-
Medical journals
- Career
Accuracy of Prehospital Diagnosis of Stroke
Authors: Sladjana Lj Andjelic
Published in: Cesk Slov Neurol N 2012; 75/108(1): 62-68
Category: Short Communication
Overview
Introduction:
Following modern trends, FAST (Face Arm Speech Test) and Cincinnati Prehospital Stroke Scale (CPSS) have been introduced at the City Emergency Medical Care Center (CEMCC) in Belgrade.Aim:
To analyze the level of agreement in acute stroke (AS) diagnosis between CEMCC medical dispatchers (EMDs), the field physicians attending to the patient and the physicians at the Hospital for Cerebrovascular Diseases and Stroke (HCDS) “St. Sava“, Belgrade.Method:
We conducted a one-year prospective study at the Belgrade CEMCC. To screen for a suspected stroke, the dispatchers use FAST criteria, emergency physicians (EP) in the field use the CPSS criteria, and additional ancillary methods are used in the SH.Results:
Within the observed period, EMDs suspected a neurological problem in 7.561 cases, 38.1% of which were suspected ASs. Attending EP confirmed the diagnosis of AS in 2.881 (71.1%) of cases. Furthermore, following completion of emergency diagnostic procedures upon an admission to the SH, the diagnosis of AS was confirmed in 1.950 (67.7%) of cases.Conclusion:
A satisfactory level of success was found when the FAST and CPSS screening scales were used for pre-hospital identification of AS symptoms.Key words:
diagnosis – stroke – stroke unitsIntroduction
In Europe, the incidence of newly diagnosed cases of acute stroke (AS) is between 635 per one million in Switzerland and 2.734 per one million in Russia [1]. The data collected by the American Heart Association (AHA) show that, in the USA, one AS occurs every 45 seconds, and that one patient dies due to AS every 3 minutes. In 2000, AS was the leading cause of reduced life expectancy in women and the second leading cause in men in Serbia. In 2001, AS became the leading cause of death in hospital settings [2]. In November 2004, National Guidelines for Treatment of Acute Stroke (NGTAS) were published in Serbia [3], intended to support physicians at all levels of health care in implementing adequate pre-hospital and hospital AS management. With an introduction of rt-PA (recombinant tissue plasminogen activator) into the Serbian market (Actilyse® – Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany), and with actual knowledge of very small therapeutic window lasting for 3–4.5 hours (ECASS III study) [4], at the beginning of 2005, the first specialized Stroke Unit (SU) was established at the Neurology Emergency Department, Institute of Neurology (DENIN), Serbian Clinical Centre in Belgrade. Similarly to the coronary unit model, SU offers 24-hour availability of a stroke team able to perform thrombolysis and all necessary diagnostic services, including an emergency computed tomography (CT) brain scan, and possessing the equipment required for intensive care and patient monitoring. In Serbia, the first thrombolysis in AS was performed at this very unit in February 2006, and it is regularly used in the management of AS since then [5].
Revolutionary changes in pre-hospital management of AS occured concurrently with the improvements in hospital management. For many years, emergency medical dispatchers (EMDs) at the City Emergency Medical Care Center (CEMCC) in Belgrade have used the Index of Urgent Treatment as the official protocol for triage and call processing [6]. According to this protocol, AS has been a condition of the second level of emergency (yellow call) (Chapter 25. Semi-conscious Conditions and Paralyses). However, following modern trends [7], AS management has been re-defined within Emergency Medical Service (EMS) as a time-dependant emergency medical condition (red call), similar to trauma or acute myocardial infarction (AMI). By accepting the slogan “time is brain”, AS became a condition treated as the first degree of emergency in Serbia. The novel role of CEMCC in Belgrade arises from the AS recovery cascade. It comprises prompt and accurate identification of AS symptoms, rapid transport of the patient and early pre-hospital therapeutic measures.
The aim of this paper was to analyze the agreement between pre-hospital diagnosis of AS symptoms by EMDs, preliminary diagnosis of AS by the physicians attending in the field, and their concordance with the final AS diagnosis made at the Hospital for Cerebrovascular Diseases and Stroke (HCDS) “St. Sava“, Belgrade. Furthermore, prehospital times and time intervals before handing the AS patient over to the Stroke Team were analyzed.
Methods
One year prospective study at Belgrade CEMCC was conducted from January 1, 2005 to December 31, 2005. City Emergency Medical Care Centre has 25 crews available 24 hours per day and covering Belgrade, a city with two-million inhabitants.
Before the start of the study, Belgrade Emergency Center Stroke Unit neurologists provided interactive training sesions to CEMCC physicians on the subject of AS. The education included pathophysiology, identification, treatment and approach to an AS patient. It has been agreed that EMDs would use the FAST criteria (Face Arm Speech Test) for the purpose of quick telephone screening of suspicious signs of AS [8]. The FAST Scale was developed in 1998 as a diagnostic instrument for prompt identification of AS symptoms. It represents modified Cincinnati Prehospital Stroke Scale (CPSS) and it is an integral part of the United Kingdom Training Package for paramedics and EMS personnel. For every patient, feedback information was obtained from the field physician in order to confirm or reject the diagnostic assumption made by EMDs. The field physician initially registered the state of consciousness of the patient using Glasgow Coma Scale (GCS) (GCS = 8 coma; GCS = 9–12 sopor; GCS = 13–15 somnolence) [9]. If GCS was >8, simple orientational neurological evaluation CPSS (facial palsy, asymmetric arm weakness, and speech abnormalities) was performed in order to make diagnosis of a suspicious AS [10]. Apart from neurological assessment, the EMS physicians performed A (airway) B (breathing) C (circulation) evaluation as well by measuring blood pressure, body temperature, glucose level, and oxygen saturation, in accordance with NGTAS and Current European Stroke Organization guidelines [11]. Anamnestic and/or heteroanamnestic data on previous AS, recent head injury and bleeding (cerebral, gastrointestinal, and urinary) were used as supplemental criteria.
After the examination, the following procedures were performed: airway support and assisted ventilation (in cases of altered state of consciousness or compromised breathing), intubation (in cases of comatose patients, those with increased risk of aspiration, and with respiratory failure), introduction of intravenous line, and electrocardiographic (ECG) monitoring. When the patient was unconscious, not breathing or pulseless, cardiopulmonary resuscitation was performed. Therapeutic measures were taken in accordance with the NGTAS recommendations: antihypertensive therapy if systolic arterial pressure (AP) was >240 mmHg and diastolic pressure >120 mmHg (level of evidence A, recommendation level I), antipyretics – paracetamol and friction when body temperature was >37.5 °C (level of evidence B, recommendation level IIa), oxygen at a dose of 3 l/min if O2 saturation was <92% (level of evidence B, recommendation level IIa), hypertonic glucose (glucose level <2.7 mmol/l) (level of evidence A, recommendation level I). Unconscious patients or those in epileptic status with spontaneous breathing were transported on their left side. Conscious patients were transported in the lateral position with head and chest elevated at 30° angle and with stabilization of a paralyzed limb. After stabilization and preparation for transport, EMS crew informed the CEMCC dispatch center that notified the Stroke Hospital and proceeded with transportation. During transport, every patient was observed clinically and diagnostically. All the data were recorded in the Internal Protocol (Figure 1) to ensure collection of all the relevant data related to AS. Data collection using this list was developed and established by the Belgrade CEMCC Education Center staff in accordance with WHO STEPwise approach to stroke surveillance [12].
Figure 1. Acute Brain Stroke List, CIEMA, Belgrade. 
Upon admission to the Stroke Hospital, the diagnosis of AS was either confirmed or rejected by the emergency diagnostic methods (Table 1) [3,11].
1. Emergency diagnostic tests in patients with acute stroke [3]. ![Emergency diagnostic tests in patients with acute stroke [3].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/30f101306aee2e3c3b1b7aca2246c233.png)
During further evaluations, head, neck and coronary blood vessels ultrasound was performed, as well as magnetic resonance imaging (MRI) and CT angiography.
Data that were collected by the protocol were implemented into a predefined database and statistically processed using SPSS 13.0 for Windows (SPSS, Chicago, IL) software standard tests: Student t-test and χ2 test. The differences were considered to be statistically significant when the p value obtained was <0.05.The results were presented in tables and graphs.
Results
During the observed period, CEMCC operators processed 78,943 calls. In 7,561 cases (10.0%), there was a suspicion of a neurological disorder. The prevalence of suspicious AS diagnosis compared to other neurological diseases was 38.1% (2,881/7,561). The AS probability index was established by EMDs using the FAST criteria (Table 2). Among other diagnoses suspected by the operators, AS was the seventh most frequent (Figure 2).
2. The FAST (Face Arm Speech Test) evaluation by a dispatch er in stroke patients (n = 2,881). 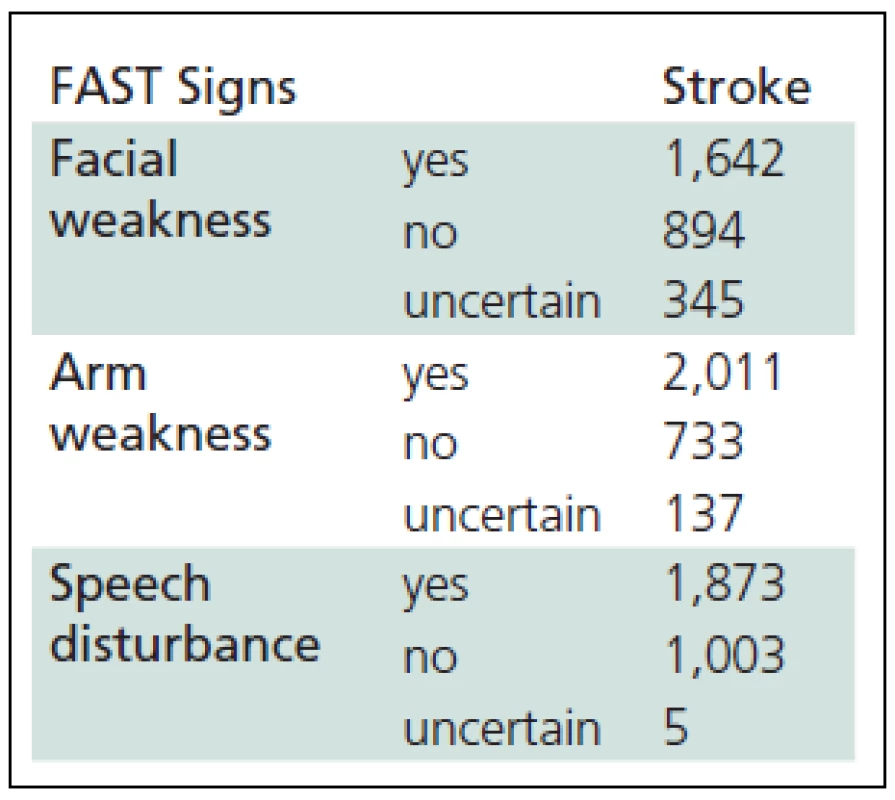
Figure 2. Acute stroke diagnosis incidence compared to other diagnostic assumptions made during emergency calls. Legend: CD – Cardiovascular Disease; TRAU – Trauma; OND – Other Neurological Disease; LG – Lung Disease; AMI – Acute Myocardial Infarction; CA – Cancer; P – Pediatric disease; GI – Gastrointestinal Disease; AP – Abdominal Pain; BL – Bleading; FU – Feel Unwell; DC – Disturbances of Consciousness; TOX – TOXicology; DER – DERmatology; PSY – PSYchiatry; R – Rheumatology; SC – SChock; ID – Infection Diseases; AL – Allergy; M/S – Murder/Suicide; OG – Obstreties and Gynecology; GU – GenitoUrinary; AC – Abstruse Conditions 
Three hundred sixty three (12.6%) suspected AS calls were accepted as the first degree emergency calls, 1,498 (52.0%) as the second degree emergency calls and the remaining calls had no emergency degree specification. In the field, emergency physicians confirmed the diagnosis of AS made by EMDs in 71.1% of cases using CPSS criteria (Table 3). The diagnosis was not confirmed in 9% of cases and no data were recorded in 19.9% of cases. In 36 patients in the group of calls with more than one diagnosis, AS was not identified before the field examination was performed. Most commonly, these were patients with altered consciousness [13], hypertensive patients and diabetics [5].
3. Agreement between FAST (Face Arm Speech Test) deficit evaluation by a dispatcher and physician examination in stroke patients pre-hospital and hospital (n = 2,881). 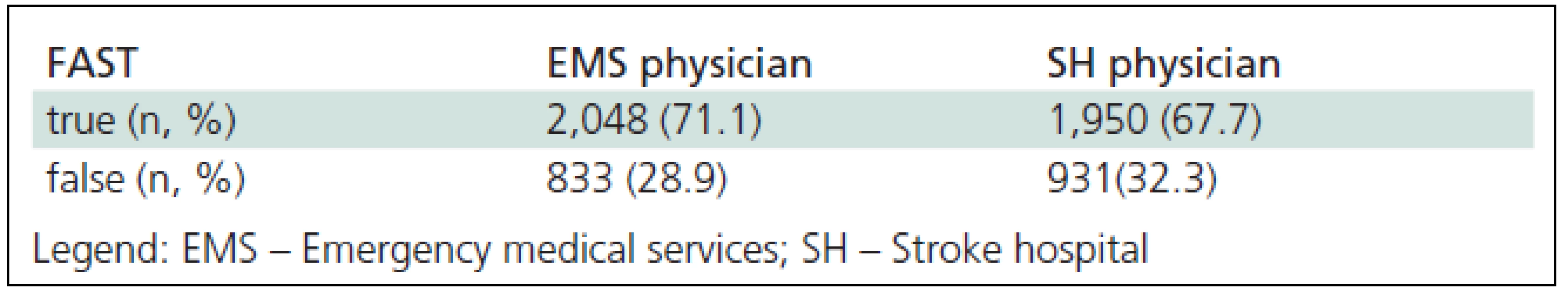
According to a specifically designed list, most common complaints that increased the index of risk for AS diagnosis by a field physician were: unilateral body paralysis in 41.1% and speech disorders in 30%. In the remaining patients, complaints included vertigo (10%), headaches (7%), consciousness crisis (10%) and other complaints (6%).
From the total number of patients with the diagnosis of suspected AS made in the field, 47.20% (1,360) were male and 52.80 (1,521) were female (χ2 = 0.392 < χ2 (1 and 0.05) = 3,841). The mean age was 68 (SD = 11.36) for the male patients, and 70 (SD = 12.47) for the female patients (t = 0.856 < t = 1.96 and p >0.05). The demographic characteristics and AS-specific variables are shown in Table 4.
4. Individual predictor variable for stroke. 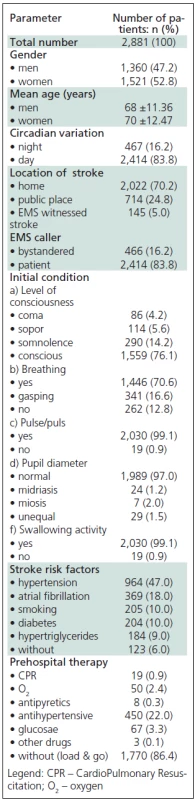
Times (in minutes) of pre-hospital portion of Chain of Recovery for Acute Stroke were extracted (Table 5). The mean time to identification of AS symptoms was 7.8 (median 5.5) minutes. The mean time before a witness or the patient called the EMS, was 2 minutes (median 1 minute), time of call forwarding to the nearest field crew was 9.2 minutes, while the duration of the call-response interval was 9.8 (median 7) minutes. The mean speed of the ES crew arrival was 11.1 (median 9) minutes, duration of the examination and the patient preparation for transport was 11.8 (median 10) minutes and the mean time before initiation of the transport was 30.5 ±21.3 minutes. The mean time elapsed from call for emergency to SH admission in patients with acute stroke was 41.1 ±12.4 minutes.
5. Times of pre-hospital portion of Chain of Recovery for Acute Stroke. 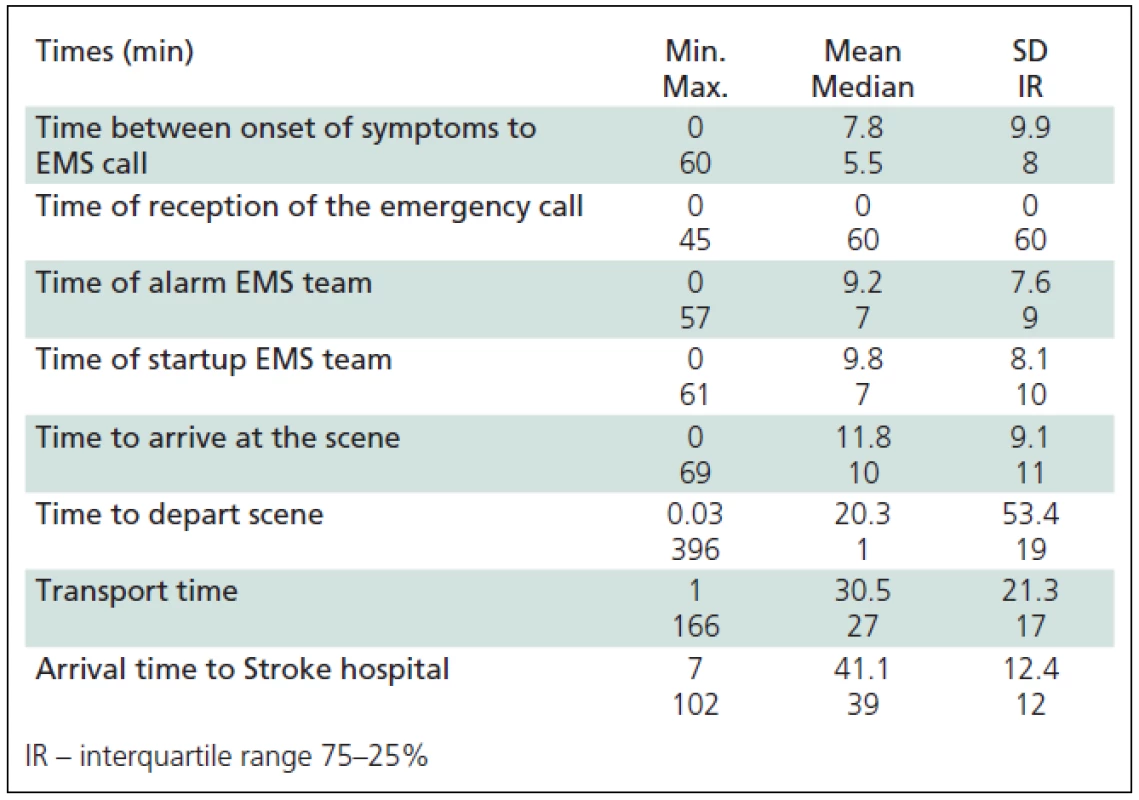
The majority of patients were transported to DENIN with a preliminary diagnosis of AS (71.1%), followed by diagnoses of vertiginous syndrome (8.9%), crisis of consciousness (10%), and epileptic seizure (10%). The working diagnosis of intracranial hemorrhage was made in approximately 1.5% of patients.
Following an admission to the Stroke Hospital and after neurological examination (NIHSS scale) and additional diagnostic procedures (Table 1), attending neurologist confirmed the diagnosis of acute stroke, initially made by the Emergency Service dispatcher, in 1950 (67.7%) [14] of patients (Figure 3).
Figure 3. Concordance between stroke diagnosis by an emergency medical dispatcher, a preliminary diagnosis by a field physician and an admission diagnosis by a physician at the the Hospital for Cerebrovascular Diseases and Stroke (HCDS) “St. Sava“ in Belgrade. Legend: FAST – Face Arm Speech Test; CPSS – Cincinnati Prehospital Stroke Scale; SH – Stroke Hospital. 
Discussion
This is the first study in Serbia that compared diagnosis of AS symptoms by EMDs, EMS field physicians and the SH neurologist. A problem emerges for the Belgrade EMS physicians when they are to make a decision whether to follow recommendations by the NINDS study [7] and treat AS as a condition of the first degree of emergency, or whether they should proceed in accordance with the Index of Urgent Treatment [6] recognized by the Serbian Ministry of Health as an official operator’s protocol. It classifies AS as a condition of the second degree of emergency. The fact that the CEMCC dispatchers processed the AS calls as the first degree emergency in 12.6% of cases only suggests that the significance of the ‘time window’ and bringing the patients with AS, AMI and trauma to the same level of emergency has not yet been fully accepted. Therefore, there is an emerging necessity for new educational seminars that would include all participants involved in the chain of treatment. Today, more than 25,000 physicians working in EMS in USA have been educated. The first medical training programs of this kind were affirmed in Cincinnati in 1969 [10]. In our study, the dispatchers made the correct diagnosis of AS in 71.1% (confirmed by the field physician), similar to the study in which the diagnosis was confirmed in 72% of cases [15]. However, in a retrospective study from San Francisco [16], the dispatchers diagnosed AS in 31% of received calls only. In CEMCC, all operators are physicians and this may explain the difference in the AS diagnosis accuracy. In the same study performed in San Francisco, EMS field physicians diagnosed AS in 62% of cases before training, while after a four-hour training, they succeeded in 91% of cases [17]. The SH neurologist confirmed the suspicion of AS made by our field physicians in 67.7% of cases. The group of 36 patients in which AS symptoms were missed by the operators resulting into a failure to diagnose AS before actually attending to the patient is especially interesting. Most commonly, these calls were processed as “felt sick on the street”, “unclear problem”, “diabetic in coma”, “hypertensive crisis”, etc.
The AS diagnosis is the seventh most frequent among other diagnoses made by CEMCC field physicians, while AMI is the fifth most frequent. It is unclear whether the proportion of the most prevalent diseases is differently defined in other studies, or the AS epidemic has not yet commenced in our city. In Croatia, the diagnosis of AMI is the second and of AS the fifth most frequent [18]. The data in Table 1 show, that following training and using the FAST scale, our operators made the diagnosis of AS based on the “A” alert sign (arm weakness) in 69.8% cases, compared to 45% in an Australian [19] and 49% in a UK study [20].
A large number of similar worldwide studies [1,6,11] suggest that the majority of AS patients are older and are female. With respect to circadian variations of AS, the majority of acute events occurred in the morning, similar to a Hong Kong study [21]. In line with the AHA observations [22], the majority of out-of--hospital AS cases in our study occurred at the place of residence (house or apartment) (70.2%), compared to somewhat lower incidence of public places (24.8%). In 83.8% of cases, the EMS callers were the patients themselves, suggesting a positive impact of a public education campaign conducted in Belgrade through public media, various seminars, online education, etc. The interesting Ansan Geriatric Study [23] showed that there was no difference in identification of AS alert signs among the educated citizens in Korea, irrespective of whether they were devoted to the traditional or to the modern Western medicine (60.2%). According to the current American guidelines, hypertension is a major risk factor for AS [14] as also suggested by the results of our study. A meta-analysis of 17 studies with hypertensive patients treated with various antihypertensive drugs (angiotensin converting enzyme inhibitors – ACE inhibitors, new generation of lipophilic calcium channel blockers, angiotensin receptor blockers) in primary AS prevention showed a reduction of the relative risk for AS by 38%. After the initial check-up, CPR was initiated in 19 patients (0.9%). In 86.4% of patients with AS, the “load & go” principle was applied (do not delay transport).
In different studies, definitions of times and time intervals associated with acute stroke vary. Time to identification of the first symptoms was extremely variable in our study and was 7.8 ±9.9 minutes on average. Since the time to symptom identification was unknown in 26% of cases (the AS patient was alone at the time of onset) and uncertain in an even higher number of patients (32%), the time intervals could only be precisely determined by considering the time of EMS call as a baseline. We compared the time intervals found in our study with similar studies in other regions (Reading, Ohio and Texas) [23]. The time from the EMS call to the arrival of the crew (response time) was 11.8 minutes in our study compared to 8.3 minutes in Texas and 3 minutes in Ohio. The longer interval measured in our study may be explained by Belgrade being a metropolis (heavy traffic, skyscrapers, insufficient number of EMS teams, etc.). Within the AHA and American Stroke Association Synopsis: “Implementation Strategies for Emergency Medical Services within Stroke Systems of Care”, the main goal of EMS is to achieve the “Response Time” (Time Call Received – Time the EMS Arrived on Scene) shorter than 9 minutes. The “Mean Scene Time” (Time the EMS Arrived on Scene – Time EMS Departed the Scene) was 20.3 min in our study compared to 17.8 min in Texas. The Transport time for our patients was 30.5 min. The “Mean Delivery Time” (Time Call Received – Time the EMS Arrived at Destination) was 41.1 minutes in our study (15 to the closest and 86 min to the most distant) compared to 45 min and 39.4 min in Ohio and Texas, respectively [24].
Although the results of other studies might be more favorable, having in mind the difficult economic situation in Serbia, the present situation may be seen as promising.
Conclusion
According to the new postulates of the CEMCC Belgrade, AS is seen as a condition of the first degree of emergency. After the training of CEMCC physicians (dispatcher and field physicians), the pre-hospital identification of AS symptoms using the FAST and CPSS scales is encouraging. Compared to other authors, the results of our study are satisfying notwithstanding the need for improvement in the AS associated time intervals.
Sladjana Lj Andjelic, MD, PhD
Aleksinackih rudara 25/4
11070 New Belgrade, Serbia
e-mail: novizivot@ptt.rs
Sources
1. Brainin M, Bornstein N. Acute neurological stroke care in Europe: results of the European Stroke Care Inventory. Eur J Neurol 2000; 7(1): 5–10.
2. Atanasković-Marković Z, Bjegović V, Janković S. Opterećenje bolestima i povredama u Srbiji (abstract). Beograd: Ministarstvo zdravlja Republike Srbije 2003.
3. Republička stručna komisija za izradu i implementaciju vodiča u kliničkoj praksi Ministarstva zdravlja Republike Srbije – rukovodilac Čovičković-Šternić N. Akutni ishemijski moždani udar – Nacionalni vodič. 1st ed. Beograd: Medicinski fakultet Univerziteta CIBID 2004.
4. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. New Engl J Med 2008; 359(13): 1317–1319.
5. Jovanović D, Beslać-Bumbaširević L, Kostić V. First experience with intravenous thrombolysis in ischemic stroke in Serbia. Srp Arh Celok Lek 2007; 135(11–12): ‚621–628.
6. The Norwegian Medical Association. Norsk indeks for medisinsk nødhjelp (The Norwegian index for medical emergencies). Stavanger: Aasmund S, Laerdal A/S 1994.
7. William G, Barsan MD. Acute medical care in United States. In: Marler JR, Jones PW, Emr M (eds). Proceedings of a National Symposium on Rapid Identification and Treatment of Acute Stroke. Bethesda Md: The National Institute of Neurological Disorders and Stroke, National Institutes of Health; August 1997. Publication No. 97-4239.
8. Nor Am, McAllister C, Louw SJ, Davis M, Jenkinson D, Ford A. Agreement between ambulance paramedic and physician-recorded neurological signs with Face Arm Speech Test (FAST) in acute stroke patients. Stroke 2004; 35(6): 1335–1339.
9. Weir CJ, Bradford AP, Lees KR. The prognostic value of the components of the Glasgow Coma Scale following acute stroke. Q J Med 2003; 96(1): 67–74.
10. Liferidge AT, Brice JH, Overby BA, Evenson KR. Ability of laypersons to use the Cincinnati Prehospital Stroke Scale. Prehosp Emerg Care 2004; 8(4): 384–387.
11. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc Dis 2008; 25(5): 457–507.
12. World Health Organization. WHO STEPS Stroke Manual: the WHO STEPwise approach to stroke surveillance. Geneva: World Health Organization 2006.
13. Azzimondi G, Bassein L, Fiorani L, Nonino F, Montaguti U, Celin D et al. Variables associated with hospital arrival time after stroke: effect of delay on the clinical efficiency ofearly treatment. Stroke 1997; 28(3): 537–542.
14. Kuljić-Obradović D, Đoković S, Labudović M. Diferencijalna dijagnoza moždanog udara u prehospitalnim uslovima. ABC – časopis urgentne medicine 2006; 6(2–3): 66–70.
15. González-Fernández M. Why Not Call 911? Stroke 2010; 41(7): 1318.
16. Porteous GH, Corry MD, Smith WS. Emergency medical services dispatcher identification of stroke and transient ischemic attack. Prehosp Emerg Care 1999; 3(3): 211–216.
17. Kothari R, Barsan W, Brott T, Broderick J, Ashbrock S. Frequency and accuracy of prehospital diagnosis of acute stroke. Stroke 1996; 26(6): 937–941.
18. Hrvatski zavod za javno zdravstvo. Hrvatski zdravstveno-statistički ljetopis za 2002. godinu. Zagreb 2003 : 39–40.
19. Reeves MJ, Hogan JG, Rafferty AP. Knowledge of stroke risk factors and warning signs among Michigan adults. Neurology 2002; 59(10): 1547–1552.
20. Nor AM, McAllister C, Louw SJ, Dyker AG, Davis M, Jenkinson D et al. Agreement between ambulance paramedic - and physician-recorded neurological signs with Face Arm Speech Test (FAST) in acute stroke patients. Stroke 2004; 35(6): 1355–1359.
21. Cheung R, Mak W. Chan KH. Circadian Variation of Stroke Onset in Hong Kong Chinese: A Hospital-Based Study. Cerebrovasc Dis 2001; 12(1): 1–6.
22. Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 2006; 113(6): e85–e151.
23. Park MH, Jo SA, Jo I, Kim E, Eun SY, Han C et al. No difference in stroke knowledge between Korean adherents to traditional and western medicine – the AGE study: an epidemiological study. BMC Public Health 2006; 6 : 153–162.
24. Bratina P, Greenberg L, Pasteur W, Grotta JC. Current emergency department management of stroke in Houston, Texas. Stroke 1995; 26(3): 409–414.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2012 Issue 1-
All articles in this issue
- Vertebroplasty – Treatment Option for Structurally Insufficient Vertebras
- Multimodal Imaging with Simultaneous EEG-fMRI
- Sonothrombolysis – Mechanisms of Action and its Use in the Treatment of Stroke
- Fiber Dissection Technique of the Tracts of the Lateral Aspects of the Hemisphere
- Variability of Angiotensinogen and Susceptibility to Multiple Sclerosis
- Carpal Tunel Syndrome and Neurosurgeon – Experience after 2,200 Surgeries
- Visual Functions in Premature Children with Perinatal Brain Injury
- Neurological Occupational Diseases in the Czech Republic in 1994–2009
- Recanalization of Acute Occlusions of Cerebral Arteries Using a Retrievable Stent
- Paraneoplastic Polymyositis – a Case Report
- Guillain-Barré Syndrome in a Patient with a Renal Carcinoma – a Case Report
- Diagnostically Specific Findings in Posturography – Two Case Reports
- Guidelines for Pharmacotherapy of Neuropathic Pain
- Diabetes and Schizophrenia
- Paediatric Intracranial Aneurysms
- Accuracy of Prehospital Diagnosis of Stroke
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Vertebroplasty – Treatment Option for Structurally Insufficient Vertebras
- Carpal Tunel Syndrome and Neurosurgeon – Experience after 2,200 Surgeries
- Guillain-Barré Syndrome in a Patient with a Renal Carcinoma – a Case Report
- Guidelines for Pharmacotherapy of Neuropathic Pain
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

