-
Medical journals
- Career
TRANSSCLERAL DIODE CYCLOPHOTOCOAGULATION IN TREATMENT OF GLAUCOMA
Authors: P. Farraová; M. Ondrejková; D. Demianová
Authors‘ workplace: OFTAL s. r. o. - Špecializovaná nemocnica v odbore oftalmológia
Published in: Čes. a slov. Oftal., 76, 2020, No. 5, p. 236-242
Category: Original Article
doi: https://doi.org/10.31348/2020/34Overview
Cyclodestructive procedures are an alternative of surgical treatment of medically refractory glaucoma.
Aim: To assess efficiency and safety of diode cyclophotocoagulation (CPC).
Methods: Retrospective study included 81 eyes with advanced glaucoma operated with CPC for elevated intraocular pressure (IOP) from January 2017 to January 2019. CPC was performed in retrobulbar anesthesia with contact diode laser FOX (A.R.C. Laser, Germany) of wavelength 810 nm, transsclerally to the ciliary body. Energy settings: intensity 2 W, exposition time 2 s, 18 applications in arc of 360°. Decrease of intraocular pressure was investigated during month 1, 6 and 12 after CPC. Safety was evaluated according to the best corrected visual acuity (BCVA) on Snellen optotypes and number of postoperative complications.
Results: Study on 31 eyes of 24 patients, 21 (68 %) women and 10 (32 %) men, with follow-up during more than 12 months. Mean follow-up time was 19.5 ± 6.1 (from 12 to 29) months. Average age was 75.9 ± 9.2 (56 - 93) years. Indication for CPC was primary open angle glaucoma in 15 eyes (49 %), primary angle closure glaucoma in 6 eyes (19 %) and secondary glaucomas in 10 eyes (32 %). All patients were on therapy of 4 antiglaucomatic drops and 10 of them (32 %) on acetazolamide pills.
IOP before CPC was 25.4 ± 11.0 (13–56) mm Hg. After 1 year IOP decreased to 16.9 ± 6.1 (8–40) mm Hg. Best corrected visual acuity (BCVA) before CPC was 0.39 ± 0.34 (0-1), 1 year after CPC 0.36 ± 0.33 (0-1). 1 year after CPC, 11 eyes (35 %) lost 0.23 ± 0.14 rows. 6 from these (19 %) due to other acquired ocular pathologies.
Hypotony occurred in 6 % and uveitis in 10 % eyes.
Conclusion: CPC is a safe and effective method of lowering IOP.
Keywords:
cyclophotocoagulation – advanced glaucoma – refractory glaucoma
INTRODUCTION
Cyclodestructive techniques represent an alternative to surgical treatment of glaucoma in the case that it is insufficiently compensated by medicamentous therapy. They reduce intraocular pressure (IOP) by reducing the production of chamber fluid through the destruction of the secretion epithelium of the ciliary body [1,2,3].
We perform transscleral diode CPC in retrobulbar or parabulbar anaesthesia, using a contact diode laser with a wavelength of 810 nm, transclerally to the region of the ciliary body, 1.5 mm from the limbus. With regard to the construction of the probe, the manufacturer states placement of the probe on the corneo-scleral interface (Fig. 1). The localisation of the corpus ciliare may differ according to different quadrants. It is necessary to exercise caution in large myopic eyes, in which it is essential to verify the localisation of the corpus ciliare by transillumination. The standard used energy of 2 W is adjusted within the range of 1.5-2.5 W in order to prevent the occurrence of audible “crackling”. The crackling sound is caused by the disruption of the surrounding tissues upon the use of excessive laser energy and its absorption by uveal melanin. The operation is performed within the range of 4-10 applications to the quadrant upon treatment within the range of 180°-360°, with omission of place no. 3 and 9 [3,4,5,6].
1. Technique of transscleral diode cyclophotocoagulation 
Laser cytophotocoagulation has been used in the following diagnoses: primary open angle glaucoma (POAG), primary angle-closure glaucoma (PACG), neovascular glaucoma (NVG), glaucoma upon a background of aphakia and pseudophakia, post-traumatic glaucoma, infantile and juvenile glaucoma, glaucoma after penetrating keratoplasty, glaucoma associated with uveitis, and glaucoma following severe chemical and thermal damage [3]. According to the American Academy of Ophthalmology, eyes in which a previous filtering operation has failed, eyes with minimal central visual acuity and decompensated IOP, painful blind eyes in which it is not possible to perform an incision operation, acute condition and eyes with permanent silicone oil (SO) tamponade are indicated for CPC [3,6].
Despite the better control and therapeutic effect of diode CPC in comparison with the previous cyclo-ablation methods there still exists a risk of postoperative complications. An overview of these is presented in table 2 [7,8].
1. Indication of transscleral cyclophotocoagulation (according to American Academy of Ophthalmology) [3,6] ![Indication of transscleral cyclophotocoagulation (according to American Academy of Ophthalmology) [3,6]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/d5661c1d0f511da4ee3df7032c84dc87.png)
2. Complications of contact transscleral diode laser cyclophotocoagulation [7,8] ![Complications of contact transscleral diode laser cyclophotocoagulation [7,8]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/e841707e940e43119b11b44a7660f200.png)
METHODS
CPC was performed in retrobulbar anaesthesia using the contact diode laser FOX (A.R.C. Laser, Germany) with a wavelength of 810 nm, transsclerally to the region of the ciliary body, 1.5 mm from the limbus within a scope of 360°, omitting no. 3 and 9 in a number of 18 blasts with an intensity of 2 W and exposure of 2 s. The effectiveness of CPC was evaluated according to the reduction of IOP one, six and twelve months after CPC. In the cohort, the scope of medicamentous therapy was observed before and after CPC. Safety was evaluated according to the incidence of complications and resulting best corrected central visual acuity (BCCVA).
RESULTS
The observed cohort included 31 eyes of 24 patients, who had an observation period of at minimum 12 months after the procedure. The mean observation period was 19.5 ± 6.1 months (range 12-29 months). There were 17 women (71 %) and 7 men (29 %) in the cohort. A total of 21 (68 %) female and 10 (32 %) male eyes were operated on. The mean age at the time of surgery was 75.9 ± 9.2 (56-93) years. 5 patients (16 %) were aged up to 65 years inclusive, and 10 patients (32 %) were aged over 80. CPC was performed on 15 eyes with POAG (49 %), on 6 eyes with PACG (19 %) and on 10 eyes with secondary glaucoma (32 %).
Previous surgery had been performed on 20 eyes (64 %). In 3 eyes a glaucoma filtering operation had been performed prior to CPC, in 6 eyes basal iridectomy or laser iridotomy, and in 11 eyes selective laser trabeculoplasty (SLT). In 3 eyes (10 %) CPC had been performed at another centre 12, 19 and 47 months previously. IOP before CPC was 25.4 ± 11.0 (13-56) mm Hg. One month after CPC, IOP was 16.2 ± 7.2 (5-42) mm Hg. Mean IOP was reduced to 16.2 mm Hg, which represents a reduction of 9.2 mm Hg (36 %). 6 months after CPC, IOP was 17.8 ± 9.3 (10-57) mm Hg (reduction of 30 %), and one year after IOP was 16.9 ± 6.1 (8-40) mm Hg (reduction of 34 %) (Graph 1). The reduction of the value of IOP after CPC is statistically significant for all the observed points in time (p < 0.001 for all points).
1. Effect of cyclophotocoagulation on reduction of intraocular pressure 
Before surgery, all the patients received local therapy of 4 antiglaucomatous agents combined in 3 phials, and 10 patients (32 %) used acetozolamide generally in tablet form.
1 month after CPC, 6 patients (25 %) used general therapy, after 6 months 1 patients (2 %), and one year after CPC none of the patients used general therapy with acetazolamide (Graph 2). After the procedure it was possible to reduce local therapy in 3 eyes (10 %).
2. Effect of cyclophotocoagulation on general medicamentous therapy 
BCCVA was from 0 to 1.0 on a Snellen chart. Mean BCCVA before surgery was 0.39 ± 0.34, after one month 0.35 ± 0.23 (0.8-1) and 6 months after the procedure 0.40 ± 0.30 (0-1). 1 year after CPC, mean BCCVA was 0.36 ± 0.33 (0-1). In 11 eyes (36 %) after 1 year there was a mean decrease of BCCVA by 0.23 ± 0.14 rows, of which in 6 eyes (19 %) this occurred as a consequence of other associated ocular pathologies (Graph 3). In 4 patients this concerned progression of cataract, which was subsequently addressed surgically. In 1 eye vision deteriorated due to the occurrence of subretinal neovascularisation upon a background of age-related macular degeneration, and in 1 eye due to branch central retinal vein occlusion. In 5 eyes (16 %) there was a deterioration of vision due to progression of glaucoma pathology (Fig. 2).
2. Perimeter of patient with progression of glaucoma pathology after cyclophotocoagulation (A, B) 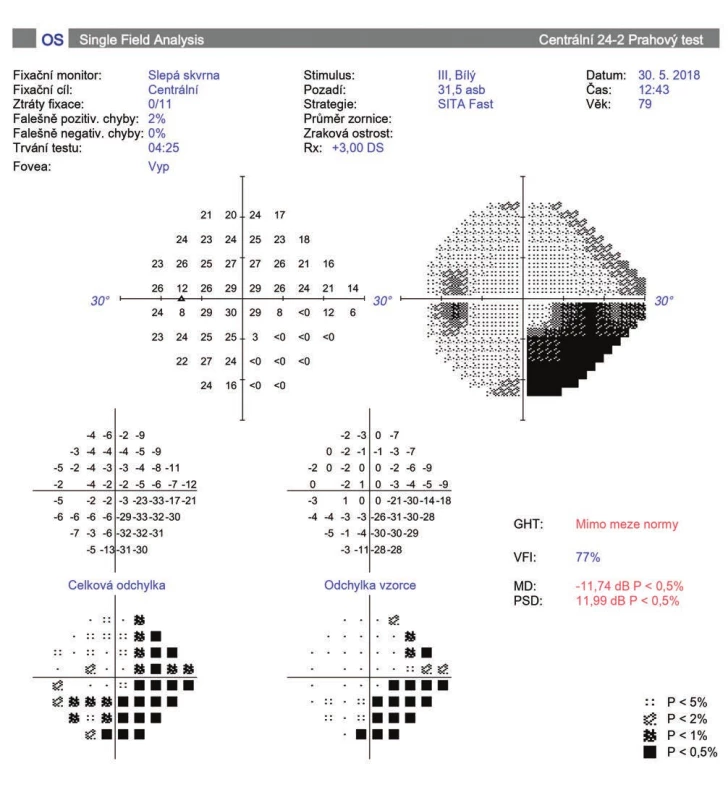
3. Best corrected central visual acuity before and after cyclophotocoagulation 
With regard to complications of CPC, in the observed cohort we recorded two cases of transitory hypotonia (6 %) and three cases of induced uveitis (10 %) (Fig. 3). All the complications were managed by local therapy. After the procedure 84 % of eyes were without complications.
3. Slight postoperative uveitis. Visible dilatation of vessels of iris and precipitates on cornea visible on first day after cyclophotocoagulation 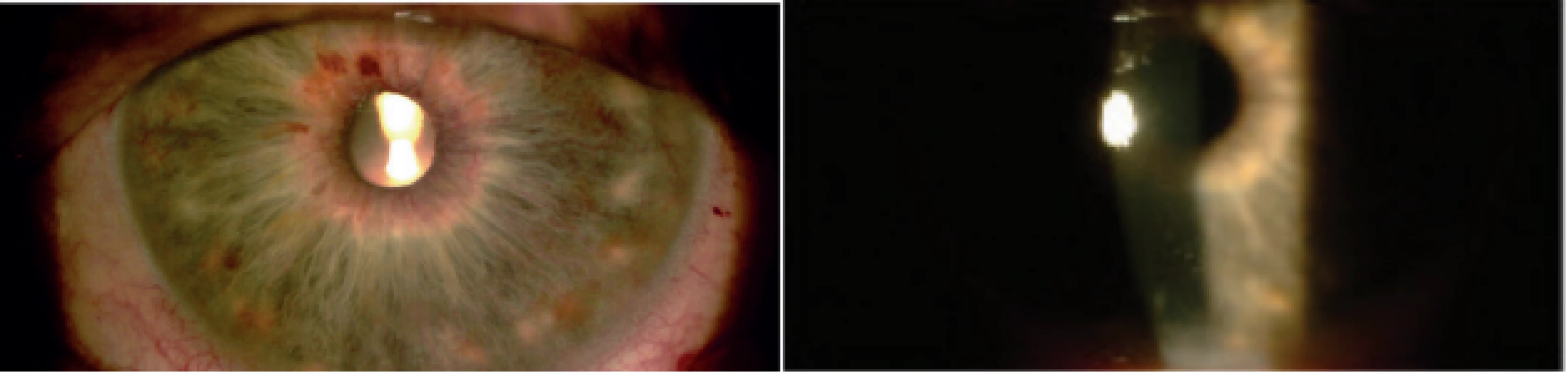
3 eyes (10 %) required repeated CPC 4, 5 and 8 months after primary CPC due to insufficient effectiveness of the primary treatment.
DISCUSSION
Transscleral diode cyclophotocoagulation is an alternative to invasive surgical treatment of glaucoma. It reduces IOP by destroying the secretion epithelium of the ciliary body. It has been performed and evaluated in various studies on several types of glaucomas. Among these, the main indication criteria include glaucomas with advanced changes, insufficiently compensated on maximal medicamentous therapy, in which previous filtration surgery had failed, or in which there was a high risk of failure for the patient [1,2,3,5,6].
In our cohort, in almost half of the cases CPC was performed for POAG (49 %), while the other half comprised eyes with PACG (19 %) and in 10 eyes secondary glaucoma (32 %). In all diagnosis the indication for the performance of surgery was insufficient compensation of IOP upon maximal medicamentous therapy, with the aim of preserving visual acuity and stability of the finding on the optic nerve papilla, in 64 % of eyes also following a previous incision or laser operation. In 36 % of eyes CPC was indicated as the primary surgical treatment for advanced stages of glaucoma with a high risk of an adverse result of a filtering operation, as well as in eyes with visual acuity on the level of practical blindness.
The literature presents the occurrence of early postoperative complications such as: pain, which is usually of a transitory character and recedes following analgesic treatment, hyphema (more common in patients with NVG), scorching of the conjunctiva (upon use of incorrect technique), high or low intraocular pressure, cataract and uveal reaction in connection with photophobia [7,8]. In our cohort only transitory hyoptonia and slight uveitis occurred, which receded after treatment
The most feared risk of CPC is postoperative hypotonia with possible progression of the finding to phthisis of the eyeball, requiring enucleation. The incidence of this complication depends on the population under study, the technique and aggressivity of treatment and the length of observation of patients [7,8].
According to the study by Contreras et al. conducted on 116 eyes, a mean reduction of IOP by 6.96 mm Hg takes place one hour after CPC. In 10.8 % of patients they also recorded a significant increase of IOP by more than 5 mm Hg. Despite this fact, they state that monitoring of IOP immediately after the procedure is not necessary. However, it is necessary to conduct a follow-up examination on patients 1 day and 1 week after the operation [9]. Regular monitoring of patients after the procedure enabled us to detect and treat hypotonia in 2 eyes (7 %) sufficiently in time.
The effectiveness of CPC is evaluated after 4 weeks [3]. Reduction of IOP to values ranging from 7-21 mmHg and/or reduction of IOP by 20-30 % as against the baseline values is considered a satisfactory result of the operation [1,10]. In our cohort of patients we achieved a reduction of IOP by 9.2 mm Hg (36 %) after one month, in which the effect of reduction of IOP persisted also after one year (34 %).
In the literature, a reduction of local and general antiglaucomatous therapy is stated after CPC [4,10,11]. In our cohort, general therapy with acetazolamide was no longer required in any of our patients after CPC, and in 10 % of cases it was possible also to reduce local therapy.
In patients with good BCCVA, a deterioration of vision is stated in 20-30 % of cases [2,4,8,11,12]. In our study a deterioration of vision occurred in 5 eyes (16 %), due to progression of glaucoma pathology.
CONCLUSION
CPC is a safe and effective method of reducing IOP. In our cohort, over the course of one year we achieved a sufficient reduction of IOP, on average by 34 %, and in 84 % of cases we also recorded stabilisation of the functional finding.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company. The authors further declare that this study has not been submitted to any other journal or printed elsewhere, with the exception of congress abstracts and recommended procedures.
Sources
1. Pastor S, Singh K, Lee D, et al. Cyclophotocoagulation. Ophtalmology. 2001 Nov;108(11):2130-2138.
2. Dastiridou AI, Katsanos A, Denis P, et al. Cyclodestructive Procedures in Glaucoma: A Review of Current and Emerging Options. Adv Ther. 2018 Nov;35 : 2103–2127.
3. Bloom PA, Negi AK, Kersey TL, Crawley L. Cyclodestructive Techniques. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG: Glaucoma Surgical Management. Philadelphia: Elsevier Saunders Ltd.; c2015. Chapter 17, Cyclodestructive procedures; p. 1150–1170. ISBN: 978-0-7020-5193-7.
4. Huang G, Lin S. When should we give up filtration surgery: Indications, Techniques and Results of Cyclodestruction. Dev Ophtalmol. 2012;50 : 173–183.
5. Giaconi J, Law S, Nouri-Mahdavi K, Coleman AL, Caprioli J. Pearls of Glaucoma Management. Berlin (Germany): Springer; c2016. Chapter 34, Procedural Treatments: Transscleral Cyclophotocoagulation. p. 311–318.
6. American Academy of Ophthalmology (AAO). Cyclodestructive Procedures in Treatment of Glaucoma. [internet]. AAO; 2019. Available from: https://eyewiki.aao.org/Cyclodestructive_ Procedures_in_Treatment_of_Glaucoma.
7. Kahook MY, Schumal JS. Complications of Cyclodestructive Procedures. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG: Glaucoma Surgical Management. Philadelphia: Elsevier Saunders Ltd.; c2015. Chapter 17, Cyclodestructive precedures; p. 1150–1170. ISBN: 978-0-7020-5193-7.
8. Aujla JS, Lee GA, Vincent SJ, Thomas R. Incidence of hypotony and symphatetic ophthalmia following trans-scleral cyclophotocoagulation for glaucoma and a report of risk factors. Clin Exp Ophtalmol. 2013;41(8):761–772.
9. Contreras I, Noval S, González Martín-Moro J, Rebolleda G, Muñoz-Negrete FJ. IOP spikes following contact transscleral diode laser cyclophotocoagulation. Arch Soc Esp Oftalmol. 2004;79(3):105–109.
10. Nutterová E, Pitrová Š, Lešták J. Our experience with micropulse cyclophotocoagulation in the therapy of glaucoma. Cesk Slov Oftalmol. 2020;76(1):29-36. Available from: http://www.cs-ophthalmology.cz/en/journal/articles/144
11. Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The diode laser ciliary ablation study group. Ophthalmology. 1996;103 : 1294–1302.
12. Fox J. Cyclophotocoagulation controversy. [Internet]. Glaucoma Today; c2017 [cited 2020 Apr 25]. Available from: https://glaucomatoday.com/articles/2017-mar-apr/cyclophotocoagulation-controversy.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2020 Issue 5-
All articles in this issue
- Carotid-cavernous fistula from the perspective of an ophthalmologist A Review
- PRESBYOPIA MANAGEMENT WITH DIFFRACTIVE PHAKIC POSTERIOR CHAMBER IOL
- HIGHLIGHTS OF HYPERTENSIVE AND NORMOTENSIVE GLAUCOMA
- HIGHLIGHTS OF ADVANCES IN MEDICAL RETINA FROM THE VIRTUAL WORLD OPHTHALMOLOGY CONGRESS 2020
- PUPILLOTONIA AND ADIE SYNDROME
- TRANSSCLERAL DIODE CYCLOPHOTOCOAGULATION IN TREATMENT OF GLAUCOMA
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- PUPILLOTONIA AND ADIE SYNDROME
- Carotid-cavernous fistula from the perspective of an ophthalmologist A Review
- TRANSSCLERAL DIODE CYCLOPHOTOCOAGULATION IN TREATMENT OF GLAUCOMA
- HIGHLIGHTS OF HYPERTENSIVE AND NORMOTENSIVE GLAUCOMA
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


