-
Medical journals
- Career
Production of isoflavonoids in the Trifolium pratense L. suspension culture
Authors: M. Kašparová; T. Siatka; J. Dušek
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy, Czech Republic
Published in: Čes. slov. Farm., 2009; 58, 67-70
Category: Review Articles
Overview
The plant Trifolium pratense L. contains a wide spectrum of constituents. Regarding the utilization of the red clover plant for therapeutic purposes, the most interesting substances are isoflavonoids. They are listed in the same class with the most important phytoestrogens. The explant cultures are a promising source of secondary metabolites, thus also of isoflavonoids. However, their production, when compared with intact plants, is usually lower and this is the reason why we are looking for different methods and approaches to increase the production. The objective of this study was to observe the influence of three elicitors – the salicylic acid, cadmium chloride, and mercury chloride – on the production of isoflavonoids by the Trifolium pratense L. suspension culture (Sprint variety). The best elicitation effect was observed with mercury chloride. The maximum increase in production was observed after a 6-hour application of the 1 μmol concentration when, after the comparison with the control sample, the production of daidzein was increased by 1300%, that of genistin by 375%, and that of genistein by 300%.
Key words:
Trifolium pratense L. suspension culture – production of isoflavonoids – biotic and abiotic elicitationIntroduction
The plant Trifolium pratense L. contains a wide spectrum of constituents. Regarding the utilization of the red clover plant for therapeutic purposes, the most interesting substances are isoflavonoids. For their ability to interact with estrogenic receptors and their structural similarity with 17 ß-estradiol, they are listed in the same class with the most important phytoestrogens. The red clover extracts, which contain the daidzein, formononetin, biochanin A, and genistein isoflavonoids, are used in female menopause non-hormonal substitution therapy. They decrease the occurrence of menopausal syndromes; have a positive effect in the prevention of osteoporosis and reduce the risk of the incidence of cardio-vascular diseases and breast cancer. A positive impact of these isoflavonoids in the prostate gland cancer prevention was also noted 1–3).
The explant cultures are a promising source of secondary metabolites, thus also of isoflavonoids. However, their production, when compared with intact plants, is usually lower and this is the reason why we are looking for different methods and approaches to increase it.
The Trifolium pratense L. suspension culture research has been focused on influencing the isoflavonoids production with the elicitation method. The principle of elicitation consists in the accumulation of the secondary substances in the plants – a process that is one part of the plant’s defense reaction against the influences caused by pathogens or by the plant’s environment 4). The factors that trigger the defensive reactions are called elicitors (stressors). These can be divided into the biotic ones (for instance, fungus, bacteria, viruses, phytohormones) and the abiotic ones (for instance, heavy metals, radiation, cold temperatures) 5).
In this study, the Trifolium pratense L. suspension culture was elicited with the salicylic acid biotic elicitor and with the salts of heavy metals (mercury, cadmium).
EXPERIMENTAL PART
Instruments
A 200S analytical scales made by Sartorius, Göttingen, Germany; a PS 20A autoclave, Chirana, Brno, Czech Republic; a HS 31A hot-air sterilizer, Chirana, Brno, Czech Republic; a laminar flow workbench, Fatran LF, Žilina, Slovakia; a roller, Vývojove Dílny AV ČR, Praha, Czech Republic; a 2010 shaker, Unimax, Heidolph, Germany; a liquid chromatograph (PU-2089 pump, MD 2015 detector, AS 2055 automatic sample injector) manufactured by Jasco, Tokyo, Japan.
Trifolium pratense L. suspension culture
The suspension cultures (Sprint variety) were derived from callus cultures. First by mechanical loosening on the shaker followed by the cultivation in Gamborg’s nutrient media 6) with an addition of 2 mg.1-1 of 2,4 dichlorophenoxyacetic acid and 2 mg.1-1 of 6 benzylaminopurine, at a temperature of 25 °C, 16-hr light/8-hr dark period. The subcultivation interval lasted for 14 days 7).
Elicitation
During the elicitation process, on the 21st day of cultivation 7), a 3-year-old suspension culture received 1.0 ml of the salicylic acid solution (in the concentrations of 10 μmol, 100 μmol, 1000 μmol, and 10000 μmol) 8, 9), cadmium chloride and mercury chloride (in the concentrations of 0.1 μmol, 1 μmol, 10 μmol, and 100 μmol) 10–13). The control cultures received 1.0 ml of distilled water. The elicitor application durations were 6, 24, 48, and 168 hours 10, 12, 14). The cells were separated from the media by vacuuming, rinsed in distilled water, and dried at the laboratory room temperature.
Determination of isoflavonoids
The elicited and the inspection samples underwent the determination of isoflavonoids via the HPLC method 15). The HPLC determination process conditions were as follows: a RP-18 Lichrospher column (250 × 4 mm, particles size 5 μm) with a precolumn made of the same material; elution: linear gradient of a mobile phase A (methanol) in phase B (water containing 0.15% of phosphoric acid) 30–80% from 0 to 9 minutes was followed by the isocratic elution mobile phase in composition of 80% of phase A in phase B from 9 to 15 minutes; the elution speed was 1.1 ml/min; the detection was carried out at the 260 nm wave length. The contents of the monitored substances were quantified by using the mathematical method of normalization and by comparing with the calibration curve drawn by the external standard of the same substance.
RESULTS
The results of the biotic and abiotic elicitations of the Trifolium pratense L. suspension culture are listed in Tables. All the listed results are the averages calculated from the results of three independent determination processes.
DISCUSSION
The objective of this study was to observe the influence of three elicitors – salicylic acid, cadmium chloride, and mercury chloride – on the production of isoflavonoids by the Trifolium pratense L. suspension culture (Sprint variety).
Salicylic acid, which is present in many vegetation species, influences a whole line of plant physiological processes. It also acts as the signal molecule during the development of plant’s defensive reactions against pathogens; it thus can be included in the biotic elicitors group 16, 17). Also, the heavy metals are known for the mechanisms that influence (in a positive or a negative way) the intensity of biosynthetic processes. If optimizing the conditions, the elicitation effects of the heavy metals ions can be used in targeted production of secondary metabolites 18).
The results of the tests show that the content of isoflavonoids (genistin, daidzein, genistein, and formononetin) in the Trifolium pratense L. suspension culture is low. The elicitation of the Trifolium pratense L. suspension culture with the salicylic acid was not a success (Table 1). The maximum content of isoflavonoids determined in the control culture after 48 hours of cultivation was not increased with this biotic elicitation. Also, the abiotic elicitor, cadmium chloride, stimulated the production very little (Table 2). Only its application of 10 μmol concentration increased the content of daidzein by 300% after 24 hours when compared with the control sample. On the other hand, the production of genistin, as well as of formononetin, was completely suppressed. The best elicitation effect was observed with mercury chloride (Table 3). All concentrations of this abiotic elicitor increased the production of the monitored isoflavonoids (with the exception of formononetin). Even the lowest mercury chloride concentrations increased the production with the application time; however, the longest application (168 hours) had a negative impact. The maximum increase in production was observed after a 6-hour application of the 1 μmol concentration when, after the comparison with the control sample, the production of daidzein was increased by 1300%, that of genistin by 375%, and that of genistein by 300%. Longer exposures to this concentration did not increase these values. The stronger concentrations of the elicitor (10 and 100 μmol) also stimulated the production, but the above-listed isoflavonoid maximums were not surpassed.
1. Production of isoflavonoids in the suspension culture of Trifolium pratense L. elicited with salicylic acid 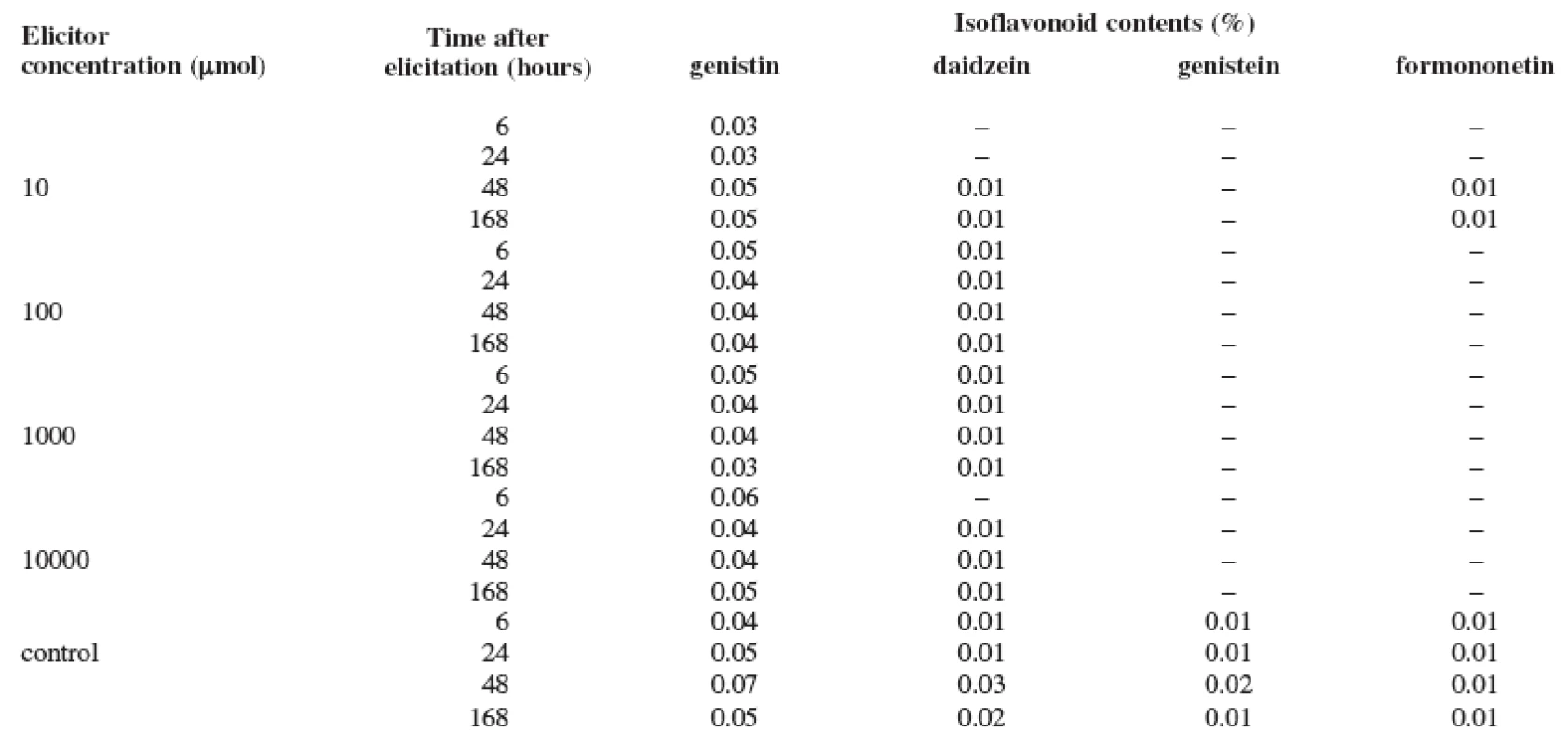
2. Production of isoflavonoids in the suspension culture of Trifolium pratense L. elicited with cadmium chloride 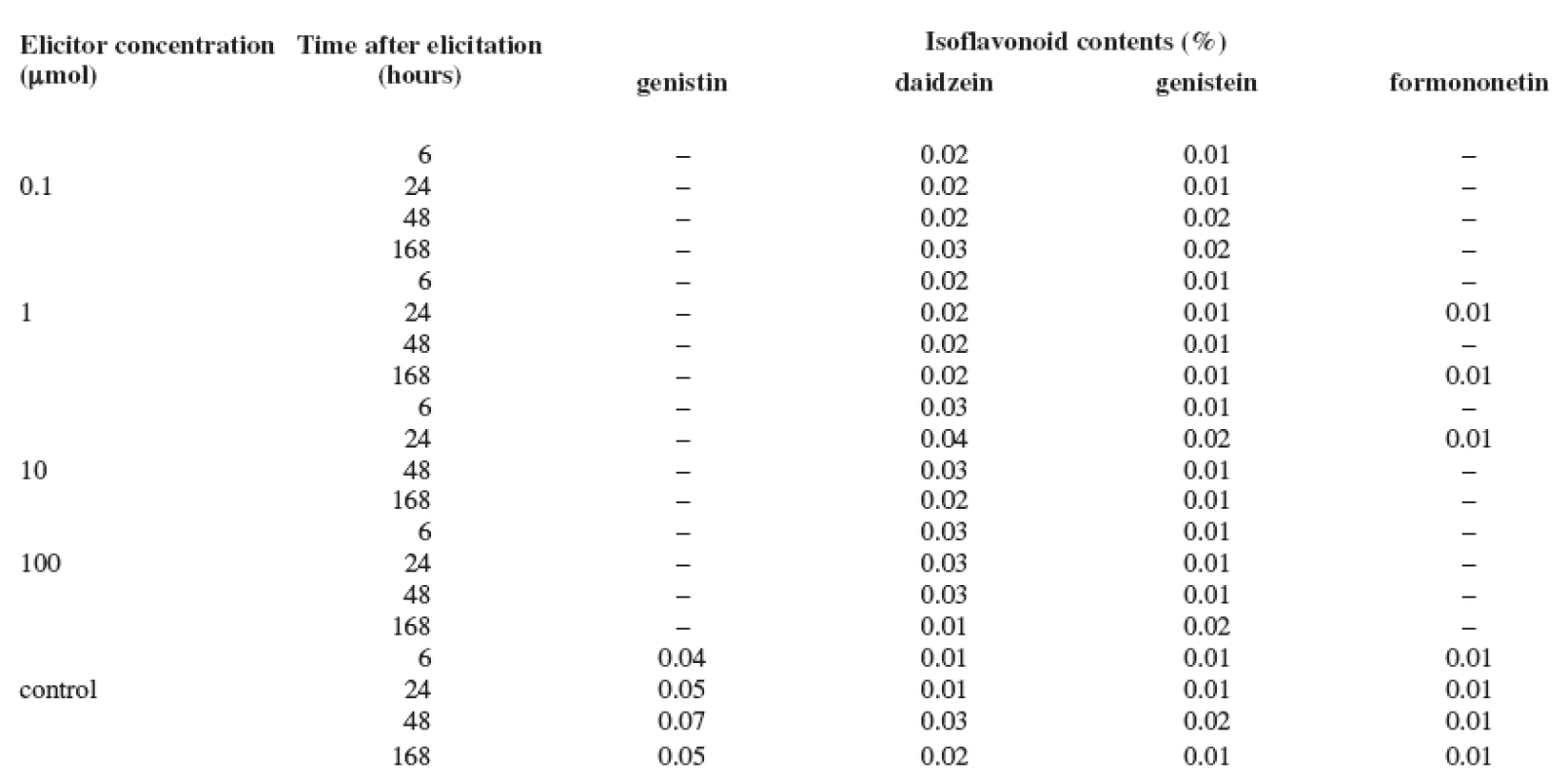
3. Production of isoflavonoids in the suspension culture of Trifolium pratense L. elicited with mercuric chloride 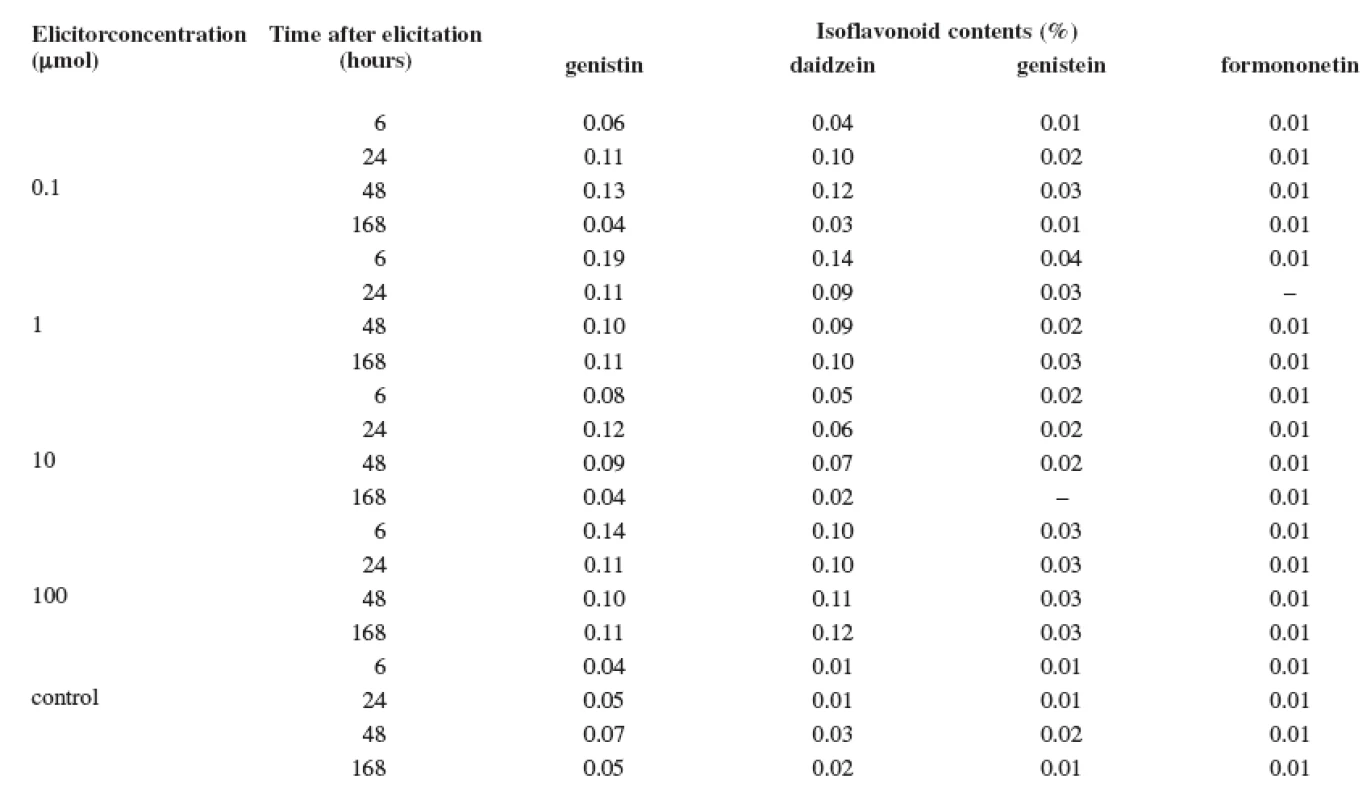
The results clearly indicate that the production of isoflavonoids in the Trifolium pratense L. (Sprint variety) suspension culture is low. One of the reasons for this can be the fact that we used a young and quickly-growing suspension culture – a culture that often accumulates only small amounts of metabolites. However, the production can be increased by introducing a certain type of stress – for instance, a decrease in the solution’s content of nutrients; leaving the growth regulator out; or applying the elicitor in the media which was, in the case of monitored elicitors, best confirmed in the case of the mercury chloride. Also, for instance, the Sesbania drummondii cell cultures reacted to mercury-induced stress by increasing their antioxidant defense mechanisms 19, 20).
This study was sponsored under the Research Project No. MSM 0021620822.
Received 13 March 2009 /Accepted 6 April 2009
Address for correspondence:
PharmDr. Marie Kašparová, Ph.D.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové
e-mail: kasparova@faf.cuni.cz
Sources
1. Dixon, R. A.: Ann. Rev. Plant Biol., 2004; 55, 225.
2. Ososki, A. L., Kennelly, E. J.: Phytoter. Res., 2003; 17, 845.
3. Tham, D. M. et al.: J. Clin. Endocrinol. Metab., 1998; 83, 2223.
4. Radman, R. et al.: Biotechnol. Appl. Biochem., 2003; 37, 91.
5. Wu, J., Lin, L.: Appl. Microbiol. Biotechnol., 2002; 59, 51.
6. Gamborg, O. L., Miller, R. A., Ojima, K.: Exp. Cell Res., 1968; 50, 151.
7. Kašparová, M., Siatka, T., Spilková, J., Dušek, J.: Čes. slov. Farm., 2006; 55, 44.
8. Pita-Alvarez, S. I., Spollansky, T. C., Giulietti, A. M.: Enzyme Microb. Technol., 2000; 26, 252.
9. Ignatov, A. et al.: Phytochemistry, 1996; 43, 1141.
10. Kašparová, M., Siatka, T., Dušek, J.: Čes. slov. Farm., 2007; 56, 225.
11. Tebayashi, S., Ishihara, A., Iwamura, H.: J. Exp. Bot., 2001; 52, 681.
12. Kartosentono, S. et al.: Biotechnol. Lett., 2002; 24, 687.
13. Tůmová, L., Blažková, R.: Čes. slov. Farm., 2002; 51, 44.
14. Kašparová, M., Siatka, T., Dušek, J.: Čes. slov. Farm., 2008; 57, 107.
15. De Rijke, E. et al.: Anal. Chim. Acta, 2002; 468, 3.
16. Thomma, B. P. H., Eggermont, K.: Proc. Natl. Acad. Sci. USA, 1998; 95, 15107.
17. Zhao, J., Davis, L. C., Verpoorte, R.: Biotechnol. Advanc., 2005; 23, 283.
18. Mithöfer, A., Schulze, B., Boland, W.: Febs Lett., 2004; 566, 1.
19. Israr, M., Sahi, S.: Plant Physiol. Biochem, 2006; 44, 590.
20. Israr, M., Sahi, S., Datta, R., Sarkar, D.: Chemosphere, 2006; 65, 591.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2009 Issue 2-
All articles in this issue
- On the terms pharmaceutical care, pharmacy care, and good pharmacy practice
- Beta-blockers
- HPLC study of temperature influence on some enantiomer separations of sulfoxides, determination of some enantiomers of sulfoxides in rat serum
- Studies of local anesthetics Part: 187 Extraction of 1-methyl-2-piperidinoethylesters of alkoxyphenylcarbamic acids from human plasma by imprinted polymers and its comparison with classic solid phase extraction
- Use radionuclide x-ray fluorescence analysis for determination of heavy metals content in dextrans in solid and liquid state
- Beneficial effects of rutin, quercitrin and quercetin on inflammatory bowel disease
- Production of isoflavonoids in the Trifolium pratense L. suspension culture
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Beta-blockers
- On the terms pharmaceutical care, pharmacy care, and good pharmacy practice
- Beneficial effects of rutin, quercitrin and quercetin on inflammatory bowel disease
- Use radionuclide x-ray fluorescence analysis for determination of heavy metals content in dextrans in solid and liquid state
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

