-
Medical journals
- Career
Imunomodulační aktivita etanolových extraktů z Galium verum L. herb.
Authors: Igor L. Shinkovenko; Natalia V. Kashpur; Tetiana V. Ilina; Alla M. Kovalyova; Olga V. Goryacha; Oleh M. Koshovyi; Olena V. Kryvoruchko; Andryi M. Komissarenko
Published in: Čes. slov. Farm., 2018; 67, 101-106
Category: Original Article
Overview
The present article discusses the results of the research into the phytochemical profile and immunomodulatory activity of Galii veri herba (Galium verum L., Rubiaceae) ethanolic extracts. The extracts under study were obtained from the plant material by means of maceration technique with 20% aqueous ethanol solution (fluid extract I), 60% aqueous ethanol solution (fluid extract II) and 96% ethanol (fluid extract III) on heating. In the substances obtained, the content of hydroxycinnamic derivatives, flavonoids and polyphenols were determined spectrophotometrically; polysaccharides were quantified gravimetrically; the immunomodulatory activity of the substances under study was determined in the reaction of lymphocyte blast transformation (RLBT). It was established that fluid extract I contained 2.4% polysaccharides, 3.1% hydroxycinnamic derivatives, 0.24% flavonoids, and 2.9% polyphenols; the fluid extract II contained 4.13% hydroxycinnamic derivatives, 0.16% flavonoids, and 3.84% polyphenols; fluid extract III contained 2.7% hydroxycinnamic derivatives, 0.18% flavonoids and 2.7% polyphenols. All the extracts under study possessed a marked stimulant effect on the transformation activity of the immunocompetent blood cells. The highest immunomodulatory activity was established for 96% ethanol extract: the percentage of lymphocytes proliferating in RLBT under the influence of this extract increased by 6.77 – 8.04 times in comparison with their spontaneous transformation and by 1.14–1.36 times in comparison with phytohemagglutinin. The results obtained give grounds for further research in the mechanisms of the immunomodulatory activity of the extracts of G. verum herb.
Key words:
ethanolic extracts • Galium verum L. • immunomodulatory activity • lymphocyte blast transformation
Introduction
The study of the development, progression and correction of conditions accompanied by malfunction of the immune system is an important challenge medical science faces today. Extensive research in theoretical and clinical immunology has identified a multitude of diseases caused by the dysfunction of various components of the immune system and requiring an immune-corrective therapy.
For the correction of immunodeficiency conditions, modern medicine has developed a wide range of medicinal products of both synthetic and natural origin. Noteworthily, developed of medicinal products of medicinal origin constitutes an especially promising direction1).
The immunomodulators of plant origin, as opposed to the synthetic ones, display a number of advantages, i.e., a mild immunomodulating effect, a low toxicity, an activation of immune system functions due to biologically active compounds of a holistic effect, etc.2–4).
In this connection, in recent years most research has been focused on the potential immunostimulatory properties of herbal phenolic compounds5–7). It was established that chlorogenic, gallic, ellagic, caffeic, protocatechuic acid and salicylic acid stimulate the production of the immunoglobulins class G. Well known is the effect of caffeic acid on the humoral component of the immune system8).
For in vitro assessment of the substances’ influence on the proliferative activity of the cells of the immune system, a reaction of lymphocytes blast transformation (RLBT) is often used. It consists in the ability of small peripheral blood lymphocytes to transform into large, undifferentiated blast cells within 72–96 hours after a primary contact with mitogens, such as phytohemagglutinin (PHA), etc., or after the second contact with microbial tissue antigens. Thus, RLBT under the influence of mitogens is viewed as an indicator of the functional activity of the T - and B-components of the immune system9).
Previously, we studied the immunomodulatory activities of Asperula L. species and of a fluid water extract from Galium verum L., Rubiaceae family10, 11).
The aim of the present article is to discuss the effect of the fluid ethanolic extracts from G. verum on the functional activity of lymphocytes in the reaction of lymphocyte blast transformation.
Experimental part
Plant materials
G. verum herb was harvested at full flowering stage in the Kharkiv region (Ukraine) in the summer of 2016. Herbarium samples (No. 20062016-25062016) are deposited at the Department of Pharmacognosy (National University of Pharmacy, Ukraine).
Equipment
Spectrophotometer EvolutionTM 60S UV-Visible (Thermo Fisher Scientific, USA), electronic analytical scales AN 100 “Axis” (AXIS, PL), electrical temperature chamber ТС80М-3 (Medlabortekhnika, UA), centrifuge OPN-3 (Phizpribor, RU), microscope ZEISS Primo Star (ZEISS, DE), pipette Thermo Scientific, Lait series 1–200 μl (Thermo Fisher Scientific, USA), pipette Thermo Scientific, Lait series 1–50 μl (Thermo Fisher Scientific, USA), pipette Thermo Scientific, Lait series 1–1000 μl (Thermo Fisher Scientific, USA), pipette Thermo Scientific, Lait series 1–20 μl (Thermo Fisher Scientific, USA), CO2 incubator (Binder, DE), bioanalyzer Agilent 2100 (Agilent, DE).
Chemicals
96% Ethanol and purified water used during extraction complied with requirements of the State Pharmacopoeia of Ukraine12, 13); chemicals used for the phytochemical screening and quantification of main groups of biologically active compounds (BACs): ethanol (ACS reagent, Fisher Scientific, USA), hydrochloric acid, p.a. (Sobstar, Zaporizhia, UA), acetic acid, puriss. (PJSC AZOT, UA), lead (II) acetate, p.a. (Unikhim Ltd., RU), aluminium chloride, p.a., granulated zinc, p.a. (PC Uralskiy zavod khimicheskih reaktivov, RU), ferric (III) chloride, puriss. (Sigma-Aldrich, USA), gallic acid, chlorogenic acid and rutin were of analytical grade (Merck, DE).
Preparation of extracts
As a solvent, ethanol at various concentrations (20%, 60% and 96%) was used; the extraction was carried out at a general ratio of the plant material : solvent of 1 : 10 on heating with reflux. The extraction was repeated three times under the same conditions (30 min each). The extracts obtained were combined, concentrated on a vacuum rotary evaporator to the ratio of plant material – finished product of 1 : 1.
Preliminary phytochemical screening of G. verum herb fluid ethanolic extracts
The preliminary phytochemical screening was performed using generally accepted methods and techniques of phytochemical analysis13, 14). Glycosides and aglycons of flavonoids were determined in extracts from G. verum herb in the reactions of identification: cyanidine reaction by Bryant (yellow-red colouring of the aqueous phase and yellow-hot colouring of the octal phase), the reaction with 3% solution of iron (III) chloride (dark green colour of flavonols, flavones); the reaction with an alkaline solution (bright yellow colour); the reaction with 5% solution of aluminium chloride (yellow-green colouring); the boric-acid reaction (yellow colouring on detection of 3 - and 5-hydroxyflavones and 5-hydroxyflavanones); the reaction with ammonia (flavones, flavonols, flavanones and flavanonols dissolve with formation of yellow colour, which, when heated, changes to orange or brown colour).
To determine tannins, the reactions of the sediment were carried out with 1% gelatine solution, 1% solution of quinine hydrochloride, 10% solution of basic acetate of lead. The group of tannins was detected by the reaction with a solution of iron ammonium alum.
Quantification of main groups of BACs
In 20% aqueous ethanol extract, polysaccharides were quantified gravimetrically after complete drying at room temperature taking into account the loss on drying15, 16). In all the fluid ethanolic extracts from G. verum herb, the sum of the hydroxycinnamic derivatives was determined by direct spectrophotometry (as chlorogenic acid, λ = 325 nm) according to Yezerska et al.17), Spagnol et al.18); flavonoids were quantified by the method of differential spectrophotometry with aluminium chloride (as rutin, λ = 410 nm)19); polyphenols were quantified by direct spectrophotometry (as gallic acid, λ = 270 nm) according to Kovalyova et al.20). All assays were performed in triplicate.
Study of immunomodulatory activity
To assess the immunomodulatory activity of the extracts obtained, in vitro RLBT with an adequate resolution was used21, 22).
As a sample for substance testing, the mononuclear cells (lymphocytes) removed from venous heparinized blood (donated blood, Kharkiv Regional Blood Banking Centre, UA) by ficoll-verographine gradient density centrifugation (density 1.077 g/mL) (Research and Production Enterprise “PanEco”, RU) by the standard technique23), were used (Protocol of Committee on Biomedical Ethics of SO “Mechnikov Institute of Microbiology and Immunology” No. 2 of May 16, 2017).
The cells obtained were cultured in medium 199 with addition of 10% bovine foetal serum (Thermo Fisher Scientific, BR), 2 mM L-glutamine (Altera Holding, RU), 100 μg/mL gentamicin (LEK (CZ). A suspension of 1 million cells per 1 mL of the culture medium with an addition of substances was incubated for 15–18 hours in a thermostat at 37 °C, in a 5% CO2 atmosphere with saturated water vapour.
The intensity of the proliferative reaction was evaluated by the indices of DNA (deoxyribonucleic acid) synthesis activation recorded by the treatment of samples with anti-BrdU (5-bromo-2’-deoxyuridine) Antibody (3H579) monoclonal antibodies (Santa Cruz Biotechnology, USA) at a concentration of 100 mg/mL. After the final sample preparation, numerical data on the total number of cells and the percentage of blast forms in the samples were established for the flow cytometric analysis with fluorescence detection.
Before the RLBT, the extracts were prepared in ratios of 1/200, 1/20, 1/10 (solvent – distilled water). 100 μL of substances were added to 100 μL of primary cultures of immunocompetent cells. The mitogenic stimulation of lymphocytes by PHA (Research and Production Enterprise “PanEco”, RU) at the concentration of 2.5 μg/mL was performed as the control. RLBT without the addition of the substances under study (spontaneous blast transformation) was also evaluated.
Statistical analysis
All statistical analyses were carried out in accordance with the requirements of the State Pharmacopoeia of Ukraine using Microsoft Office Excel 200716, 24). Differences between groups were statistically analysed using one-way analysis of variance (ANOVA). The results were expressed as mean ± standard deviation (SD). P values less than 0.05 were considered statistically significant.
Results and discussion
Phytochemical screening of G. verum herb fluid ethanolic extracts
The phytochemical screening of G. verum herb fluid ethanolic extracts revealed the presence of flavonoids (flavonols and flavones) and condensed tannins and the results obtained correspond with previous studies25–27).
Quantification of main groups of BACs
The content of main groups of BACs in G. verum herb fluid ethanolic extracts is given in Table 1.
1. The content of main groups of BACs in G. verum herb fluid ethanolic extracts 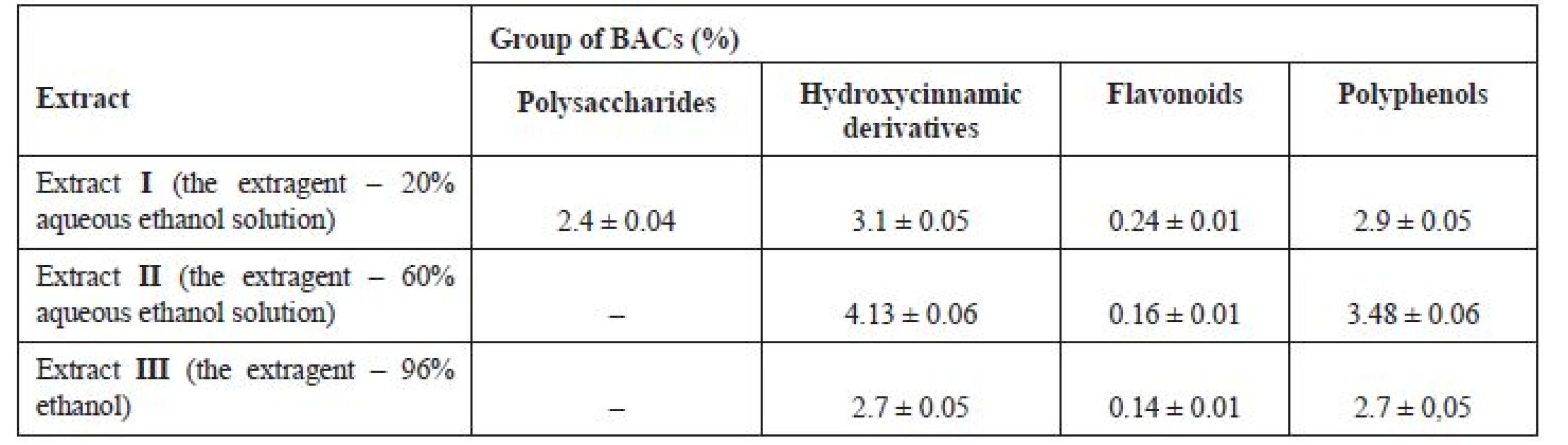
– this group of BACs is not contained in extracts The highest content of hydroxycinnamic derivatives is found in fluid extract II and it is comparable with the content of hydroxycinnamic derivatives in the fluid water extract II), the lowest content of this group of BACs being found in the 96% ethanol extract. Among the fluid ethanolic extracts, the content of flavonoids is the highest in the extract obtained with 20% aqueous ethanol solution, and it is significantly lower than that in the fluid water extract II). The content of polyphenols is the highest in the extract obtained with 60% aqueous ethanol solution and it is slightly higher than that in the water extract II).
The data obtained display some differences from those reported by other researchers28, 29), which may result from various factors, such as growth conditions of the plants under study or the methods of extraction and analysis.
In vitro reaction of lymphocyte blast transformation
The research presented is the first known study of the immunomodulatory activity of G. verum herb fluid ethanolic extracts.
It was established that all substances under study considerably stimulate the transformational activity of peripheral blood mononuclear cells. Under the influence of the extracts under study, 41.7–66.7% of mononuclear cells were involved in the proliferation process, which indicates the stimulating effect of the substances on T - and B-lymphocytes (Table 2). The activity under the influence of substances increases from 33.4% (extract II at a dilution of 1/200) to 58.4% (extract III at a dilution of 1/20) compared with spontaneous lymphocyte blast transformation.
2. The effect of fluid ethanolic extracts of G. verum on the indices of lymphocyte blast transformation (X ± m), n = 5 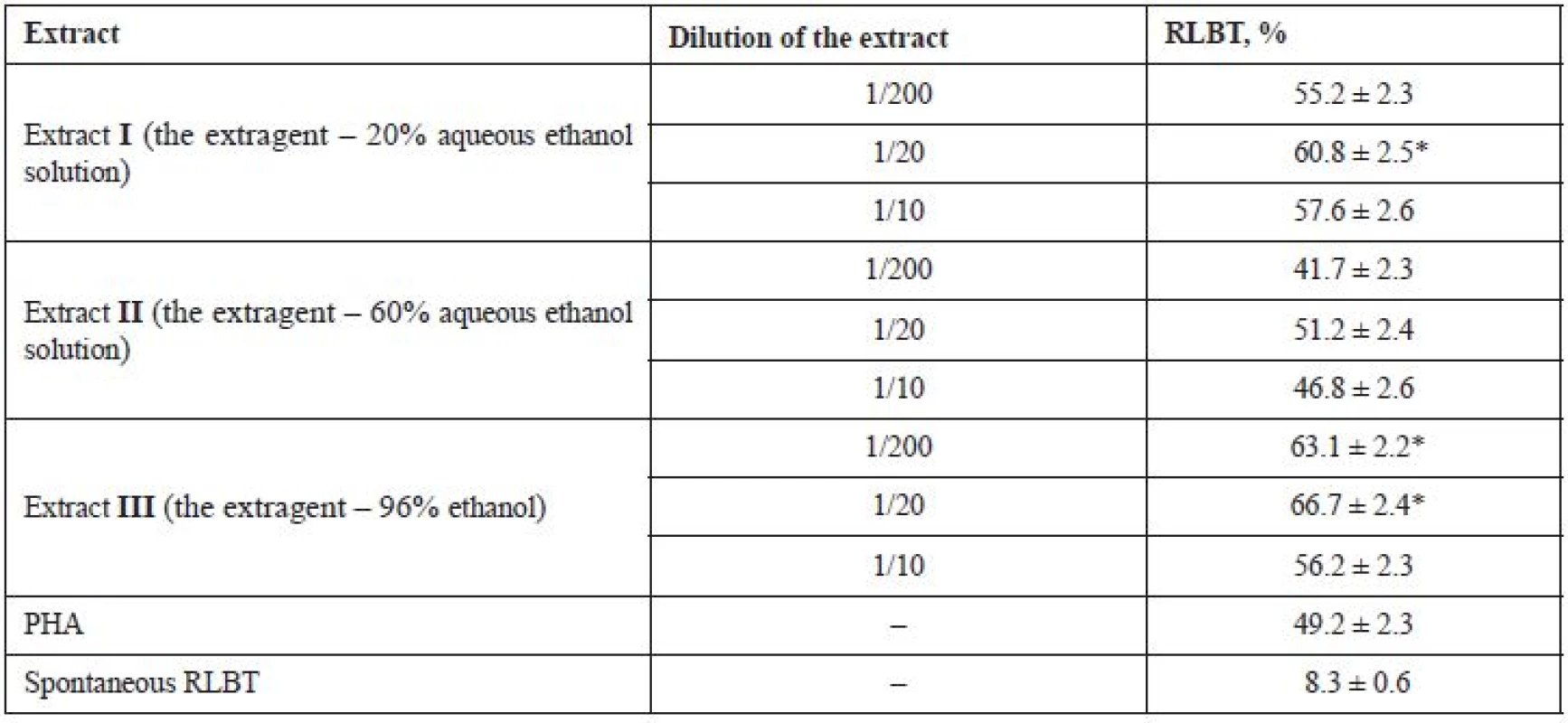
PHA – phytohemagglutinin, RLBT – the reaction of lymphocyte blast transformation
* Р < 0.05 in comparison with the indices of controlThe highest activity was established for extract III at a dilution of 1/20, its activity being by 58.4% higher than that of the lymphocyte spontaneous transformation and by 17.5% higher than that of the reference substance PHA. Somewhat lower indices were established for the extract at a dilution of 1/200, its activity being by 54.8% higher than that of the lymphocyte spontaneous transformation and by 13.9% higher than that of PHA; the lowest activity was exhibited at a dilution of 1/10, its activity being by 47.9% higher than that of the lymphocyte spontaneous transformation and by 7.0% higher than that of PHA.
Extract I showed a significant activity at a dilution of 1/20 (its activity was by 52.5% higher than that of the lymphocyte spontaneous transformation and by 11.6% higher than that of PHA), at a dilution of 1/10 (its activity was by 49.3% higher than that of the lymphocyte spontaneous transformation and by 8.4% higher than that of PHA), at a dilution of 1/200 (its activity was by 46.9% higher than that of the lymphocyte spontaneous transformation and by 6.0% higher than that of PHA).
The lowest activity was established for extract II: at a dilution of 1/20 its activity was by 42.9% higher than that of the lymphocyte spontaneous transformation and by 2.0% lower than that of PHA; at a dilution of 1/10 its activity was by 38.5% higher than that of the lymphocyte spontaneous transformation and by 2.4% lower than that of PHA; at a dilution of 1/200 its activity was by 33.4% higher than that of the lymphocyte spontaneous transformation and by 7.5% lower than that of PHA.
On average, the studied substances (at the concentration of 100 μL) showed the most potent stimulation on the functional activity of immunocompetent cells at a dilution of 1/20, which corresponds with the data obtained in the study of the immunomodulatory activity of the water extract from G. verum herb and its components11).
Only extract III (the extragent – 96% ethanol) at dilutions of 1/20 and 1/200 possesses almost the same stimulant effect on the functional activity of lymphocytes as the water extract; however, at a dilution 1/10 its effect is somewhat lower11). The levels of the immunomodulatory activity established for extracts I and II are lower than that of the water extract. The activity of extract I (the extragent – 20% aqueous ethanol solution) is comparable with the activity of the polysaccharide complex11).
While the immunomodulatory effects of a number of plants of the Rubiaceae family have been reported in several studies10, 30, 31), there is only one study on the immunomodulatory effects of the species of genus Galium – Galium mite32). Although the inhibitive effect of the plant on lymphocyte proliferation via induction of apoptosis has been described, there are no reports regarding the constituents of G. mite.
A number of previous studies have established the presence of flavonoids and hydroxycinnamic acids in the herb G. verum33, 34). As flavonoids are known to exhibit immunostimulatory and/or immunosuppressive effects35, 36), this could account for the results of the immunological analysis of the plant extracts.
Up to date, there have been no publications testifying for the immunomodulatory activity of extracts from G. verum. Our data, however, correlate with those of the immunostimulatory effects of extracts containing polyphenolic compounds, i.e. hydroxycinnamic acids, flavonoids, etc., from other plants, such as, Trigonella foenum graecum and Leonurus cardiacа37–40).
The data obtained indicate that ethanolic extracts from G. verum herb intensively stimulate the blast transformation of lymphocytes at the initial low level, which gives grounds for further research into the influence of these extracts on the blast transformation of lymphocytes at the initial high level.
Conclusion
First-obtained fluid ethanolic extracts from G. verum herb were studied for their chemical composition and immunomodulatory activity. All ethanolic extracts from G. verum herb significantly stimulate the transformational activity of immunocompetent blood cells, with 96% ethanolic extract being most active. The percentage of lymphocytes proliferating in RLBT under the influence of 96% ethanolic extract increased by 8.04–6.77 times compared with the spontaneous transformation and by 1.36–1.14 times compared with PHA. The data obtained give grounds for further research into the mechanisms of immunomodulatory activity of extracts from G. verum herb.
Acknowledgments
This study was supported by the Laboratory of Immunorehabilitology of the Mechnikov Institute of Microbiology and Immunology of National Academy of Sciences of Ukraine.
Authors wish to thank Igor V. Ilyin for professional language editing service.
Conflicts of interest: The authors have declared no financial relationships with any organizations that might have an interest in the submitted work; no any other relationships or activities that could appear to have influenced the submitted work.
Received: April 24, 2018
Accepted: July 30, 2018
Igor L. Shinkovenko
National University of Pharmacy
53-Pushkinska str., 61002 Kharkiv, Ukraine
N. V. Kashpur
Laboratory of Immunorehabilitology of the Mechnikov Institute of Microbiology and Immunology of National Academy of Sciences of Ukraine, 14/16-Pushkinska str., 61057 Kharkiv, Ukraine
Prof. T. V. Ilina, D.Sc. (∗) • A. M. Kovalyova • O. V. Goryacha • O. M. Koshovyi • O. V. Krivoruchko • A. M. Komissarenko
National University of Pharmacy
4-Valentynivska str., 61168 Kharkiv, Ukraine
e-mail: ilyinatany86@gmail.com
Sources
1. Aliyev S. C., Chalilov E. N., Veliyeva M. N., Memedova-Ozel H., et al. The implementation of immunotropic and anti-inflammatory activities of “Azeomed” and “Glycyrrhiza glabra” preparations. In: Abstracts of International Symposium on Medicinal Chemistry Research and Development. Kuşadası 2005; 83.
2. Bałan B. J., Różewski F., Zdanowski R., Skopińska-Różewska E. Immunotropic activity of Echinacea. Part I. History and chemical structure. Centr. Eur. J. Immunol. 2012; 37(1), 45–50.
3. Nagarathna P. K. M., Reena K., Sriram R, Wesley J. Review on Immunomodulation and Immunomodulatory Activity of Some Herbal Plants. Int. J. Pharm. Sci. Rev. Res. 2013; 22(1), 223–230.
4. Nfambi J., Bbosa G. S., Sembajwe L. F., Gakunga J., et al. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J. Basic Clin. Physiol. Pharmacol. 2015; 26(6), 603–611.
5. Barcz E., Rogala E., Glinkowska G., Strzelecka H., et al. Immunotropic activity of plant extracts. IV. The effect of phenolic compounds of poplar leaves (Populus nigra L.) extract on human leukocytes movement. Herba Pol. 1998; 44, 45–51.
6. Chiang L. C., Ng L. T., Chiang W., Chang M. Y., et al. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003; 69 (7), 600–604.
7. Grigore A. Plant Phenolic Compounds as Immunomodulatory Agents, Phenolic Compounds – Biological Activity. https://www.intechopen.com/books/phenolic-compounds-biological-activity/plant-phenolic-compounds-as-immunomodulatory-agents.
8. Lukashov P. I., Moiseev D. V., Stolyarova V. N., Makaremko M. N. Pharmacological activity of caffeic acid. Vestnic Pharmacii 2012; 3(57), 61–65 (in Russian).
9. Dabrowski M. P., Stankiewicz W., Kubacki R., Sobiczewska E., et al. Immunotropic effects in cultured human blood mononuclear cells pre-exposed to low-level 1300 M Hz pulse-modulated microwave field. Electromagnetic Biol. Med. 2003; 22 (1), 1–13.
10. Kashpur N. V., Yurchenko N. S., Ilyina T. V., Kovalyova A. M., et al. The immunomodulatory effect of Asperula odorata L. and Asperula humifusa M. Bieb. Besser dry extracts. Clinical Pharmacy 2015; 19(1), 56–58.
11. Shinkovenko I. L., Kashpur N. V., Ilyina T. V., Kovalyova A. M., et al. The immunomodulatory activity of the extracts and complexes of biologically active compounds of Galium verum herb. Čes. slov. Farm. 2018; 67, 25–29.
12. The State Pharmacopoeia of Ukraine/State enterprise “Scientific and Expert Pharmacopoeial Centre”, First edition, fourth supplement. Kharkiv: RІREG 2011; 538 p. (in Ukrainian).
13. Korulkin D. Y., Abilov D. A., Muzychkina R. A., Tolstikov G. A. Natural flavonoids. Novosibirsk: Geo 2007.
14. Mykchailenko O. O., Kovalyov V. M. Phenolic compounds of the genus Iris plants (Iridaceae). Čes. slov. Farm. 2016; 65, 70–77.
15. The State Pharmacopoeia of Ukraine/State enterprise “Scientific and Expert Pharmacopoeial Centre”, First edition. Kharkiv: RІREG 2001; 556 p. (in Ukrainian).
16. The State Pharmacopoeia of Ukraine/State enterprise “Scientific and Expert Pharmacopoeial Centre”, First edition, first supplement. Kharkiv: RІREG 2004; 494 p. (in Ukrainian).
17. Yezerska O., Kalynyuk T., Vronska L. Quantitative determination of hydroxycinnamic acids in Chicory root. Chemistry and Chemical Technology 2013; 7(3), 247–250.
18. Spagnol C. M., Oliveira Th. S., Lucia Borges V. I., Corrêa M. A., et al. Validation of caffeic acid in emulsion by UV-Spectrophotometric method. Physical Chemistry 2015; 5(1), 16–22.
19. The State Pharmacopoeia of Ukraine/State enterprise “Scientific and Expert Pharmacopoeial Centre”, First edition, second supplement. Kharkiv: RІREG 2008; 617 p. (in Ukrainian).
20. Kovalyova A. A., Georgievskiy G. V., Kovalyov V. M., Komisarenko A. M., et al. Development of new piflamin medicine standardization methods. Farmakom. 2002; 2, 92–97 (in Ukrainian).
21. Korneeva M. N., Novokhatskii A. S., Grebenyuk V. N., Kerimov S. G. Use of the lymphocyte blast transformation reaction to assess the state of cellular immunity. Bulletin of Experimental Biology and Medicine 1989; 107(4), 533–535.
22. Bashirova D. K., Kochnev O. S., Davletkil’deev F. A., Lagutina M. V. Immunologic activity of human lymph cells in the lymphocyte blast transformation reaction. Biull. Eksp. Biol. Med. 1980; 89(1), 33–35.
23. Bulanova E. G., Budagyan V. M., Yarilin A. A., Mazurenko N. N. Expression of Protooncogenes during Lymphocyte Activation by Growth Factors. http://protein.bio.msu.ru/biokhimiya/contents/v62/full/62091191.html
24. Zulfiqar A., Bhaskar S. B. Basic statistical tools in research and data analysis. Indian J. Anaesth. 2016; 60(9), 662–669.
25. Zhao C., Shao J., Cao D., Zhang Y., et al. Chemical constituents of Galium verum. Zhongguo Zhong Yao Za Zhi 2009; 34(21), 2761–2764.
26. Vlase L., Mocan A., Hanganu D., Benedec D., et al. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Digest Journal of Nanomaterials and Biostructures 2014; 9(3), 1085–1094.
27. Ghiţă G., Necula R., Trifan A., Gille E., et al. Investigations regarding the phytochemical study of some samples of Galium verum L. and Galium album Mill. Analele Ştiinţifice ale Universităţii “Al. I. Cuza” Iaşi s. II a. Biologie vegetală 2012; 58(1), 45–50.
28. Tamas M., Stana D., Timis S. Comparative phytochemical research of Galium verum and G. mollugo L. Not. Bot. Hort. Agrobot. Cluj 2006; 34, 18–20.
29. Layali I., Ebrahimzadeh M. A., Joulaei M. Antioxidant properties of Galium verum. International Journal of Life Science and Pharma Research 2016; 6(3), 31–37.
30. Zeng K., Thompson K. E., Yates C. R. Miller D. D. Synthesis and Biological Evaluation of Quinic Acid Derivatives as Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2009; 19, 5458–5460.
31. Amaral S., Mira L., Nogueira J. M., da Silva A. P., et al. Plant extracts with anti-inflammatory properties – a new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships. Bioorg. Med. Chem. 2009; 17, 1876–1883.
32. Amirghofran Z., Javidnia K., Bahmani M., Azadmehr A., et al. The effect of the methanol extract of Galium mite on the cellular immunity and antibody synthesis. Journal of Immunoassay and Immunochemistry 2011; 32, 157–169.
33. Vlase L., Mocan A., Hanganu D., Benedec D., et al. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Dig. J. Nanomater Biostruct. 2014; 9, 1085–1094.
34. Matei A. O., Gatea F., Dumitra T. E., Radu G. L. Polyphenols analysis from different medicinal plants extracts using capillary zone electrophoresis (CZE)/Revista de Chimie 2016; 67(6), 1051–1055.
35. Raso G. M., Meli R., Di Carlo G., Pacilio M. et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001; 68(8), 921–931.
36. Sharma M. L., Singh B., Chandan B. K., Khajuria A., et al. Actions of some flavonoids on specific and non-specific immune mechanisms. Phytomedicine 1996; 3(2), 191–195.
37. Sneha J. Anarthe S., Sandhya Rani D., Ganga Raju M. Immunomodulatory activity for methanolic extract of Trigonella foenum graecum whole plant in wistar albino rats. Am. J. Phytomed. Clin. Therapeutics 2014; 2(9), 1081–1092.
38. Tripathi S., Maurya A. K., Kahrana M., Kaul A., et al. Immunomodulatory activity of ethanolic extract of Trigonella foenum graecum leaves on mice. Sch. Res. Lib. 2012; 4(2), 708–713.
39. Sadowska B., Micota B., Różalski M., Redzynia M., et al. The immunomodulatory potential of Leonurus cardiaca extract in relation to endothelial cells and platelets. Innate Immunity 2017; 23(3), 285–295.
40. Gonzalez-Gallego J., Garcıa-Mediavilla M. V., Sanchez-Campos S., Tunon M. J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010; 104, 15–27.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2018 Issue 3-
All articles in this issue
- Inflammatory bowel disease: factors involved in pathogenesis
- Biosurfactants and their role in the inhibition of the biofilmforming pathogens
- Pharmacy, pharmacists and drugs in the Terezín ghetto
- Imunomodulační aktivita etanolových extraktů z Galium verum L. herb.
- Výzkum chemického složení kůry Sorbus aucuparia
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Inflammatory bowel disease: factors involved in pathogenesis
- Pharmacy, pharmacists and drugs in the Terezín ghetto
- Biosurfactants and their role in the inhibition of the biofilmforming pathogens
- Imunomodulační aktivita etanolových extraktů z Galium verum L. herb.
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

