatively intact control, #p < 0.05 relatively control (wounds without treatment) On the 3rd, 7th, 14th day from the beginning of the wound process modeling, the content of TBARS in the serum of rats with wounds without treatment exceeded in the rats of the intact control by 116.2% (p < 0.001), 83.3% (p < 0.001) and 40.4% (p < 0.05), respectively. On the day of complete closure of the wound bed, the content of TBARS in the serum of rats with wounds without treatment was at the level of the intact control. The use of GB did not affect the study indicator compared with the control group.
During the whole healing period, due to «Saprogel» actions the content of TBARS in the serum of rats was lower compared to the control untreated rats: on the 3rd, 7th, and 14th day from the start of the wound process modeling, it was reduced by 33.7% (p < 0.05), 20.8% (p < 0.05) and 19.5% (p < 0.05), respectively. The difference in action of «Saprogel» and «Pantestin-Darnitsa» was observed only in the I-st phase of healing, in which «Pantestin-Darnitsa» was ineffective. On the day of complete closure of the wound in both groups the content of TBARS in the serum of rats was at the level of the intact control.
The content of SchB in the rat serum of the control group was increased during the I-st phase (on the 3rd day by 43.7% (p < 0.05)) and at the beginning of the II-nd phase of healing (on the 7th day by 44.6%) (p < 0.05), by the end of the II-nd phase it returned to the level of the intact control. GB did not affect the content of SchB in the serum of rats with full-thickness wounds. However, in the I-st phase of the wound process «Saprogel» reduced the content of SchB in the rat serum relative to the control group by 21.37% (p < 0.05), and at the beginning of the II-nd phase it was decreased to the level of the intact control. Similarly, «Pantestin-Darnitsa» gel influenced the healing process.
Thus, the depletion of the antioxidant system was observed in rats with wounds without treatment, as confirmed above.
In rats of the control group (with wounds without treatment), superoxide dismutase (SOD) activity was reduced during all days of the observation. On the 3rd, 7th, 14th day from the beginning of the wound process modeling and on the day of complete closure of the wound bed, it was lower by 84.9% (p < 0.001), 86.4% (p < 0.001), 51.8% (p < 0.01) and 59.4% (p < 0.01), respectively. We have confirmed the results of other authors, in which an experimental chronic wound is accompanied by a decrease in the activity of antioxidant enzymes in serum compared to healthy animals48, 49). Daily application of GB to the wound did not affect SOD activity (Table 3).
4. Activity of SOD and CAT in the serum of rats with full- thickness wound (M ± m, n = 7) 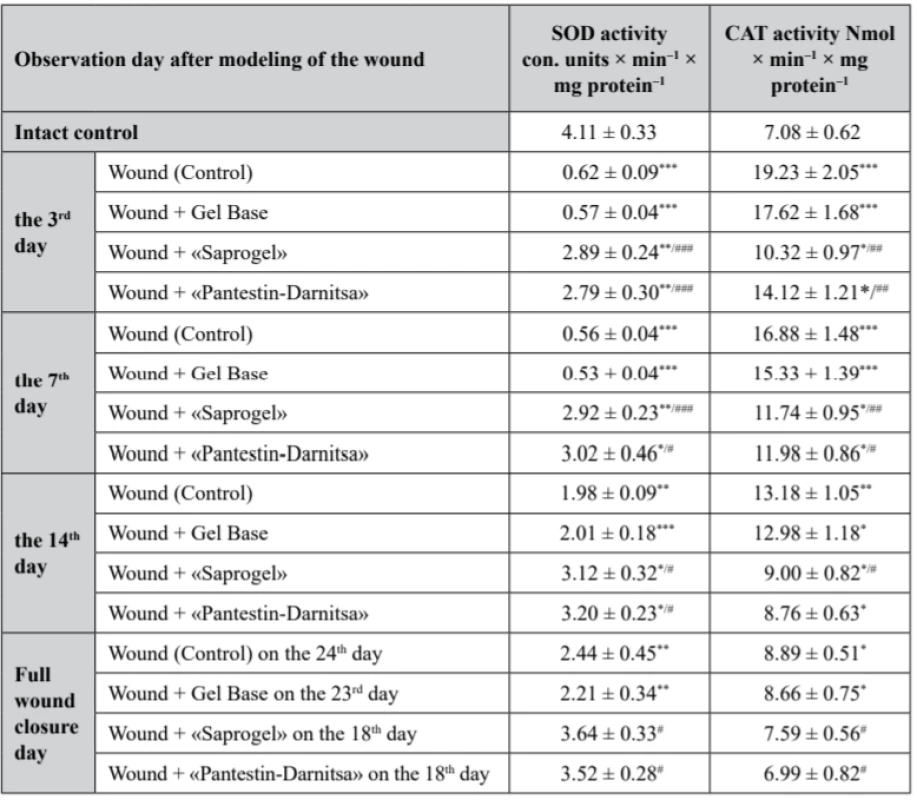
*р < 0.05, **р < 0.01 – relatively the intact control, #p < 0.05 relatively the control (wounds without treatment) In the group of rats whose wounds were treated with «Saprogel», SOD activity was significantly higher than in the rats of the control group. Thus, on the 3rd, 7th, 14th day from the beginning of the experiment compared to the intact control, it was lower by 29.7% (p < 0.001), 30.0% (p < 0.001) and 24.1% (p < 0.01), respectively. The effect of the «Pantestin-Darnitsa» use was almost the same as after «Saprogel». On the day of complete wound closure, both after «Saprogel» treatment and «Pantestin-Darnitsa» treatment, SOD activity was at the level of the intact control.
With regard to CAT, in the control group rats, it was higher than that in the intact control rats in all phases of the observation. On the 3rd, 7th, 14th day from the beginning of the experiment and on the day of complete epithelialization, the activity of CAT increased by 172.5% (p < 0.001), 138.4% (p < 0.001), 86.2% (p < 0.01) and 25.6% (p < 0.05), respectively. Changes in the studied indicator under the influence of GB in all observation periods were slightly different from the control (p > 0.05).
The use of «Saprogel» affected the activity of CАТ in all phases of healing. On the 3rd, 7th, 14th day from the beginning of the experiment, the activity of the CAT was higher than the given indicator in the intact control group by 45.8% (p < 0.05), 65.8% (p < 0.01) and 27.1% (p < 0.05), respectively. On the day of full epithelialization after the use of «Saprogel», the activity of CАТ was at the level of the intact control. Thus, with using «Saprogel», the activity of CAT increased to a lesser extent than in the rats in the self-healing process. In addition, with «Saprogel» actions, the recovery of CAT was much faster than in the control group rats.
Compared to the «Saprogel» effect on the activity of CAT, the effect of the reference sample on this enzyme activity was approximately the same in the II-nd phase and the III-rd phase of the healing process. However, in the I-st phase, «Saprogel» was more effective in relation to the CAT. Actually, these data confirm on the wound healing effect of «Saprogel» and «Pantestin-Darnitsa» gel in different phases of the wound healing process.
Conclusion
«Saprogel» treatment of full-thickness excised plane wounds in rats reduced their healing time by 24.5% (p < 0.05). There was no statistically significant difference between the effect of «Saprogel» gel and the comparator product on the duration of wound healing. «Saprogel» showed wound healing effect in all phases of the healing process with the most pronounced effect in the II-nd and the III-rd phases of wound healing. Unlike «Saprogel», the «Pantestin-Darnitsa» gel showed wound healing effect only in the II-nd and the III-rd phases of wound healing with the most pronounced effect in the II-nd phase. The demonstrated effectiveness of «Saprogel» in the I-st phase of healing process indicates its antibacterial effect.
With «Saprogel» action the content of LPO products is reduced in rats with full-thickness wounds in the serum and the enzymes activity of the antioxidant protection SOD and CAT is normalized, indicating that one of the mechanisms of wound healing action of «Saprogel» is its antioxidant properties. In its antioxidant properties, «Saprogel» compared to «Pantestin-Darnitsa» gel, was more effective in the I-st phase of healing. In the II-nd and the III-rd phases of wound healing, there was no difference between the preparations.
Acknowlegments
We are thankful to Zander – Ukraine LTD for providing free samples of sapropel to carry out the research.
Conflict of interest: none.
Assoc. Prof. Oksana Strus, PhD (∗)
Department of Drug Technology and Biopharmaceutics
Danylo Halytsky Lviv National Medical University
Pekarska str. 69, 79010 Lviv, Ukraine
e-mail: oxana.strus@ukr.net
N. Polovko
Department of Drug Technology
National University of Pharmacy, Kharkiv, Ukraine
Sources
1. Mohanty C., Sahoo S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017; 22, 1582–1592. doi:10.1016/j.drudis.2017.07.001
2. Strus O., Polovko N., Yezerska O. Justification of technological parameters of the cream production with sapropel extract. Pharmacia 2019; 66(1), 19–25 doi:10.3897/pharmacia.66.e35022
3. Velnar T., Bailey T., Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009; 37, 1528–1542. doi:10.1177/147323000903700531
4. Ebadi M. Desk reference of clinical pharmacology. Taylor & Francis Group, LLC 2008.
5. Hilman А. Г., Khardman D., Limberd L. Clinical farmacology by Hudman and Hilman. Moscow: Practika 2006.
6. Blautin L. A. Local drug treatment of wounds. Problems and new possibilities of their solution. Consilium medicum: surgery (application) 2007; 1, 9–16.
7. Girgolava S. S. A gunshot wound. Moscow: Medicine 2006.
8. Rudenko V. V., Shmatenko O. P., Prytula R. L. Pharmacoeconomic analysis of local use medicinal products in II-nd phase of the wound process. Topical Issues in Pharmaceutical and Medical Science and Practice. 2013; 2 (12), 121–124.
9. State Register of Medicinal Products of Ukraine, January, 2020 http://www.drlz.com.ua/
10. Strus O., Polovko N. The composition and technology development of the gel dermatotropic product with sapropel extract. World J. Pharm. Pharma. Sci. 2020.
11. Bilous S. B., Kalynyuk T. H., Gudz N. I. Aktualni pytannia farmatsevtychnoi rozrobky miakykh likarskykh zasobiv dlia zovnischnoho zastosuvannia. Farmatsevtychnyi zhurnal 2010; 2, 17–27.
12. Encyclopedia of pharmaceutical technology. Ed. By J. Swarbrick. 3rd ed. N.Y: In-forma Halthcare USA, Inc. 2007; 4372 p.
13. Pharmaceutical Manufacturing Handbook. Production and Processes 2008 https://www.uv.mx/personal/izcamacho/files/2012/02/Pharmaceutical-Manufacturing-Handbook-Production-and-Processes-Wiley-2008.pdf
14. Kala K. J., Prashob P. K. J., Chandramohanakumar N. Humic substances as a potent biomaterials for therapeutic and drug delivery system-a review. Int. J. App. Pharm. 2019; 11(3), 1–4. doi:http://dx.doi.org/10.22159/ijap.2019v11i3.31421
15. Schepetkin I., Khlebnikov A., Kwon B. S. Medical drugs from humus matter: Focus on mumie. Drug Dev. Res. 2002; 57(3), 140–159. doi:10.1002/ddr.10058
16. Chauke T. L. Evaluating the efficacy, safety and possible mechanism of action of potassium humate with selenium. Dissertation (MSc) University of Pretoria 2014. http://hdl.handle.net/2263/40844
17. Gomes de Melo B. A., Lopes Motta F., Andrade Santana M. H. Humic acids: Structural properties and multiple functionalities for novel technological developments Materials Science and Engineering C 2016; 62, 967–974. http://dx.doi.org/10.1016/j.msec.2015.12.001 0928-4931/
18. Aykac A., Becer E., Okcanoglu T. B., Guvenir M., Suer K., Vatansever S. The cytotoxic effects of humic acid on human breast cancer cells. Proceedings 2018; 2, 1565.
19. Jurcsik I. Possibilities of applying humic acids in medicine (wound healing and cancer therapy), In: Senesi N., Milano T. M. (eds.) Humic Substances in the Global Environment. Amsterdam, London, New York, Tokyo: Elsevier 1994; 1331–1336.
20. Ji Y., Zhang A., Chen X., Che X., Zhou K., Wang Z. Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm. Sin. B (APSB) 2016; 6(2), 132–140. doi:10.1016/j.apsb.2016.01.009
21. Metin Çalışır, Aysun Akpınar, Ahmet Cemil Talmaç, Aysan Lektemur Alpan, Ömer Fahrettin Göze Humic acid enhances wound healing in the rat palate evid based complement. Alternat. Med. 2018 : 1783513. doi:10.1155/2018/1783513
22. Kodama H., Denso Okazaki F., Ishida S. Protective effect of humus extract against Trypanosoma brucei infection in mice. J. Vet. Med. Sci. 2008; 70(11), 1185–1190. doi:10.1292/jvms.70.1185
23. van Rensburg C. E. J., van Straten A., Dekker J. An in vitro investigation of the antimicrobial activity of oxifulvic acid J. Antimicrob. Chemother. 2000; 46(5): 853–854. doi:10.1093/jac/46.5.853
24. Avvakumova N. P., Gerchikov A. Y., Khairullina V. R., Zhdanova A. V. Antioxidant properties of humic substances isolated from peloids. Pharm. Chem. J. 2011; 45, 192.
25. Kitapova R. R., Ziganshin A. U. Biologic activity of humic substances from peat and sapropel. Каzаnsky meditcinsky zhurnal 2015; 96(1), 184. https://kazanmedjournal.ru/kazanmedj/article/view/1495/1112
26. van Rensburg C. E. J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015; 29(6), 791–795. doi:10.1002/ptr.5319
27. Naudé P. J. W., Cromarty A. D., van Rensburg C. E. J. Potassium humate inhibits carrageenan-induced paw oedema and a graft-versus-host reaction in rats. Inflammopharmacology 2010; 18(1), 33–39. doi:10.1007/s10787-009-0026-8
28. Jooné G. K., van Rensburg C. E. J. An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation 2004; 28(3), 169–174. doi:10.1023/B:IFLA.0000039563.90066.5d.
29. Junek R., Morrow R., Schoenherr J. I., Schubert R., Kallmeyer R., Phull S., et al. Bimodal effect of humic acids on the LPS-induced TNF-α release from differentiated U937 cells. Phytomedicine 2009; 16, 470–476.
30. Pastar I., Nusbaum A. G., Gil J. Interactions of Methicillin Resistant Staphylococcus aureus USA 300 and Pseudomonas aeruginosa in Polymicrobial Wound Infection. PLoS One 2013; 8(2), 1–11.
31. Klöcking R, Sprössig M, Wutzler P, Thiel K-D, Helbig B. Antiviral wirksame Huminsäuren und huminsäureähnliche Polymere. Zeitschrift für Physiotherapie 1983; 35, 95–101. doi:10.1055/s-2008-1065760
32. Schynkarenko A. L., Milenina N. G. Organic substances of therapeutic mud and their role in the mechanism of therapeutic effect on the body Sb. nauch. trudov Piatihorskoho NII kurortolohii I fizioterapii «Griazevie preparaty». Тоmsк 1981; 30–33.
33. Strus O. Y. Study of sapropel extracts from Prybych natural deposits. J. Chem. Pharm. Res. 2015; 7(6), 133–137. http://www.jocpr.com/articles/study-of-sapropel-extracts-from-prybych-natural-deposits.pdf
34. Strus O., Polovko N., Plaskonis Y. The investigation of the development of a cream composition with the sapropel extract. Asian J. Pharm. Clin. Res. 2018; 11(7), 147–150. doi:10.22159/ajpcr.2018.v11i7.23575
35. Council Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Communities 2010; L 276, 33–79.
36. European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Council of Europe, Strasbourg 1986; 53 p.
37. Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Eighth Edition, 220 s.
38. Law of Ukraine »On Protection of Animals from Cruel Treatment« of February 21, 2006, No. 3447-IV/ Data of the Verkhovna Rada of Ukraine. Official edition, 2006. No. 27. https://zakon.rada.gov.ua/laws/show/3447-15/
39. First national congress on Bioethics. Weekly Journal Pharmacy 2001; 308(37) (date 24. 09. 2001).
40. Henry S. L., Concannon M. J., Yee G. J. The effect of magnetic fields on wound healing. Experimental study and review of the literature. Eplasty 2008; 8, 393–399.
41. Gavrylov V. B., Gavrylova A. R., Khmara N. F. Determination of diene conjugates in blood plasma by UV absorption of heptane and isopropanol extracts. Laboratory Work 1988; 2, 60–63.
42. Zymon A. D., Leschenko N. F. Colloidal chemistry. Moscow: Khimiia 1995; 336.
43. Stalnaia I. D., Garischvili T. G. Moderm methods in biochemistry. Moscow: Medidcine 1977; 66–68.
44. Chevari S., Chaba I., Sekey Y. The role of superoxide dismutase in the oxidative processes of a cell and a method for determining it in biological materials. Laboratory Work 1985; 11, 678–681.
45. Korolyuk M. A., Ivanova L. I., Mayorova I. G. Method for determining the activity of catalase. Laboratory Work 1988; 1, 16–18.
46. Taburets O. V., Morgaienko O. O., Kondratiuk T. O., Beregova T. V., Ostapchenko L. I. The effect of «Melanin-gel» on the wound healing. Res. J. Pharm. Biol. Chem. Sci. 2016; 7(3), 2031–2036.
47. Wagener F. A., Carels C. E., Lundvig D. M. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013; 14, 9126–9167.
48. Shaik M. M., Dapkekar A., Rajwade J. M., Jadhav S. H., Kowshik M. Antioxidant - antibacterial containing bi-layer scaffolds as potential candidates for management of oxidative stress and infections in wound healing. J. Mater Sci. Mater Med. 2019; 11, 30(1). doi:10.1007/s10856-018-6212-8
49. Rajagopalan P., Jain A. P., Nanjappa V., Patel K., Mangalaparthi K. K., Babu N., Cavusoglu N., Roy N., Soeur J., Breton L., Pandey A., Gowda H., Chatterjee A., Misra N. Proteome-wide changes in primary skin keratinocytes exposed to diesel particulate extract-A role for antioxidants in skin health. J. Dermatol. Sci. 2019. pii: S0923-1811(19)30273-7; doi:10.1016/j.jdermsci.2019.08.009
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 2-
All articles in this issue
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
- Nové knihy
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- The influence of «Saprogel» in the wound healing process on rats with a full-thickness wound model
- A cost minimization analysis of α2b-interferon supplementation in complex pharmacotherapy of rotavirus infection in newborns
- Formulation and technology development of vaginal pessaries with probiotic activity
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Formulation and technology development of vaginal pessaries with probiotic activity
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

