-
Medical journals
- Career
Olfactory dysfunction in a cohort of Czech patients with idiopathic REM sleep behaviour disorder
Authors: I. Dall‘antonia; P. Dušek; A. Tesař; J. Nepožitek; S. Dostálová; V. Ibarburu Lorenzo Y Losada; I. Příhodová; O. Bezdicek; T. Nikolai; P. Peřinová; E. Růžička; K. Šonka
Authors‘ workplace: Department of Neurology and Center of Clinical Neuroscience, 1st Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic
Published in: Cesk Slov Neurol N 2019; 82(4): 415-419
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2019415Overview
Aim: Rapid eye movement (REM) sleep behaviour disorder (RBD) is a sleep abnormality heralded by the absence of physiological atonia in REM sleep and dream enactment behaviour. Idiopathic RBD (iRBD), diagnosed when no primary RBD cause can be identified, is a marker of prodromal synucleinopathy with a high conversion rate to overt neurodegenerative disorders from the synucleinopathy group. The aim of this cross-sectional study was to investigate olfactory function in iRBD patients and its relation to other symptoms.
Patients and methods: This study included 54 iRBD patients with a median age of 67 (IQR 63–72) years; the 37 control subjects, matched by gender and age, had a median age of 67 (57.5–70.0) years. All subjects underwent a complex examination, which included olfactory testing using the University of Pennsylvania Smell Identification Test (UPSIT).
Results: In total, 62.9% of iRBD patients had either total loss of olfactory function or severe hyposmia. In contrast, only 8.1% of controls showed such a degree of olfactory dysfunction. Furthermore, we found that the percentage of REM sleep without atonia on polysomnography negatively correlates with the UPSIT score (P < 0.01).
Conclusion: This study demonstrated a significantly lower olfactory function in the iRBD group compared to controls.
捷克特发性REM睡眠行为障碍患者队列中的嗅觉功能障碍
目的:快速眼动睡眠行为障碍(Rapid eye movement sleep behavior disorder, RBD)是一种睡眠异常,其特征是在快速眼动睡眠中缺乏生理上的弛豫和做梦行为。特发性RBD (Idiopathic RBD, iRBD)是一种前驱性共核蛋白病的标志,在共核蛋白病组中,其转化为明显的神经退行性疾病的几率很高。本横断面研究的目的是探讨iRBD患者的嗅觉功能及其与其他症状的关系。
患者和方法:本研究纳入了54名iRBD患者,中位年龄为67岁(IQR 63–72)岁。根据性别和年龄相匹配的37名对照受试者的中位年龄为67岁(57.5–70.0)。所有受试者均接受了复杂的检查,其中包括使用宾夕法尼亚大学气味识别测试(UPSIT)进行的嗅觉测试。
结果:共有62.9%的iRBD患者出现嗅觉功能完全丧失或严重的嗅觉减退。相比之下,只有8.1%的对照组表现出这种程度的嗅觉功能障碍。此外,我们发现在多导睡眠图上,无肌张力的REM睡眠百分比与仰卧评分呈负相关(P < 0.01)。
结论:这项研究表明,与对照组相比,iRBD组的嗅觉功能明显降低。
关键词:表型转换–低渗–突触核蛋白病
Keywords:
hyposmia – phenoconversion – synucleinopathy
Introduction
Rapid eye movement (REM) sleep behaviour disorder (RBD) is a parasomnia manifesting with a loss of physiological atonia in REM sleep associated with dream enactment [1]. The prevalence of RBD is estimated as 1–2% of the population over 50 years of age [2,3]. In the majority of RBD patients, no cause of this abnormality can be identified and this disorder is considered as idiopathic RBD (iRBD) [4]. Following the discovery of RBD, it became clear that the vast majority of iRBD subjects convert to overt synucleinopathy disorders, such as Parkinson’s disease (PD) or dementia with Lewy bodies (DLB) and multiple system atrophy; iRBD is therefore considered as a marker of the prodromal phase of neurodegeneration [5]. A high rate of phenoconversion to synucleinopathies has been confirmed in several longitudinal studies with RBD patients [6–8], including our recent study, where a conversion rate of 32.4% within 5 (median, range 1–14) years follow-up was observed [9].
In addition to RBD, a wide array of symptoms, including olfactory and autonomic impairment, have been shown to precede or occur in parallel with the manifestation of motor symptoms in PD and other synucleinopathies. Olfactory threshold has been shown to correlate with the Hoehn and Yahr classification of disease progression in PD patients [10]. Given the extensive clinical evidence, olfactory dysfunction is included in the supportive diagnostic criteria for PD by the European Federation of Neurological Societies [11]. Furthermore, hyposmia has been shown to precede other markers of prodromal neurodegeneration by several years [12], allowing the early identification of patients at a high risk of future synucleinopathy diag-
nosis. The recent largest multicentre study of iRBD phenoconversion predictors has shown that impaired olfaction represents the hazard ratio of phenoconversion into PD or DLB of 2.62 (1.7–4.1) in the mean follow-up of 4.6 (range 1–19) years [13]. The aim of this cross-sectional study was to detail properties of olfactory dysfunction and its associations with other relevant symptoms in a cohort of iRBD patients compared to control subjects.Methods
Patients were screened in a stepwise manner following a public advertisement and internet survey [14]; RBD was diagnosed in accordance with the International Classification of Sleep Disorders, third edition [15]. Exclusion criteria were: overt parkinsonism (diagnosed according to the Movement Disorder Society [MDS] criteria [16]) or dementia (diag-
nosed according to the Diagnostic and Statistical Manual of Mental Disorders [17]), severe obstructive sleep apnea defined by the Apnea-Hypopnea Index (AHI) ≥ 30, and the presence of other disorders that could cause secondary RBD (for example, narcolepsy or lesions in the brainstem on brain MRI).Patients in the study underwent a single--night video-polysomnography (PSG), which was performed according to the 2014 Academy of Sleep Medicine (AASM) guidelines, including the superficial EMG of bilateral flexor digitorum superficialis muscles. Sleep analysis was done in standard 30-s epochs. Increased tonic or phasic activity recorded on the EMG of the mentalis muscle during PSG, known as REM sleep without atonia (RWA), was thoroughly investigated. A 30-s epoch of REM sleep was scored positive for RWA when at least 50% of its duration had a chin EMG amplitude greater than the minimum amplitude in non-REM sleep or excessive transient muscle activity was found in at least 50% of the epoch divided into 3-s mini epochs [18]. RWA is presented as the percentage of epochs scored as RWA from the total number of epochs scored as REM sleep. Sleep efficacy (SE), the proportion of total sleep time relative to the time in bed, was used as a simple marker of sleep quality. AHI represents the mean number of apnea and hypopnea events in 1 h of sleep.
The cognitive performance was evaluated by the Montreal Cognitive Assessment (MoCA) Czech version [19], which was administered by a clinical neuropsychologist. The autonomic symptom questionnaire, Scales for Outcomes in Parkinson‘s Disease--Autonomic (SCOPA-AUT), was completed by participants and consisted of a 23-item questionnaire with a maximum score of 69 points [20,21]. To assess motor function, patients were examined by a certified neurologist using the Movement Disorder Society-sponsored revision of the Unified Parkinson‘s Disease Rating Scale (MDS-UPDRS), part III [22].
To analyse olfaction, the University of Pennsylvania Smell Identification Test (UPSIT) was used. It entails the identification of 40 different smells in a multiple-choice manner [23]. The UPSIT has been validated for detection of olfactory dysfunction and proved to possess high sensitivity and high reliability in various populations and disorders [24]. The Czech translation of the German version of the UPSIT (manufactured by Sensonics International, Haddon Heights, NJ, USA) was administered in a standard examination room in the morning hours. The total score ranging from 0 to 40 points was calculated using an answer key from the supplier. The results were then evaluated using the administration manual obtained from the manufacturer, according to age and gender specific cut-off scores. Subjects were divided into five categories according to their UPSIT score: normosmic (men: >33, women: >34), mild microsmic (men: 30–33, women: 31–34), moderate microsmic (men: 26–29, women: 26–30), severe microsmic (men: 19–25, women: 19–25), and anosmic (men: <19, women: <19) [25].
Age and gender-matched controls underwent the same protocol as RBD patients. The control group was composed of healthy volunteers without a history of major neurological or sleep disorders. RBD in control subjects was excluded by PSG.
The protocol was approved by the Hospital Ethics Committee; all participants gave written consent before being enrolled in the study.
The SPSS 20 (IBM, Armonk, NY, USA) was used to perform all statistical analysis. First, we applied the Shapiro-Wilk test of normality. Depending on their distribution, data are presented as mean and standard deviation or median and interquartile range. For between-group comparison we used the
t-test for variables with normal distribution and the Mann-Whitney U test for non-normally distributed variables. P-values below 0.05 were regarded as significant. We also studied Pearson correlation coefficients to identify linear correlation between individual variables or Spearman correlation analysis for variables with non-normal distribution in both groups.Results
A total of 54 RBD patients and 37 healthy controls were included in the study; their demography and clinical characteristics are summarized in Tab. 1. Several statistically significant variables were calculated in iRBD patients compared to controls. RBD patients had a higher median MDS-UPDRS III and a higher median SCOPA-AUT score than control subjects (P < 0.01). The median MoCA score was found to be lower in iRBD patients (P = 0.02). Of note, the median RWA was considerably higher in iRBD patients at 50.7% compared to 2.0% in controls (P < 0.01). Lastly, the median AHI and body mass index (BMI) in iRBD patients was considerably lower than in controls (P < 0.01).
1. Clinical characteristics of iRBD and control subjects. Variables are expressed as mean and standard deviation or as median and interquartile range. Statistically signifi cant results (P < 0.05) are marked in bold text. 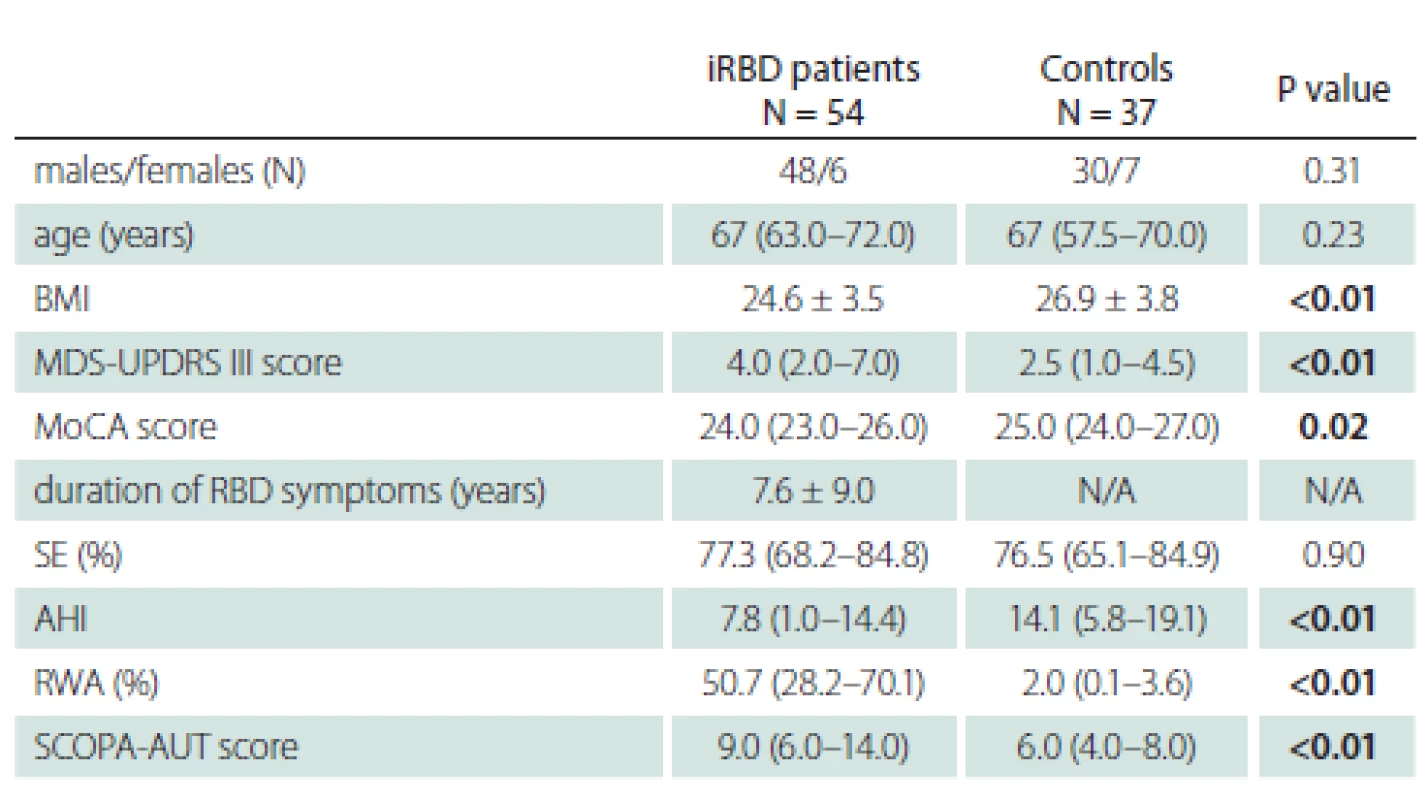
AHI – apnoe-hypopnoe index; BMI – body mass index; iRBD – idiopathic REM sleep be haviour disorder; MDS-UPDRS III – Movement Disorder Society Unified Parkinson’s Disease Rating Scale, part III; MoCA – Montreal Cognitive Assessment; N – number; N/A – not applicable; RWA – REM sleep without atonia; SE – sleep efficacy; SCOPA-AUT – Scales for Outcomes in Parkinson‘s Disease-Autonomic UPSIT scores for patients and controls are shown in Tab. 2. A lower UPSIT score was observed in iRBD patients.
2. UPSIT score. Statistically signifi cant results (P < 0.05) are marked in bold text. 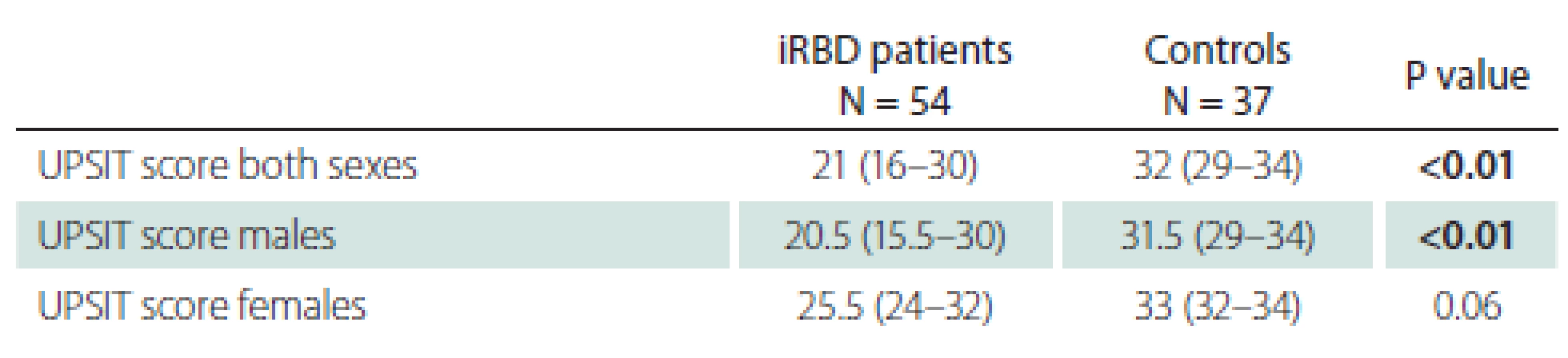
iRBD – idiopathic REM sleep behaviour disorder; N – number; UPSIT – University of Pennsylvania Smell Identification Test Classification of olfactory function into five groups according to UPSIT scores is shown in Tab. 3. Markedly, 37.0% of iRBD patients were found to be anosmic, whereas only 2.7% of controls were anosmic. Similarly, 25.9% of iRBD patients suffered from severe microsmia, compared to 5.4% of control subjects.
3. Classification of UPSIT results. 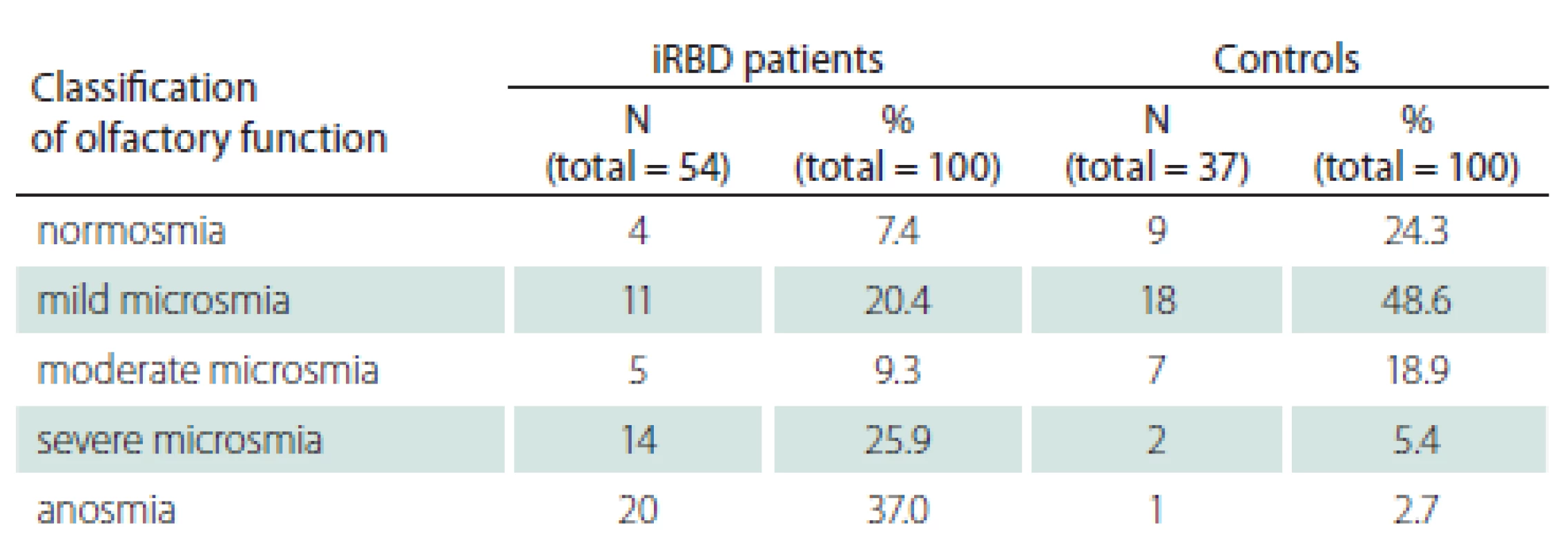
iRBD – idiopathic REM sleep behaviour disorder; N – number The associations between UPSIT and other relevant clinical parameters are displayed in Tab. 4.
4. Correlation of the UPSIT score and other clinical variables in iRBD patients. Statistically significant results are marked in bold text. 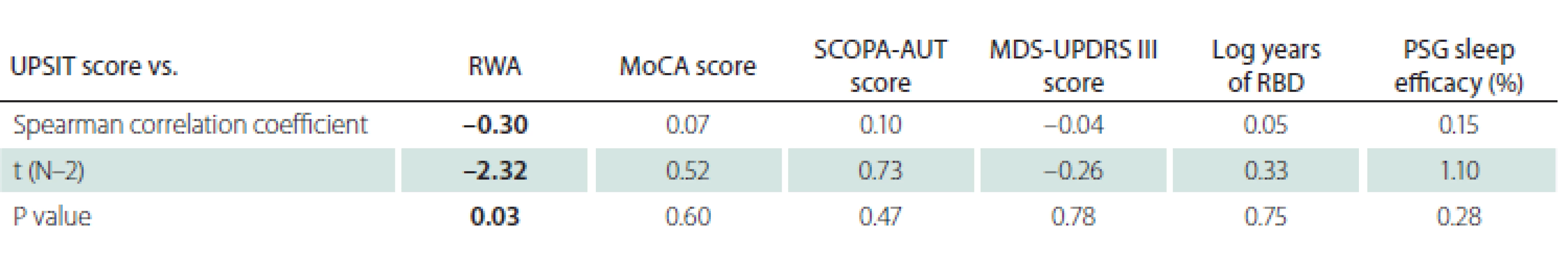
iRBD – idiopathic REM sleep behaviour disorder; MDS-UPDRS III score – Movement Disorder Society Unifi ed Parkinson’s Disease Rating Scale, part III; MoCA – Montreal Cognitive Assessment; N – number; PSG – polysomnography; RBD – REM sleep behaviour disorder; RWA – percentage of REM sleep without atonia; SCOPA-AUT – Scales for Outcomes in Parkinson‘s Disease-Autonomic; UPSIT – University of Pennsylvania Smell Identifi cation Test A negative correlation was found between the UPSIT score and RWA (Fig. 1). The associations between UPSIT and other examined parameters were not statistically significant.
1. Scatter graph of Spearman correlation between RWA and UPSIT. RWA – percentage of REM sleep without atonia out of total REM sleep duration; UPSIT – University of Pennsylvania Smell Identification Test score
Obr. 1. Bodový graf znázorňující korelaci mezi RWA a UPSIT skóre. RWA – podíl REM spánku bez svalové atonie vzhledem k celkovému trvání REM spánku v procentech; UPSIT – skóre University of Pennsylvania Smell Identification Test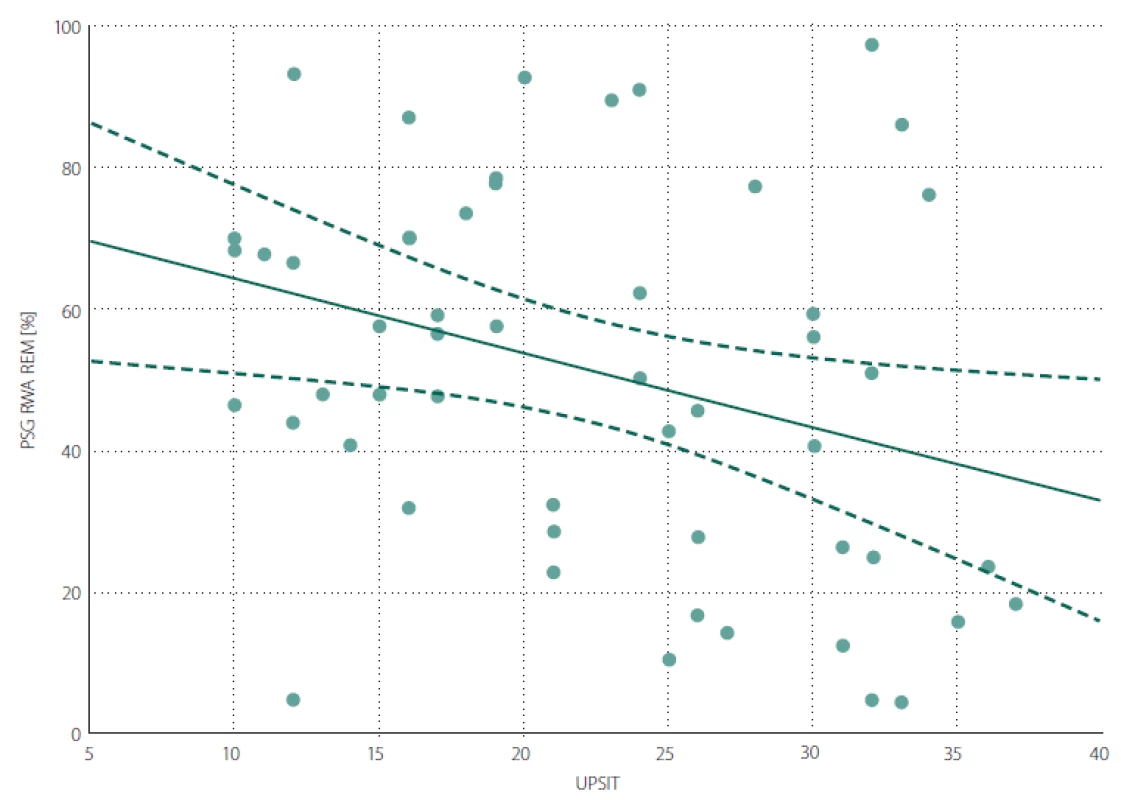
Discussion
This study found lower olfactory identification capabilities in iRBD patients compared to controls. In the iRBD group, 62.9% of patients were classified as having severe microsmia or anosmia. In contrast, only 8.1% of our control subjects had either severe microsmia or anosmia. These results are in line with a previous Italian and French Canadian study, where 61.1% of iRBD patients vs. 16.6% of controls showed abnormal olfactory function [26]. Similarly, in the Japanese population, a significant olfactory dysfunction in iRBD patients compared to that of control subjects was found [27]. Of note, iRBD patients with olfactory function in the lowest tercile compared to other iRBD patients, have been shown to have a significantly higher relative risk of conversion to DLB and therefore can predict phenoconversion into overt synucleinopathies in a relatively short time frame [28]. More pronounced brain abnormalities typical for synucleinopathies were found in iRBD patients with hyposmia compared to those with normal olfactory function using fluorodeoxyglucose PET imaging [29].
As expected, olfactory function differed between genders in our study. The median UPSIT score was higher for females in both the control and iRBD groups. This is in accordance with studies in various ethnic groups, which have consistently shown that olfactory identification capabilities are higher in women than in men [30]. This implicates that different UPSIT cut-off values for identification of a high phenoconversion risk have to be defined for men
and women.A lower UPSIT score was associated with a decreased ability to maintain REM sleep atonia. This finding supports the theory postulated by Braak et al stating that in the first stage of the spread of synuclein pathology, the anterior olfactory nucleus and lower parts of the brainstem are affected, followed by affliction of areas in the rostral pons [31] responsible for maintenance of REM sleep atonia. The negative association between olfactory function and severity of RWA indicates that areas of the brain ensuring olfaction and sleep are affected in close succession and/ or similar intensity in the early phases of the synucleinopathy disease process. The relevance of RWA as an early sign of synuclein-mediated neurodegeneration has been highlighted as an intriguing area for further investigation [32]. We found no significant correlation between UPSIT and the duration of RBD symptoms. This could be influenced by unreliable recall of the RBD duration by
patients.Some limitations of the study should be noted. Firstly, the iRBD and control group contained a low number of female subjects and thus results for the female cohort have a limited validity. A further limitation is the statistically lower AHI in iRBD patients compared to control subjects despite the inclusion criteria concerning AHI in both groups were identical. This difference can be explained by the statistically higher BMI in control subjects compared to iRBD patients. A higher BMI is associated with the presence and severity of obstructive sleep apnea [33]. Lastly, this study did not include odour threshold testing, which could theoretically bring additional information regarding olfactory function.
In summary, this study shows that Czech iRBD patients have significantly impaired olfaction compared to control subjects, supporting results from previous studies in other populations.
This study was supported by The Ministry of Health of the Czech Republic, grant number 16-28914A; and by the Czech Science Foundation, grant number 16-07879S.
We would like to express a special thanks to the study nurses, Jana Brdková and Petra Nesvačilová, for their help in this study.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
MUDr. Irene Dall‘Antonia
Department of Neurology and Center of Clinical Neuroscience 1st Faculty of Medicine
Charles University
and General University Hospital
Kateřinská 30
120 00 Praha 2
Czech Republic
e-mail: irenedallantonia@yahoo.com
Accepted for review: 4. 3. 2019
Accepted for print: 11. 6. 2019
Sources
1. Howell MJ, Schenck CH. Rapid Eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol 2015; 72(6): 707–712. doi: 10.1001/ jamaneurol.2014.4563.
2. Haba-Rubio J, Frauscher B, Marques-Vidal P et al. Prevalence and determinants of REM sleep behavior disorder in the general population. Sleep 2018; 41(2). doi: 10.1093/ sleep/ zsx197.
3. Kang SH, Yoon IY, Lee SD et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep 2013; 36(8): 1147–1152. doi: 10.5665/ sleep.2874.
4. Ferini-Strambi L, Marelli S, Galbiati A et al. REM sleep behavior disorder (RBD) as a marker of neurodegenerative disorders. Arch Ital Biol 2014; 152(2–3): 129–146. doi: 10.12871/ 000298292014238.
5. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013; 14(8): 744–748. doi: 10.1016/ j.sleep.2012.10.009.
6. Iranzo A, Fernandez-Arcos A, Tolosa E et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014; 9(2): e89741. doi: 10.1371/ journal.pone.0089741.
7. Postuma RB, Gagnon JF, Bertrand JA et al. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015; 84(11): 1104–1113. doi: 10.1212/ WNL.0000000000001364.
8. Li Y, Kang W, Yang Q et al. Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology 2017; 88(16): 1493–1500. doi: 10.1212/ WNL.0000000000003838.
9. Peřinová P, Plchová L, Bušková J et al. Follow-up pacientů s idiopatickou poruchou chování v REM spánku – fenokonverze do parkinsonského syndromu a demence. Cesk Slov Neurol N 2018; 81/114(2): 205–207. doi: 10.14735/ amcsnn2018205.
10. Radil T, Roth J, Růžička E et al. Olfactory dysfunction: symptom of Parkinson’s disease? Cesk Slov Neurol N 1995 : 286–289. 1995; 58/91(6): 286–289.
11. Berardelli A, Wenning GK, Antonini A et al. EFNS/ MDS-ES/ ENS [corrected] recommendations for the diagnosis of Parkinson‘s disease. Eur J Neurol 2013; 20(1): 16–34. doi: 10.1111/ ene.12022.
12. Postuma RB, Gagnon JF, Vendette M et al. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011; 69(5): 811–818. doi: 10.1002/ ana.
22282.13. Postuma RB, Iranzo A, Hu M et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019; 142(3): 744–759. doi: 10.1093/ brain/ awz030.
14. Buskova J, Ibarburu V, Sonka K et al. Screening for REM sleep behavior disorder in the general population. Sleep Med 2016; 24 : 147. doi: 10.1016/ j.sleep.2016.07.003.
15. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien: American Academy of Sleep Medicine 2014.
16. Postuma RB, Berg D, Stern M et al. MDS clinical diag-
nostic criteria for Parkinson‘s disease. Mov Disord 2015; 30(12): 1591–1601. doi: 10.1002/ mds.26424.17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington: Ameriacan Psychiatric Association 1994.
18. American Academy of Sleep Medicine (AASM). The AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine 014.
19. Kopecek M, Stepankova H, Lukavsky J et al. Montreal cognitive assessment (MoCA): normative data for old and very old Czech adults. Appl Neuropsychol Adult 2017; 24(1): 23–29. doi: 10.1080/ 23279095.2015.1065
261.20. Visser M, Marinus J, Stiggelbout AM et al. Assessment of autonomic dysfunction in Parkinson‘s disease: the SCOPA-AUT. Mov Disord 2004; 19(11): 1306–1312. doi: 10.1002/ mds.20153.
21. Kaiserova M, Opavsky J, Maertin JJ et al. Czech version of the Autonomic Scale for Outcomes in Parkinson‘s Disease (SCOPA-AUT) – Questionnaire to Assess the Presence and Severity of Autonomic Dysfunction in Patients with Parkinson‘s Disease. Cesk Slov Neurol N 2014; 77/ 110(1): 96–99.
22. Goetz CG, Tilley BC, Shaftman SR et al. Movement disorder society-sponsored revision of the Unified Parkinson‘s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23(15): 2129–2170. doi: 10.1002/ mds.22340.
23. Doty RL, Shaman P, Applebaum SL et al. Smell identification ability: changes with age. Science 1984; 226(4681): 1441–1443.
24. Doty RL. Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol Head Neck Surg 2015; 1(1): 28–33. doi: 10.1016/ j.wjorl.2015.09.007.
25. Doty R. Der Geruchsidentifikationstest Prufungs-
handbuch. In: Pennsylvania Uo (ed). White Horse Pike,
Haddon Heights, New Jersey: Sensorics Inc. 2000:
8–9.26. Fantini ML, Postuma RB, Montplaisir J et al. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull 2006; 70(4–6): 386–390. doi: 10.1016/ j.brainresbull.2006.07.008.
27. Miyamoto T, Miyamoto M, Iwanami M et al. Olfactory dysfunction in Japanese patients with idiopathic REM sleep behavior disorder: comparison of data using the university of Pennsylvania smell identification test and odor stick identification test for Japanese. Mov Disord 2010; 25(10): 1524–1526. doi: 10.1002/ mds.23
170.28. Mahlknecht P, Iranzo A, Hogl B et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015; 84(7): 654–658. doi: 10.1212/ WNL.0000000000001265.
29. Meles SK, Vadasz D, Renken RJ et al. FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov Disord 2017; 32(10): 1482–1486. doi: 10.1002/ mds.27094.
30. Doty RL, Applebaum S, Zusho H et al. Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia 1985; 23(5): 667–672.
31. Braak H, Del Tredici K, Rub U et al. Staging of brain pathology related to sporadic Parkinson‘s disease. Neurobiol Aging 2003; 24(2): 197–211.
32. Stefani A, Gabelia D, Hogl B et al. Long-term follow--up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med 2015; 11(11): 1273–1279. doi: 10.5664/ jcsm.5184.
33. Unal Y, Ozturk DA, Tosun K et al. Association between obstructive sleep apnea syndrome and waist--to-height ratio. Sleep Breath 2019; 23(2): 523–529. doi: 10.1007/ s11325-018-1725-4.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2019 Issue 4-
All articles in this issue
- Intracranial stenosis – the best conservative treatment should be combined with a stent - YES
- Intracranial stenosis – the best conservative treatment should be combined with a stent - NO
- Intracranial stenosis – the best conservative treatment should be combined with a stent
- Multiple system atrophy
- Two original Czech tests for memory evaluation in three minutes – Amnesia Light and Brief Assessment (ALBA)
- Hypersomnia in acute bilateral thalamic ischemic stroke
- Hearing loss after spinal anesthesia
- Superior semicircular canal dehiscence
- The spectrum of MRI findings of progressive multifocal leukoencephalopathy in patients with multiple sclerosis in the Czech Republic
- Sense of smell examination before and after surgery for nasal polyposis
- Retrospective autoevaluation of the results of intrinsic brain tumor surgeries – consecutive cohort of 270 surgeries within one neurosurgical center of the NOS ČOS (Neurooncological section of the Czech Oncology Society) from 2015–2017
- Spina bifida in the Czech Republic – incidence and prenatal diagnostics
- Intrathecal baclofen pump for the treatment of severe spasticity – 15 years of experience
- Behavioral manifestation profi le in idiopathic REM sleep behavior disorder
- Effect of subcutaneously administered interferon β-1a on disease activity in patients with clinically isolated syndrome – ATRACT observational study
- Is it necessary to perform irrigation of a haematoma during the operation of a chronic subdural haematoma via burr hole drainage?
- Inter-rater reliability between paramedics and neurologists in the assessment of severe hemiparesis in acute stroke
- Olfactory dysfunction in a cohort of Czech patients with idiopathic REM sleep behaviour disorder
- Giant frontal sinus pneumocele with massive intracranial spread imitating lumboperitoneal shunt malfunction in a predisposed patient with Marfan syndrome
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Multiple system atrophy
- Superior semicircular canal dehiscence
- Spina bifida in the Czech Republic – incidence and prenatal diagnostics
- Hearing loss after spinal anesthesia
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

