-
Medical journals
- Career
Urgent Surgical Treatment of GIST of Esophago-Gastric Junction in a Patient with Giant Hiatal Hernia
Authors: Ivan Romic 1; Goran Pavlek 1; Marijan Romic 2; Trpimir Moric 1; Mirna Bajt 3; Petra Puz 4; Dora Grgic 1; Igor Petrovic 1
Authors‘ workplace: Department of Surgery, University Hospital Centre Zagreb, Croatia 1; Department of Surgery, University Hospital Sestre milosrdnice, Croatia 2; Medical School Zagreb, Croatia 4 General Hospital Koprivnica, Croatia 3
Published in: Klin Onkol 2019; 32(4): 306-309
doi: https://doi.org/10.14735/amko2019306Overview
Gastrointestinal stromal tumors (GISTs), being the most common mesenchymal tumors of the gastrointestinal tract, arise most commonly in stomach (60–70%) and small intestine (20–25%) while other sites of origin are rare. In most cases, they are diagnosed accidentally due to their indolent clinical course; however, 10–30% have malignant potential. Gastric and esophageal GISTs carry a better prognosis than small bowel GISTs of similar size and mitotic rate. Complete surgical resection is the only potentially curative procedure, but despite its success, at least 50% of patients develop recurrence or metastases. Tyrosine kinase inhibitor imatinib gave positive results in treatment of unresectable, metastatic or recurrent GISTs. We present the case of a 69-year-old woman with a large unresectable GIST of esophago-gastric junction with multiple bilobar liver metastases who underwent an emergent palliative surgery due to diffuse bleeding from the tumor. Twelve months after the surgery, patient is still alive and stable under imatinib therapy with no signs of local recurrence of the disease. This example suggests that patients with locally advanced GISTs with distant metastases may benefit from surgery in terms of prolonged survival and quality of life.
Keywords:
resection – hiatal hernia – Stomach – GIST
Introduction
Gastrointestinal stromal tumors (GISTs), classified into soft tissue sarcomas according to the National Comprehensive Cancer Network (NCCN) classification, are the most common mesenchymal tumors arising within the wall of the gastrointestinal (GI) tract. GISTs account for 1–3% of all GI malignancies. They occur with almost the same frequency in men and women with an incidence of 2–3/100 000, usually in the sixth decade of life [1].
They arise from the interstitial cells of Cajal, “pace-maker” cells, placed in the muscularis propria of the GI tract, responsible for regulation of peristalsis. They go through “gain of function” mutation of KIT protooncogene responsible for activation of protein CD117, a transmembrane tyrosine kinase growth factor receptor, which leads to proliferation of tumor cells and inhibition of their apoptotic death. Positive immunoreactivity for CD117 is the main parameter which separates GISTs from other mesenchymal tumors of the GI tract and the basis for targeted therapy with tyrosine kinase inhibitors (TKI) (imatinib – Gleevec, sunitinib – Sutent) [1,2].
GIST may appear throughout the entire length of the GI tract, but the most common site of origin is stomach (60–70%), followed by small intestine (20–25%), and rarely colon, rectum, esophagus or appendix. Primary localization is closely related to the clinical manifestations and biological behavior of the tumor. It has been shown that even small GIST of the small intestine is far more likely to grow progressively and disseminate, compared to gastric GIST bigger in size, which more frequently has indolent clinical course and is diagnosed accidentally [2].
It is estimated that only 10–30% of GISTs exhibit their malignancy, where the main predictors of such behavior are the number of mitosis and the size of tumor. In contrast to other soft tissue tumors, most metastasis of GIST are liver metastasis or peritoneal seeding, while lymph node involvement is rare, occurring in only 0–8% cases [2,3].
Since GIST develops as a submucosal and intramural process, it takes some time for it to become clinically expressed, while 10–30% of patients may be completely asymptomatic. When symptoms do appear, the most common are hematemesis or melena in 40–65% of patients. Bleeding occurs because of pressure necrosis and ulceration of the overlying mucosa with resultant hemorrhage from disrupted vessels. Obstructive symptoms are usually site-specific and can result from intraluminal growth of an endophytic tumor or from extraluminal compression from an exophytic lesion. Other common symptoms include abdominal pain, anemia, anorexia, nausea, vomiting, weight loss and dysphagia [2,4,5].
The “gold standard” for therapy in GIST is complete surgical resection of localized tumor; it offers the only chance for cure from GIST. Even after complete resection of primary GIST, at least 50% of patients develop recurrence or metastasis in the first 2 years. Locally recurrent tumors are not usually suitable for complete resection because of peritoneal implantation. Survival after complete surgical resection ranges from 48 to 80% at 5 years. If resection is not complete, only 9% of patients survive for an average of 12 months. Imatinib mesylate is the first effective drug with response rate of 54% in the treatment of metastatic GIST; nowadays, it is the drug of choice for unresectable, metastatic, or recurrent GISTs [2,4].
Case report
A 69-year-old woman was referred to our surgical department due to the GI stromal tumor which was found 2 months earlier on upper endoscopy (Fig. 1). Initial symptoms included epigastric pain, dysphagia and rapid weight loss. Computed tomography scan showed multiple bilobar liver metastases and a large 8.2 × 11.1 cm tumor located in esophago-gastric junction (Fig. 2). Cardia and most of the fundus were displaced intrathoracally due to the large sliding hiatal hernia. The tumor was considered inoperable initially, and chemotherapy with imatinib was started. After four cycles of chemotherapy, patient developed mycrocitic anemia which could not be managed by blood transfusions. Endoscopy confirmed diffuse bleeding from the tumor. Considering this, we indicated urgent surgical exploration and planned a palliative gastric resectional procedure. Intraoperatively, there was a large hiatal hernia and an expansive tumor at the gastroesophageal junction which infiltrated the edges of hiatal orifice, cardia and upper part of fundus (Fig. 3a). After the partial resection of the diaphragm, we performed proximal esophago-gastrectomy with esophago-gastric anastomosis on the middle part of stomach corpus (Fig. 3b). Surprisingly, 12 months after the treatment, the patient was still alive and there were no signs of local (gastric) tumor recurrence while hepatic metastases remained stable under imatinib therapy.
1. Endoscopic appearance of gastrointestinal stromal tumor. 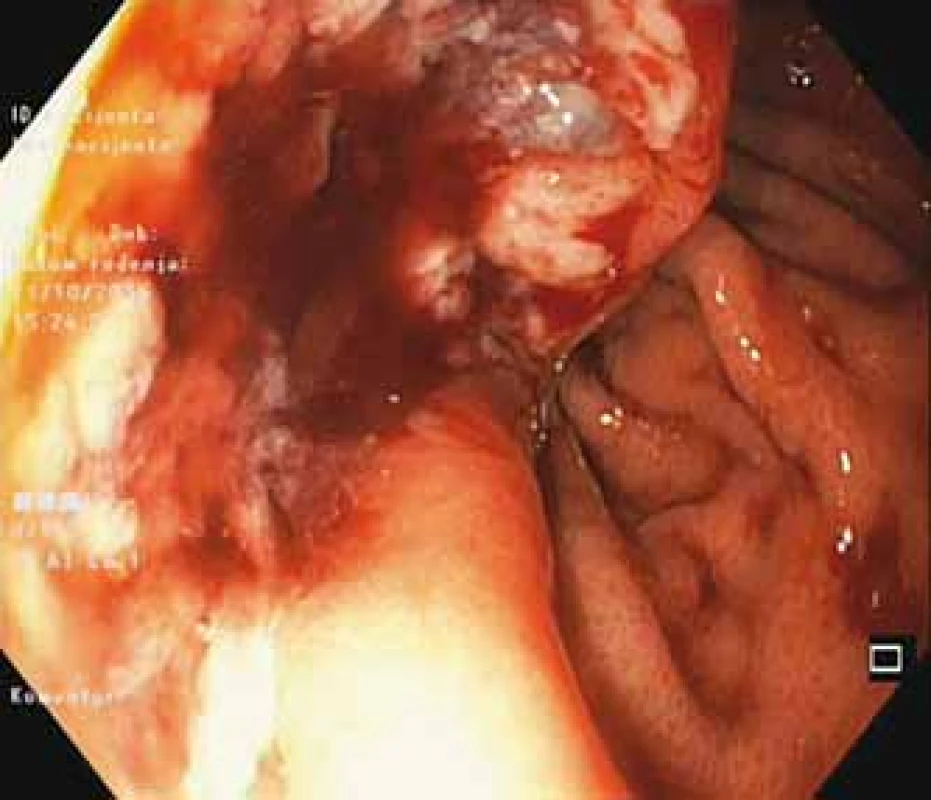
2. Positron emission tomography scan showing stomach gastrointestinal stromal tumor and liver metastases. 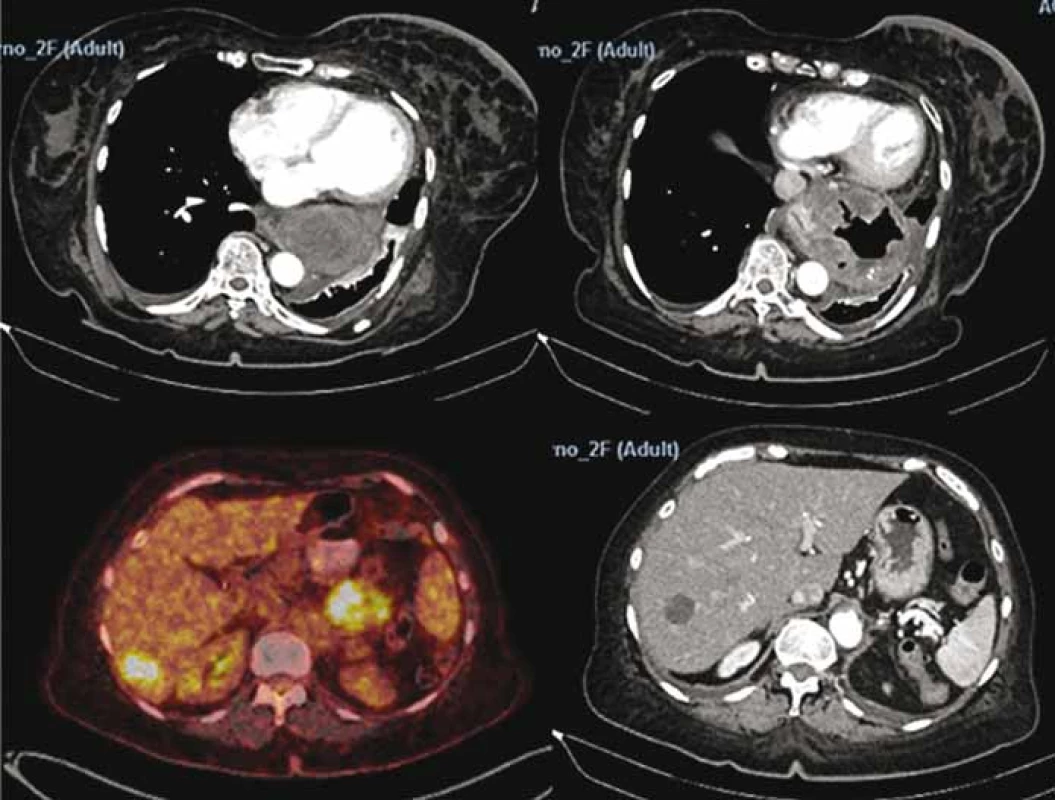
3. A. Intraoperative photo showing gastrointestinal stromal tumor and diaphragmatic crus. 3B. Specimen after proximal gastrectomy showing “en-block” tumor and esophago-gastric junction. 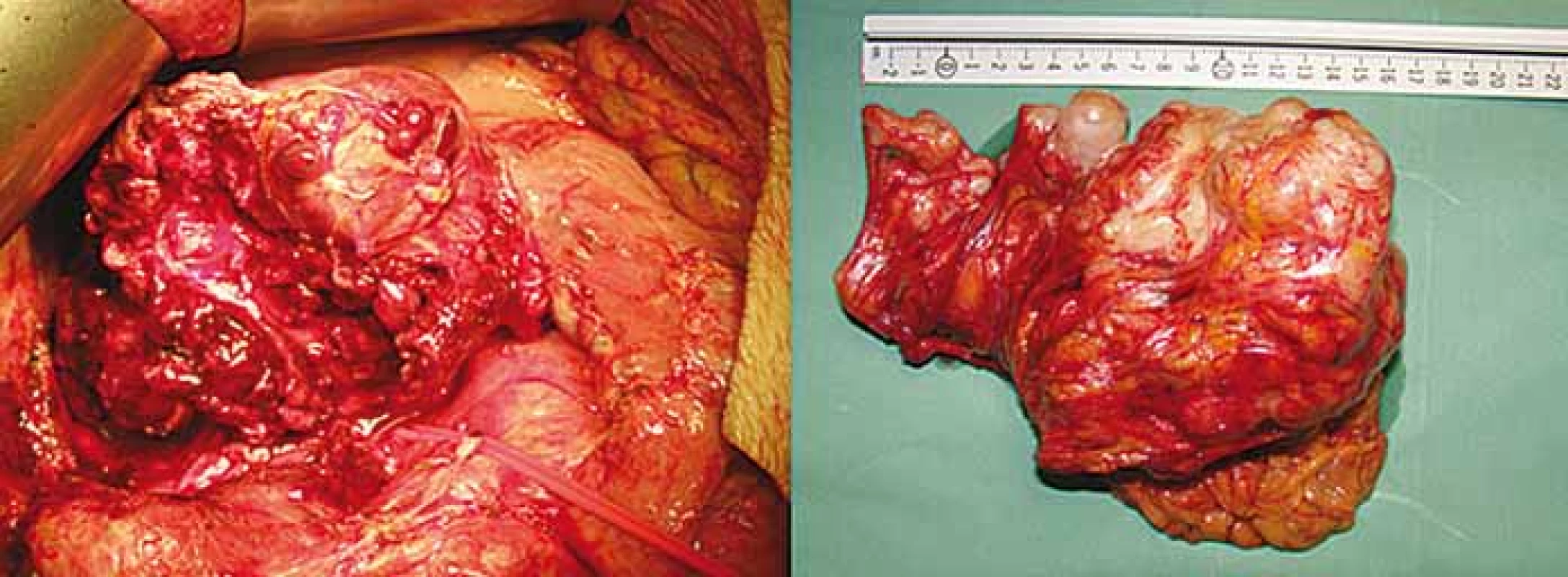
Discussion
GISTs are the most common mesenchymal tumors of the GI tract and account for about 1–3% of all GI malignancies. In total, 60–70% of GISTs are gastric neoplasms, from which 15% affect cardia and fundus, 70% body and 15% antrum. Small intestine is the second most common site of origin in about 20–30% of cases, mainly in jejuno-ileum. Other literature supports the fact that tumors at other primary sites are very rare, 5–15% in colon and rectum, and less than 5% in the esophagus. Several cases of other locations have also been reported [6,7].
Currently available literature presents only few case reports of GIST in the esophago-gastric junction, while others classify them among gastric or esophageal GISTs without precise anatomical localization. Concurrent involvement of stomach and esophagus, as presented in our case, is extremely rare. One paper gives an overview of seven case reports of GIST in hiatal hernia, and several ones are related to spontaneous bleeding of the gastric GIST, either caused by spontaneous rupture of giant gastric GIST or intratumoral bleeding due to necrosis. Symptoms of lumen obstruction due to large extraluminal expansive process have also been reported, as GISTs may vary in size from 1 to 40 cm in diameter. Our case report sums up all of those unconventional presentations of GIST and puts them into context of clinical emergency in an inoperable disease, as that is the point in which subject of GIST treatment becomes a bit controversial [8,9].
NCCN guidelines put surgery as the initial treatment for a localized and resectable disease. The goal should be complete resection with an intact pseudocapsule. Compared to other stomach malignancies, there is no indication for routine lymphadenectomy due to low frequency of lymph node involvement.
Speaking of advanced GIST, in the last few years, surgical treatment has gone through many improvements since primary chemotherapy and neoadjuvant therapy with TKI gave possibility to make locally advanced primary GIST resectable, allowing for less invasive procedures or promoting preservation of function.
TKI (imatinib, sunitinib, and regorafenib) target KIT and/or platelet-derived growth factor receptors and they are indicated for unresectable, metastatic or recurrent GISTs. With regards to adjuvant therapy, oncologists reserve imatinib for those patients who meet criteria for “high-risk” based on tumor size and mitotic index.
It is thought that surgery may have a limited positive impact on progression-free survival and overall survival in cases of good response to imatinib therapy, or those with limited focal progression. Thus, at present, surgery may be discussed with the patient, but not recommended based on a definitive proof of benefit. The case of our patient favors performing surgery even in stage IV disease with hepatic metastases as it turned out to be a good emergent and palliative procedure. Patient is alive 12 months after the procedure and stable under imatinib therapy without signs of recurrence. For patients who do not respond to imatinib therapy, surgery should not be offered, unless as a palliative intervention. In those cases, it is recommended to give sunitinib as the drug of second and regorafenib as the 3rd line of choice [7,9]. Major causes of such drug resistance are additional mutations (primary or secondary) in the kinase domains of the KIT genes [10].
The primary location of GIST presented in our case is esophago-gastric junction. It is shown that gastric and esophageal GISTs have better prognosis than GISTs of non-gastric origin. One study concluded that in both gastric and non-gastric group, the size of tumor and mitotic activity were the most important prognostic factors related to progression-free survival. Risk of progression for GISTs larger than 10 cm with mitotic index less than 5/50 high-power field is 10% for gastric and 34–57% for GISTs of other origin. For GISTs larger than 10 cm and mitotic index above 5/50 HPF, risk of progression is equally high (86%) regardless of localization.
Even though some other papers gave opposed results regarding anatomic localization being a prognostic factor for survival independent of tumor size, more of them represented thesis that patients with small intestinal GISTs had poorer outcomes than those with gastric tumors [7,9]. GISTs are 4 times more likely to recur if the primary site is the intestine compared to stomach. These data should be considered when evaluating success of surgical resection of recurrent tumors since the primary site could potentially condition the ultimate outcome in form of overall survival and recurrence-free survival [10–15].
Conclusion
Our case confirms that patients with locally advanced, stage IV disease with hepatic metastases may benefit from surgery in terms of prolonged survival and quality of life. We also showed that urgent surgery for GIST complications may not just be a life-saving surgery, but also a good palliation procedure and, as our case shows, it may be a beneficial R2 resection which allows normal feeding and prevents fatal consequences of tumor bleeding. Consequently, it affects overall survival and symptoms-free period. It must also be emphasized that this success would not be possible without positive response on imatinib therapy which could have been facilitated with “debulking” effect of our surgery.
Hence, we consider that treatment options of stomach GIST, even in advanced stages, should be interpreted differently than other stomach malignancies considering different pathological and oncological nature of tumors. Multidisciplinary and individualized approach is mandatory as well as meticulous pre-and postoperative planning and adequate follow-up plan.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Ivan Romic, M.D.
Department of Surgery
University Hospital Centre Zagreb
Kišpatićeva ul. 12
10000 Zagreb, Croatia
e-mail: i.romic@gmail.com
Submitted: 10. 3. 2019
Accepted: 14. 5. 2019
Sources
1. Al-Thani H, El-Menyar A, Rasul KI et al. Clinical presentation, management and outcomes of gastrointestinal stromal tumors. Int J Surg 2014; 12 (10): 1127–1133. doi: 10.1016/j.ijsu.2014.08.351.
2. Machishi H, Okada Y, Nagai M et al. A rare case of huge gastrointestinal stromal tumor (GIST) of the stomach extending into the posterior mediastinum. Dig Dis Sci 2002; 47 (7): 1492–1497.
3. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006; 130 (10): 1466–1478. doi: 10.1043/1543-2165 (2006) 130.
4. Nishida T, Blay JY, Hirota S et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016; 19 (1): 3–14. doi: 10.1007/s10120-015-0526-8.
5. Demetri GD, von Mehren M, Blanke CD et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347 (7): 472–480. doi: 10.1056/NEJMoa020461.
6. Mullady DK, Tan BR. A multidisciplinary approach to the diagnosis and treatment of gastrointestinal stromal tumor. J Clin Gastroenterol 2013; 47 (7): 578–585. doi: 10.1097/MCG.0b013e3182936c87.
7. Melo C, Canhoto C, Manata F et al. Surgical treatment of giant gist with acute gastrointestinal bleeding: Case report. Int J Surg Case Rep 2018; 53 : 354–357. doi: 10.1016/j.ijscr.2018.11.021.
8. Liu Q, Kong F, Zhou J et al. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res 2018; 10 : 735–743. doi: 10.2147/CMAR.S159689.
9. Ford SJ, Gronchi A. Indications for surgery in advanced/metastatic GIST. Eur J Cancer 2016; 63 : 154–167. doi: 10.1016/j.ejca.2016.05.019.
10. Schwameis K, Fochtmann A, Schwameis M et al. Surgical treatment of GIST – an institutional experience of a high-volume center. Int J Surg 2013; 11 (9): 801–806. doi: 10.1016/j.ijsu.2013.08.016.
11. Akahoshi K, Oya M, Koga T et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018; 24 (26): 2806–2817. doi: 10.3748/wjg.v24.i26.2806.
12. Fujisawa R, Akiyama Y, Iwaya T et al. Giant gastrointestinal stromal tumor of the mediastinum associated with an esophageal hiatal hernia and chest discomfort: a case report. Surg Case Rep 2018; 4 (1): 144. doi: 10.1186/s40792-018-0553-x.
13. Mehta RM, Sudheer VO, John AK et al. Spontaneous rupture of giant gastric stromal tumor into gastric lumen. World J Surg Oncol 2005; 3 (1): 11. doi: 10.1186/1477-7819-3-11.
14. Včev A et al. Gastrointestinalni stromalni tumor (GIST). Med Vjesn 2003; 35 (1–4): 55–58.
15. Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother 2014; 15 (14): 1979–1989. doi: 10.1517/14656566.2014.937 707.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2019 Issue 4-
All articles in this issue
- Current Perspective on HPV-Associated Oropharyngeal Carcinomas and the Role of p16 as a Surrogate Marker of High-Risk HPV
- Role of the Microbiome in the Formation and Development of Colorectal Cancer
- Methods of Classical and Molecular Cytogenetics Suitable for Biodosimetry of Persons with Professional Exposure to Carcinogens
- BMI and Odds of Endometrial Adenocarcinoma in Czech Women – a Case Control Study
- The Pharmacoeconomic Analysis of Cetuximab and Panitumumab in the 1st Line Treatment of mCRC in Real Clinical Practice in the Czech Republic
- Incidence and Risk Factors of Distant Metastases of Head and Neck Carcinoma
- Oncology Case Report – When Is the Appropriate Time to Integrate Palliative Care?
- Farmakoekonomické studie a procesy HTA při hodnocení nákladů a benefitů nákladné inovativní léčby u nás i ve světě
- Aplikovat jeden cyklus nebo jednu sérii chemoterapie?
- Zemřel prof. MUDr. Josef Koutecký, DrSc., zakladatel dětské onkologie
- Onkologie v obrazech Kožní toxicita při cílené léčbě generalizovaného melanomu
- P21-Associated ncRNA DNA Damage-Activated Expression in Bladder Cancer
- The Role of Radiotherapy in Skull Metastasis of Thyroid Follicular Carcinoma
- Urgent Surgical Treatment of GIST of Esophago-Gastric Junction in a Patient with Giant Hiatal Hernia
- Proposed Strategies for Improving Adherence to Tyrosine Kinase Inhibitors in Patients with Chronic Myeloid Leukaemia
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Role of the Microbiome in the Formation and Development of Colorectal Cancer
- Current Perspective on HPV-Associated Oropharyngeal Carcinomas and the Role of p16 as a Surrogate Marker of High-Risk HPV
- Oncology Case Report – When Is the Appropriate Time to Integrate Palliative Care?
- Incidence and Risk Factors of Distant Metastases of Head and Neck Carcinoma
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

