-
Medical journals
- Career
Serum anti-Müllerian hormone levels in multiple sclerosis – a multicenter case-control study
Authors: M. Mehrpour 1; F. Taherian 2; P. Arfa-Fatollahkhani 2; A. N. Moghadasi 3; S. Mokhtar 4; H. Keivani 5
Authors‘ workplace: Department of Neurology, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran 1; Department of Neurology, Rasoul-e-Akram Hospital, Iran University of Medical Sciences, Tehran, Iran 2; MS Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran 3; Department of Infertility, Avicenna Infertility Center, Tehran, Iran 4; Department of Virology, Iran University of Medical Sciences, Tehran, Iran 5
Published in: Cesk Slov Neurol N 2018; 81(2): 199-202
Category: Original Paper
doi: https://doi.org/10.14735/amcsnn2018199Overview
Introduction:
Multiple sclerosis (MS) is considered as an inflammatory demyelinating disease with a 3-fold risk in females of child-bearing age that imposes deleterious pregnancy-related concerns for patients. We aimed to clarify the correlation of anti-Müllerian hormone (AMH) level with clinical and gynaecological characteristics of relapsing-remitting MS (RRMS) patients.Methods:
Fifty consecutive RRMS female patients and 50 age-matched healthy controls from among the hospital staff were enrolled in the study. The serum AMH levels of all subjects were evaluated by ELISA. T-tests and Mann-Whitney U tests were used for the quantitative variables. Pearson’s chi-squared test and Fisher’s exact test were used for qualifying variables. Regression analysis was performed for AMH values.Results:
The mean ± SD AMH level for the case group was 3.12 ± 3.6 and for the control group was 3.65 ± 2.06. Regression analysis detected a significant difference in AMH values between the case and control groups (p = 0.034). There was no significant influence for disease activity and therapy on AMH level in the patients. Age was the only demographic factor that predicted the AMH values in both groups (p = 0.001).Conclusion:
The results showed that serum AMH values were significantly lower in MS patients; however, this finding does not necessarily have an effect on fertility.Key words:
anti-Müllerian hormone – infertility – multiple sclerosisIntroduction
Multiple sclerosis is an inflammatory, neurodegenerative disease affecting the CNS. The first manifestations commonly appear within the second and third decades of life. There is a 3-fold level of risk in females [1–3], making fertility and pregnancy challenging concerns for MS patients.
Limited data are available on fertility in MS patients, but a decrease in the number of children and reduced spontaneous fertility has been reported in several studies. Auto-immunity, sexual disturbances, and ovarian neurologic dysfunction are recognized as suspicious etiologies [3 – 5]. Moreover, studies evaluating MS patients – treated with immunosuppressive or immunomodulating agents like mitoxantrone and interferon β, resp. – have uncovered diminished follicular reserves and fertility in the treatment groups compared to the age-matched healthy controls [6,7].
Recently, endocrine disturbances have been suggested as the pathology of MS infertility. Among the different biomarkers, one of the most reliable predictors of the ovarian reserve is the anti-Müllerian hormone (AMH). This is a dimeric glycoprotein expressed from 36 weeks of gestation that dramatically increases during puberty and persists to the age of 30 years with a gradual decline to menopause. As AMH is a relatively cycle-independent hormone expressed by the antral follicles, there is a strong correlation between serum AMH level and the number of antral follicles [8 – 11].
Some studies have highlighted the role of AMH in MS infertility [9 – 11]. One pilot study detected no differences between AMH values in relapsing-remitting MS (RRMS) cases and age-matched healthy controls; however, they found lower AMH levels and ovarian reserves in patients with higher MS activity [10]. Conversely, another recent case control study reported significantly lower AMH values in RRMS patients compared to the control group [11]. The present study aimed to clarify the correlation of AMH levels with clinical and gynaecological characteristics of RRMS patients in greater detail.
Materials and methods
This analytic case-control study was carried out from June 2014 through January 2015. Fifty consecutive RRMS female patients - admitted at the Hospitals in Tehran, Iran - were enrolled along with 50 age-matched healthy controls from among the hospital staff.
The exclusion criteria for both groups were a diagnosis of non-RR type MS, being over 35 years old, being pregnant or breastfeeding, undertaking infertility treatments and any autoimmune disease or abnormal thyroid, kidney or liver function.
Study design
All patients were interviewed by an expert to gather complete demographics and the clinical, obstetrical and gynaecological histories of all subjects, including age, body mass index (BMI), cigarette use, age of menarche, menstrual cycle regularity, dysmenorrhea, amenorrhea, marital status, number of children, desire to bear a child, infertility history and treatment. Also recorded were the method of contraception used, oral contraceptive pill use, abortion history, disease history in terms of duration, type and treatment (disease-modifying therapy; DMT), pulse therapy with immunosuppressive agents, and untreated status (defined as no treatment with a DMT during the 16 weeks prior to AMH evaluation). The Expanded Disability Status Scale (EDSS) score of the patients was obtained by an experienced neurologist.
Sample collection and ELISA
Venous blood samples (3 cc) were collected from the antecubital fossa and maintained in heparinized tubes at room temperature. They were transferred to the ELISA laboratory in less than 1 hour and centrifuged. Laboratory cut points were 0.8 – 1.5 ng/ mL for ages 15–24, 0.8 – 10 ng/ mL for ages 25–29, 0.4 – 9.3 for ages 30–34, and 0.15 – 7.3 ng/mL for ages 35–39.
Statistical analysis
Between-group comparisons were applied using the t-test for quantitative variables, which were normally contributed. The Mann-Whitney U test was assigned for abnormally contributed quantitative variables. Pearson’s chi-square test and Fisher’s exact test were performed to analyse the qualifying variables. Regression analysis was performed on ELISA results. Descriptive analysis of the data was expressed as frequency and mean ± SD; p < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS (Version 22, SPSS Inc., Chicago, IL, USA) and R software (The R Foundation, Vienna, Austria).
Ethics
This work was approved by the Local Medical Ethics Committee of Iran University of Medical Sciences.
Results
Demographic and gynaecological characteristics
Demographic and gynaecological characteristics of all subjects are listed in Tab. 1. None of the subjects have a history of using illegal drugs or smoking cigarettes. Of the 100 participants, one used an oral contraceptive pill for contraception and one was trying to conceive. Importantly, none of the healthy controls were infertile, and the infertility rate of the participants was 6%. Nine participants in the case group and two people in the control group had an abortion; however, the Pearson chi-square analysis revealed no significance for both (p = 0.24, p = 0.19, resp.).
1. Demographic and gynaecologic characteristics of RRMS patients and healthy controls. 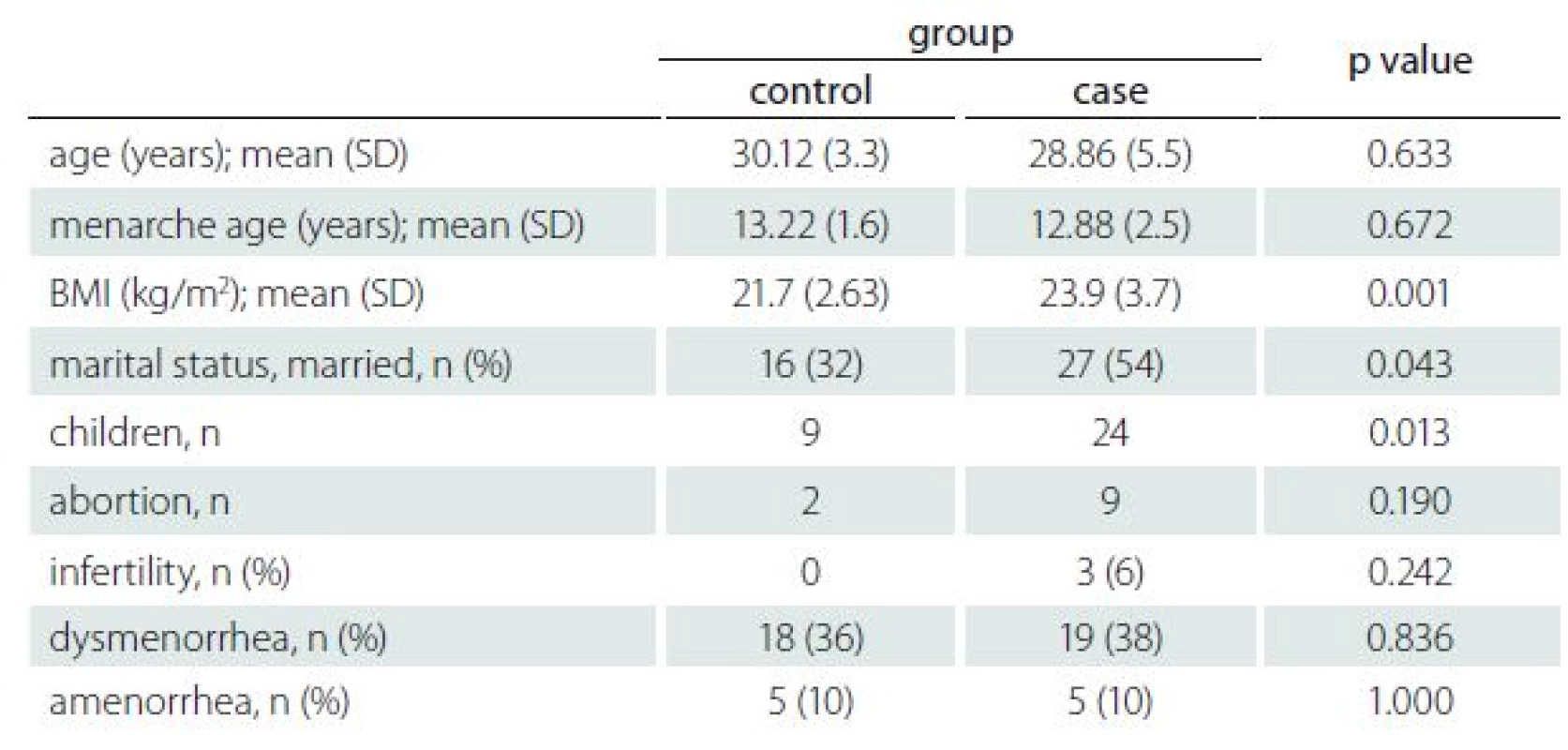
BMI – body mass index; n – number of patients; RRMS – relapsing-remitting multiple sclerosis; SD - standard deviation The BMI, marital status and number of children were significantly different between the case and control groups. The t-test results revealed that BMI was significantly higher in the case group (p = 0.001). Interestingly, the case group had a higher number of children than their age-matched controls, which was statistically significant in chi-square analysis (p = 0.013). Of the 50 participants 27 (54%) and 16 (32%) were married in the case group and control group, resp., which was significant at p = 0.043 (Tab. 1).
Clinical characteristics of patients
In the case group, three participants were new cases of RRMS in whom the treatment had not commenced. The level of medication was categorized as: 1. low frequency and included Avonex (22 patients); 2. high frequency and included glatiramer acetate, Betaseron and Rebif (22 patients); 3. the last group used fingolimod (3 patients). The EDSS scores of patients had a mean ± SD of 1.80 ± 0.95, with a maximum of 4.5 and minimum of 0. The duration of the disease in the case group was 40 days to 14 years (3.7 ± 3.10 years).
AMH analysis between groups
In the case group, the mean ± SD for AMH level was 3.12 ± 3.6 and in the control group was 3.65 ± 2.06. The serum AMH levels were normal in 38 cases. However, analysis revealed lower AMH levels in nine patients and higher levels in three patients among the case group. Only two patients from the control group had AMH results outside the normal range; one was above and one was below the normal range. Regression analysis detected a significant difference in AMH values between the case and control groups (adjusted R2 = 0.05403; p = 0.034). There was no significant difference for age of subgroups between groups (Tab. 2).
2. Correlation of demographic and gynaecologic factors and AMH level. 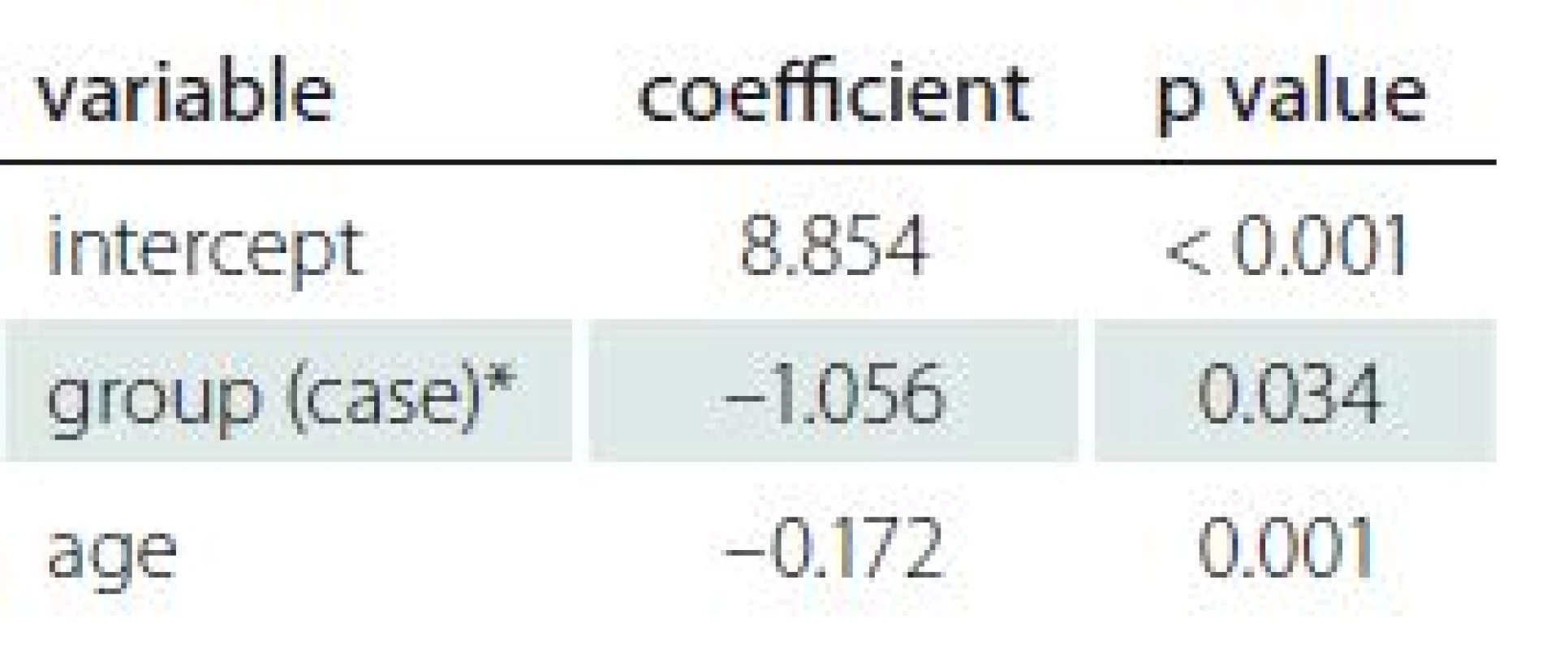
AMH – anti-Müllerian hormone * reference category is ”control” Correlation of demographic and gynaecologic characteristics
The number of children, infertility rate, number of abortions and marital status were not statistically significant predictors of AMH values according to regression analysis (p > 0.2). In the backward method, age was the only demographic factor that significantly predicted AMH values in both groups (adjusted R2 = 0.05403; p = 0.001). Analysis showed that advanced age was a significant predictor of AMH level. Details are available in Tab. 2.
Influence of disease activity and therapy
Table 3 shows the results of regression analysis for treatment, duration of disease and EDSS score versus AMH values in the case group. There was no significant influence of the disease activity or therapy on the AMH levels found (adjusted R2 = – 0.1149).
3. Influence of disease activity and therapy on AMH level. 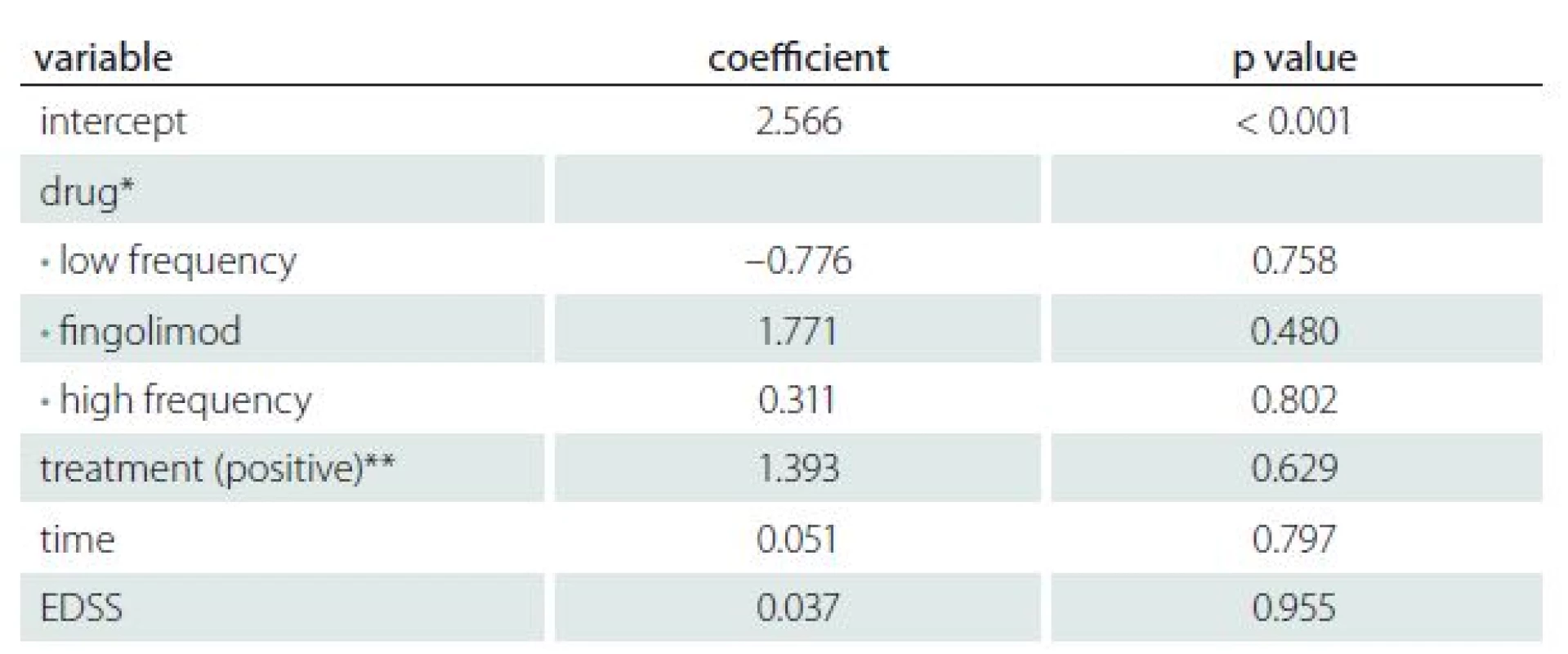
AMH – anti-Müllerian hormone; EDSS – Expanded Disability Status Scale * reference category is “no drug” ** reference category is “negative” Discussion
This cross-sectional case-control study evaluated the relationship of AMH values and demographic, gynaecological and clinical features of RRMS patients in comparison to age-matched healthy controls. The findings showed a significantly lower serum AMH levels in the case group compared to the controls (p = 0.034). Notably, the results revealed no correlation between the low AMH level and the rate of infertility or abortion in the case group. The results suggest that advanced maternal age and MS were the only significant predictors of decreased AMH level.
A recent cross-sectional pilot study evaluated 76 RRMS patients and 58 controls and reported a lower mean of the AMH levels for the RRMS patients; however, it also detected very low AMH levels in patients not currently under treatment with a DMT [11]. In contrast, the current study revealed no effect for the type of MS therapy on the AMH values. Another recent case-control study found no significant difference in AMH values between the RRMS patients and healthy populations. Moreover, they found that higher disease activity might result in poorer ovarian reserves in RRMS patients [10]. The results of the present study found no potential effect for EDSS score and duration of the disease on AMH levels as a reliable predictor of ovarian reserves [8].
More studies are needed to elucidate the correlation between the clinical features of the disease and fertility in MS patients. In keeping with previous studies, the results illustrated that AMH levels declined significantly with age in both the case and control groups. As shown, AMH levels peaked dramatically during puberty, persisted till the age of 30, and gradually declined to menopause [8].
A higher mean BMI was recorded in the case group as compared with the control group. In line with previous studies, high BMI and obesity are significant and possible risk factors for MS that have deleterious functional effects on patients [12 – 14]. Surprisingly, and in contrast with previous studies reporting childlessness in MS patients [3,15], the current findings were consistent with a recent pilot study, showing a higher rate of marriage and number of children in the case group [10]. This difference could be attributed to the lifestyle of the healthy controls, who were primarily members of the hospital staff.
This work was limited by a small sample size because of the large number of exclusion criteria that aimed to achieve exact evaluations without the potential effects of co-morbidity on fertility and AMH values of the participants.
Conclusions
This study showed that serum AMH values decreased significantly in the RRMS group; however, this finding might not have a potential impact on fertility. Because this work was one of the first to evaluate AMH as an influential factor on fertility in MS patients, more studies are needed to produce practical and effective reproductive programmes for MS sufferers.
The authors wish to thank Rasoul-e-Akram Hospital Clinical Research Development Center and Ms Zahra Torabi-Goodarzi (clinical psychologist) for their technically-supported implementation of the project.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 27. 7. 2017
Accepted for print: 12. 2. 2018
Fatemeh Taherian, MD
Department of Neurology Rasoul-e-Akram Hospital Iran University of Medical Sciences
Niyayesh St., Satarkhan St.
Tehran, Iran
e-mail: research.rasoul@gmail.com
Sources
1. Chen YH, Lin HL, Lin HC. Does multiple sclerosis increase risk of adverse pregnancy outcomes? A population-based study. Mult Scler 2009; 15(5): 606 – 612. doi: 10.1177/ 1352458508101937.
2. Greer JM, McCombe PA. Role of gender in multiple sclerosis: clinical effects and potential molecular mechanisms. J Neuroimmunol 2011; 234(1 – 2): 7 – 18. doi: 10.1016/ j.jneuroim.2011.03.003.
3. Cavalla P, Rovei V, Masera S et al. Fertility in patients with multiple sclerosis: current knowledge and future perspectives. Neurol Sci 2006; 27(4): 231 – 239.
4. Dahl J, Myhr KM, Daltveit AK et al. Planned vaginal births in women with multiple sclerosis: delivery and birth outcome. Acta Neurol Scand Suppl 2006; 183 : 51 – 54.
5. Hellwig K, Brune N, Haghikia A et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand 2008; 118(1): 24 – 28. doi: 10.1111/ j.1600-0404.2007.00978.x.
6. Cocco E, Sardu C, Gallo P et al. Frequency and risk factors of mitoxantrone-induced amenorrhea in multiple sclerosis: the FEMIMS study. Mult Scler 2008; 14(9): 1225 – 1233. doi: 10.1177/ 1352458508094882.
7. Cil AP, Leventoğlu A, Sönmezer M et al. Assessment of ovarian reserve and Doppler characteristics in patients with multiple sclerosis using immunomodulating drugs. J Turk Ger Gynecol Assoc 2009; 10(4): 213 – 219.
8. Bhide P, Homburg R. Anti-Müllerian hormone and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2016; 37 : 38 – 45. doi: 10.1016/ j.bpobgyn.2016.03.004.
9. Thöne J, Kleiter I, Stahl A et al. Relevance of endoglin, IL-1α, IL-1β and anti-ovarian antibodies in females with multiple sclerosis. J Neurol Sci 2016; 362 : 240 – 243. doi: 10.1016/ j.jns.2016.01.057.
10. Sepúlveda M, Ros C, Martínez-Lapiscina EH et al. Pituitary-ovary axis and ovarian reserve in fertile women with multiple sclerosis: a pilot study. Mult Scler 2016; 22(4): 564 – 568. doi: 10.1177/ 1352458515602339.
11. Thöne J, Kollar S, Nousome D et al. Serum anti-Müllerian hormone levels in reproductive-age women with relapsing-remitting multiple sclerosis. Mult Scler 2015; 21(1): 41 – 47. doi: 10.1177/ 1352458514540843.
12. Cambil-Martín J, Galiano-Castillo N, Muñoz-Hellín E et al. Influence of body mass index on psychological and functional outcomes in patients with multiple sclerosis: a cross-sectional study. Nutr Neurosci 2016; 19(2): 79 – 85. doi: 10.1179/ 1476830514Y.0000000156.
13. Kvistad SS, Myhr KM, Holmøy T et al. Body mass index influence interferon-beta treatment response in multiple sclerosis. J Neuroimmunol 2015; 288 : 92 – 97. doi: 10.1016/ j.jneuroim.2015.09.008.
14. Taylor KL, Hadgkiss EJ, Jelinek GA et al. Lifestyle factors, demographics and medications associated with depression risk in an international sample of people with multiple sclerosis. BMC Psychiatry 2014; 14 : 327. doi: 10.1186/s12888-014-0327-3.
15. Nielsen NM, Jørgensen KT, Stenager E et al. Reproductive history and risk of multiple sclerosis. Epidemiology 2011; 22(4): 546 – 552. doi: 10.1097/ EDE.0b013e31821c7adc.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2018 Issue 2-
All articles in this issue
- Glucose transporter-1 deficiency syndrome – expanding the clinical spectrum of a treatable disorder
- Carpal tunnel syndrome within the context of functional disorders of the musculoskeletal system
- Identification of pediatric patients with pharmacoresistant epilepsy and selection of candidates of non-pharmacological therapy
- Neurofilament light chains in serum and cerebrospinal fluid and status of blood-CSF barrier in the selected neurological diseases
- Physiotherapy in Parkinson’s disease in the Czech Republic – a demographic study
- Patients with idiopathic REM sleep behavior disorder follow-up – phenoconversion into parkinsonian syndrome and dementia
- Czech version of the Edinburgh Cognitive and Behavioral Amyotrophic Lateral Sclerosis Screen – a pilot study
- Acute pediatric myelitis – cohort of 20 patients
- Granular cell tumor in the pituitary stalk
- Brain biopsy in 10 key points – what can a neurologist expect from the neurosurgeon and the neuropathologist?
- Ataxia
- Anticoagulation therapy in patients with atrial fibrillation and cerebral amyloid angiopathy - NO
- Anticoagulation therapy in patients with atrial fibrillation and cerebral amyloid angiopathy
- Anticoagulation therapy in patients with atrial fibrillation and cerebral amyloid angiopathy - YES
- Fabry disease, an overview and the most common neurological manifestations
- Is the role of amyloid in senile dementia substantial?
- Serum anti-Müllerian hormone levels in multiple sclerosis – a multicenter case-control study
- Invasive primary intracerebral infections in women caused by Streptococcus intermedius manifesting as purulent meningitis and intracerebral abscess
- Malignant melanotic schwannoma of the vertebral body in a patient with Carney complex
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Ataxia
- Brain biopsy in 10 key points – what can a neurologist expect from the neurosurgeon and the neuropathologist?
- Fabry disease, an overview and the most common neurological manifestations
- Glucose transporter-1 deficiency syndrome – expanding the clinical spectrum of a treatable disorder
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

