-
Medical journals
- Career
Screening of Psychological Distress 4.5 Years after Diagnosis in Breast Cancer Patients Compared to Healthy Population
Authors: T. Sverak 1; K. Skrivanova 2; L. Anderkova 3,4; M. Bendová 5; D. Brancikova 6; N. Elfmarkova 3,4; H. Peterkova 2; Jiří Jarkovský 7; K. Benesova 7; L. Minar 5; L. Dusek 7; J. Nedvěd 8; M. Protivánková 6
Authors‘ workplace: Department of Psychiatry, Faculty of Medicine, Masaryk University, Brno, Czech Republic. 1; Department of Psychology and Psychosomatics, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Applied Neuroscience Research Group, CEITEC – Central European Institute of Technology, Masaryk University, Brno, Czech Republic 3; First Department of Neurology, Faculty of Medicine, Masaryk University and St. Anne’s University Hospital Brno, Czech Republic 4; Department of Gynecology and Obstetrics, Faculty of Medicine, Masaryk University and University Hospital Brno, Czech Republic 5; Department of Internal Medicine, Hematology and Oncology, Faculty of Medicine, Masaryk University and University Hospital Brno, Czech Republic 6; Institute of Biostatistics and Analyses, Faculty of Medicine and Faculty of Science, Masaryk University, Brno, Czech Republic 7; Psychological ambulance PhDr. Dagmar Konecna s. r. o., Olomouc, Czech Republic 8
Published in: Klin Onkol 2016; 29(3): 210-215
Category: Original Articles
doi: https://doi.org/10.14735/amko2016210Overview
Background:
Survival rate of breast cancer patients has improved significantly in recent years. Cancer diagnosis represents a great psychological distress for patients which may not stem solely from the disease itself. Patients may experience higher distress even several years after treatment.Patients and Methods:
The study was carried out at the Department of Obstetrics and Gynecology and Department of Internal Medicine, Haematology and Oncology, Faculty Hospital Brno. Results of 85 patients at 4.5 years after diagnosis of breast cancer compared to 72 healthy controls are presented in this paper. The data were collected in the form of semi-structured interviews, from the patients’ medical records and by Symptom Check List-90.Results:
The overall rate of psychological distress (GSI) 4.5 years after breast cancer diagnosis does not differ significantly (p = 0.703) from the healthy population. Also, we did not find any statistically significant relationship between the observed factors and the level of psychological distress in breast cancer patients.Conclusion:
Screening investigation showed no difference in the psychological distress in breast cancer patients 4.5 years following diagnosis, compared with the healthy population.Key words:
breast cancer – psychological stress – screening
The incidence of breast cancer diagnoses increases in the Czech Republic every year [1]. Cancer diagnosis represents a great psychological distress for patients and may not stem solely from the disease itself. Many distress factors can emerge afterwards, such as the fear of death or relapse. Patients may also suffer from a damaged body image, changes in their femininity, sexuality and attractiveness. These factors cause psychological distress, which may predispose the affected person to mental disorders, including depression and anxiety, even many years after treatment [2 – 4]. These mental disorders and high psychological distress also reduce a patient’s quality of life [5 – 7].
Grabsch et al. [6] observed a 42% incidence of psychiatric disorders in breast cancer patients (97 of 227). Reich and Lesur [2] noted a significantly higher prevalence of depression and mood disorders in the meta-analysis, ranging from 16% to 60%. Thus, the exact designation of the prevalence of psychiatric disorders in breast cancer patients is still quite difficult. Unfortunately, there are several factors which affect the patients’ condition and their level of psychological distress.
These factors can be divided into three categories. The first category of factors includes factors relating to the patients themselves. The next category describes the extent, severity and other factors relating to the individual disease, and the last category includes factors relating to the treatment of the disease [8]. Some authors [8,9] conclude that the risk factors affecting the level of psychological distress and the prevalence of anxiety and depression are more closely related to the patient, rather than the manner of treatment or condition of the disease.
Among the factors that increase psychological distress and are associated with the patient are low self-esteem, low emotional support, poor body image [10], the patient’s perception of his or her prognosis [11] and anxiety level as a personality trait [12]. Cohen et al. [13] found negative correlation between age and psychological distress. Older women had lower level of psychological distress than younger women. Bleiker et al. [3] also described the intrusive thoughts related to the illness and anxiety as factors of similar importance. According to his study, these intrusive thoughts together with a patient’s health problems and sleep disorders can be treated as the best predictors of the prevalence of psychological distress. Also, the diagnosis of depression or psychiatric illness before the cancer diagnosis increases the level of psychological distress [14]. It is still unknown what the influence of stressful events is before the disease. Some studies have asserted the significance of such events, especially in the number of stress episodes [14,15], while other studies found no connection between their number and disease [3]. More studies are needed to determine the effect of stressful events on psychological distress and the disease. The influence of surgical treatment, such as mastectomy compared to lumpectomy, on the psychological distress is still disputable. Some literature is inclined to state that there are no significant differences in psychological distress between these two methods [16,17]. Other literature sources find higher psychological distress in younger women (> 50) who received mastectomy. However, for older women (< 50), it was less stressful to receive mastectomy than lumpectomy [13]. Adjuvant chemotherapy may entail more side effects (such as hair loss, digestive problems etc.), which are associated with higher psychological distress and higher prevalence of depression and anxiety disorders [11].
This article maps psychological distress in breast cancer patients and compares it with healthy controls.
Sample and methods
Sample
The study was carried out at the Department of Obstetrics and Gynecology and the Department of Internal Medicine, Haematology and Oncology, Faculty Hospital Brno of the University Hospital Brno (FNB). The inclusion criteria were age (30 to 90 years) and patient history with histologically confirmed breast cancer. The inclusion criteria for healthy controls were also age (30 to 90 years) and no oncological or psychiatric diagnosis.
Overall, 172 breast cancer patients and 80 healthy controls participated in the study. The completed data from 85 breast cancer patients and from 72 healthy volunteers are presented here. Refusal rate was less than 1%. The average time from diagnosis to the participation in the study was 4.5 years. These data are part of a larger four-year running project aimed at measuring quality of life, psycho-neuroimmunologic markers and coping strategies related to Type C coping behavior in breast cancer patients. The study was approved by the Ethics Committees of the Faculty of Medicine and FNB, and informed consent was obtained from every patient beforehand.
Methods
The anamnestic data were collected at the clinic in the form of semi-structured interviews with qualified psychologists and by Symptom Check List-90 (SCL-90) inventory completed by the patients. Clinical oncologists and onco-gynecologists completed a questionnaire about the patient‘shealth status according to their medical records. The anamnestic data from healthy controls were collected by structured questionnaires, which were created on the basis of semi-structured interview.
In the semi-structured interview for patients and in the structured questionnaire for healthy controls, there were questions about socio-demographic and lifestyle-related factors, such as age, educational level, employment status, monthly income, residence size, marital status, number of children, age at first birth, age at menarche, duration of breastfeeding, smoking habit, spirituality and others.
The questionnaire on the health status of patients was focused on factors such as BMI, time of diagnosis and start of treatment, menopausal status, currently used medication, histological type of cancer and its stage, which kind of therapies were used (surgery, chemotherapy, radiotherapy, hormonal biological therapy), disease relapse, co-morbid diseases and therapeutic response.
SCL-90 is a self-report inventory with 90 items for assessing the nine basic dimensions of symptoms (psychoticizm, paranoid thoughts, obsessive-compulsive symptoms, interpersonal sensitivity, hostility, anxiety, phobias, depression, and somatization). Symptom index (GSI) is created by averaging all 90 items and is considered a sensitive indicator of general psychological distress [18,19]. SCL-90 works on the basis of a five-point Likert scale, where the participant assesses whether and how much he/ she suffers from the mentioned symptoms (0 = never, 4 = strongly). Participants were given the Czech version of this checklist [20,21].
Statistical analysis
Analysis of the data utilized descriptive and comparative statistics. For categorical variables, absolute and relative frequencies, standard deviation (SD) or median complete the 5th and 95th percentile for continuous variables and scores were used. Statistical significance between groups of patients were tested using Man-Whitney U, Fisher‘s exact test and the Kruskal-Wallis test. The significance level α was set at 0.05 for all of the above tests. Statistical data processing and analysis was carried out using statistical software SPSS version 22 (IBM Corporation, 2013).
Results
Description of the sample
The average age was 63 for patients and 67 for healthy controls. Seventy--eight (91.8%) patients had children, which is nearly the same as the healthy controls (71; 98.6%; p = 0.071). The number of patients who breastfed was also nearly equal to the number of healthy controls (90.3% vs. 82.4%; p = 0.173). No differences were found in either the number of smokers (18.8% vs. 18.1%; p = 0.999) and in the highest level of education achieved (p = 0.052). No difference between groups was found in marital status (p = 0.5), when most women were still married (54.1% of patients and 59.7% of healthy controls). The only statistically significant difference was found in employment status, in which cancer patients were receiving disability pension (20% vs. 0%) and healthy controls were more frequently employed (37.5% vs. 5.9%). Differentiation between the descriptive data of patients and that of healthy controls was minimal. Detailed basic, sociodemographic characteristics of the sample are presented in Tab. 1.
1. Basic characteristics of the sample. 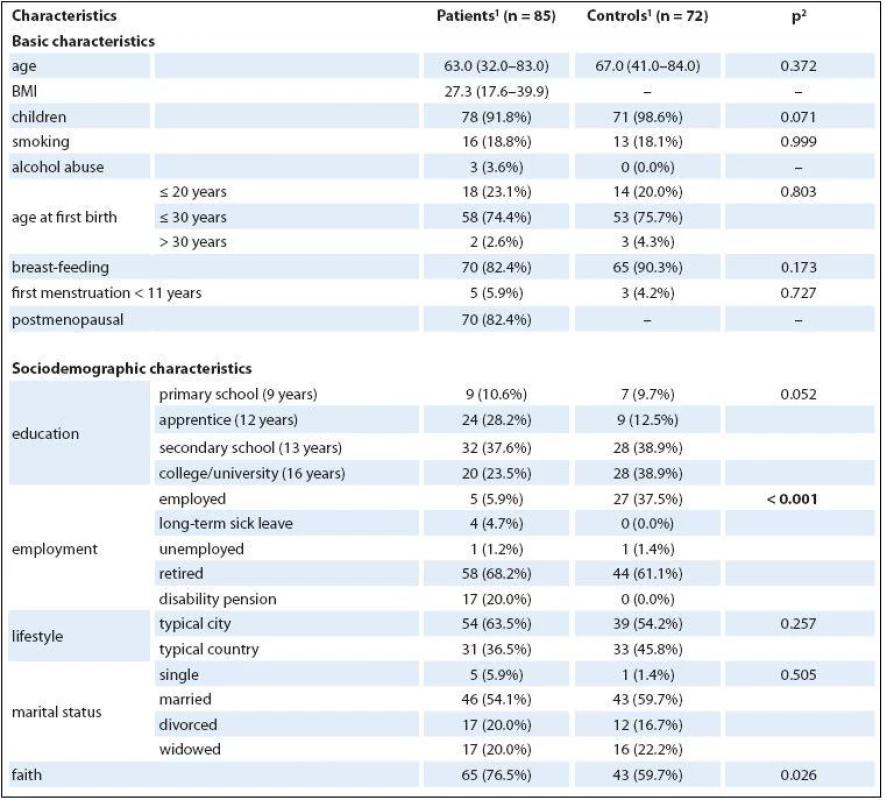

<sup>1</sup> median (min.–max.) or n (column %) <sup>2</sup> p-value of Mann-Whitney U test for continuous variables and p-value of Fisher’s exact test for categorical variables Medical characteristics of the patients
Patients were most often in stage II – III of cancer (51; 61.4%), fewer patients were in stage I (22; 26.5%) and stage IV was the least common (10; 12%). Seventy-nine patients (92.9%) underwent surgery intervention: 61 (71.8%) chemotherapy, 70 (82.4%) radiotherapy, 19 (22.4%) biological therapy, and 70 (82.4%) hormonal therapy. Relapse had been experienced by 20 patients (24.7%). More medical characteristics of the patients are presented in Tab. 2.
2. Medical characteristics of the patients. 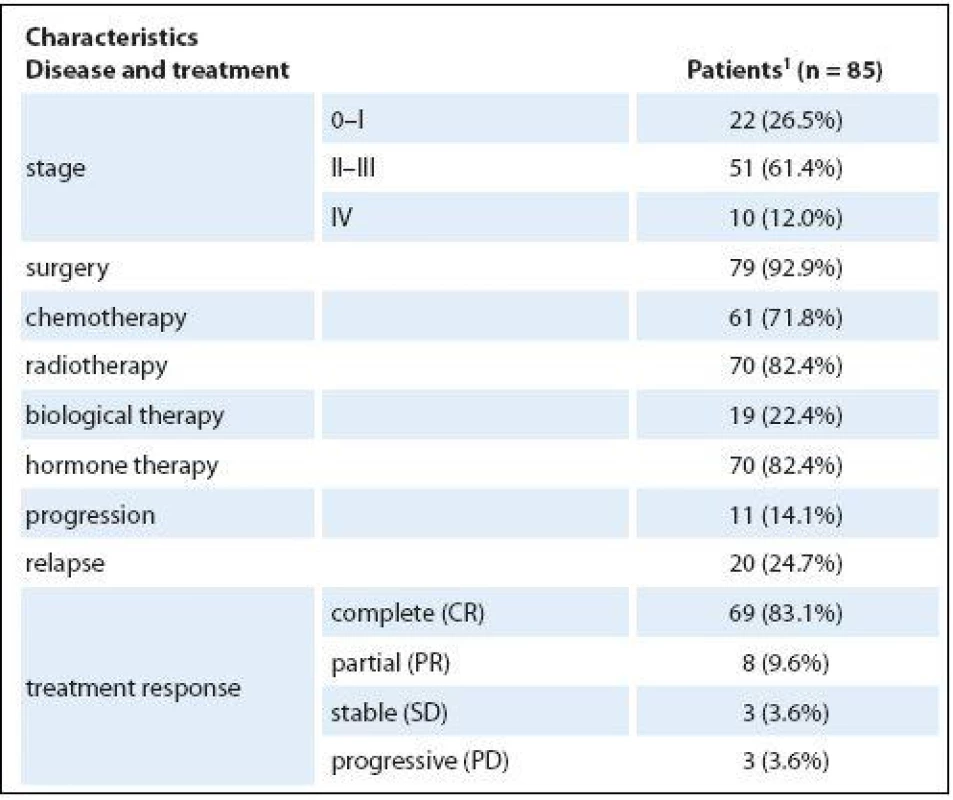
1 median (min.–max.) or n (column %) Psychological distress
The age of patients did not affect significantly the level of psychological distress (p = 0.602), though the younger patients (less than 65 years) achieved higher score (57.8 points) in psychological distress than older woman (> 65 years; 54.6 points). There was no significant effect of the stage of the cancer (p = 0.455). The highest level of distress was reached by patients in stage I (58.1 points), followed by the patients in stages II and III (56.3), and finally in stage IV (52.6). The current level of psychological distress exhibited a negligible effect from the experience of a significant stressful situation in the last five years (p = 0.712). Increased, but still not statistically significant, values of psychological distress (60.1 vs. 54.9 points; p = 0.212) were observed in patients with history of psychological or psychiatric problems compared with patients without such history. The level of distress was not affected by whether patients underwent chemotherapy or not (55.3 vs. 56.7; p = 0.910). Slightly increased values of distress were reported by patients who had suffered a relapse compared to patients who had not (58.7 vs. 55.4; p = 0.280). No differences were found (p = 0.957) between the patients with medical comorbidities and those without.
A slightly higher level of distress was experienced by patients living at home alone compared to patients living with their husbands or their children in a shared household (57.7 vs. 55.8; p = 0.257). The same results were found in the healthy population (58.9 vs. 53.5; p = 0.109), so the differences between them and patients are therefore not statistically significant (p = 0.906 for patients and controls who live alone; p = 0.555 for patients and controls who live with someone).
No significant differences at each SCL-90 scale were found between patients and healthy controls (Tab. 3), and the scale that dealt with anxiety was the only one to exhibit any increase (p = 0.053). The differences in GSI (56.3 vs. 54.8) were not statistically significant between patients and healthy controls (p = 0.703).
3. Comparison of psychopathology between patients and healthy controls. 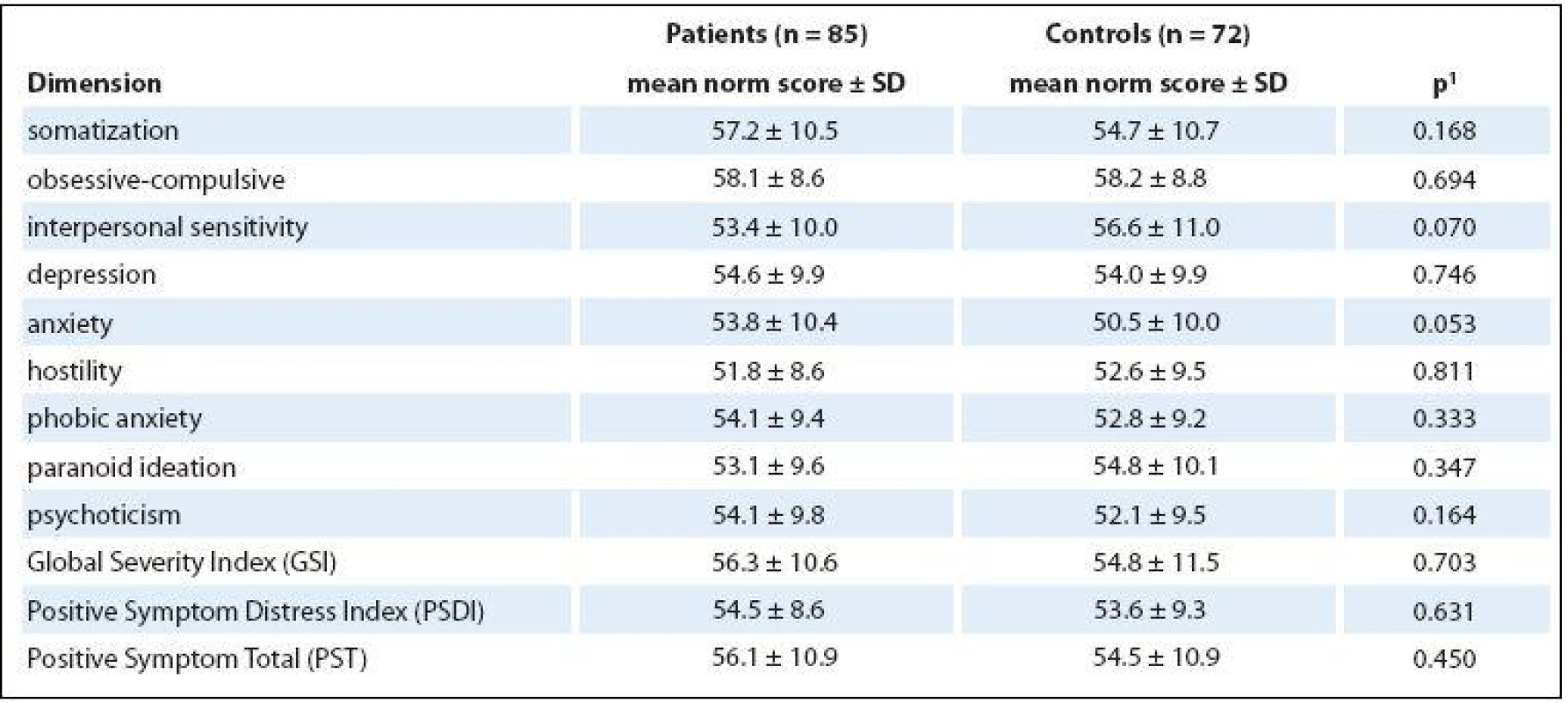
1 p-value of Mann-Whitney U test Limits of the study
One limit of our study is that psychological distress was measured only by one screening. A better way for future research could be use of more methods, such as other questionnaires or structured diagnostic interview.
Another limit is that we did not specify surgical intervention or the type of chemotherapy. These variables could be another potential indicators of the level of psychological distress and they could play an important role in the patient’s recovery.
Important limit is also the size of the research sample, when we did not have fully relevant representation of the patients in the subgroups, which could affect our statistical results.
Discussion
We did not observe any significant differences in psychological distress in breast cancer patients compared to healthy controls after an average of 4.5 years after diagnosis, nor did we find any significant factor that could directly affect psychological distress. Based on our inconclusive results, we can say that the level of psychological distress could be reduced over time after diagnosis, which is consistent with the results of Burgess et al. [22], where the rate of depression and anxiety in breast cancer patients progressively decreased every year after diagnosis. As is mentioned in the paper by Burgess et al, prevalence of depression and anxiety or both is twice as high in breast cancer patients than in the general female population.
Unusually, recent articles [13,23] suggested that older patients have lower level of psychological distress or better adjustment than younger woman. According to our results, we did not find significant differences relative to age, but younger women had higher score in psychological distress than older woman. One possible explanation of these results is that our cut-off for patients comparison was 65 years due to the balancing of our sample (46 patients under 65 vs. 39 patients over 65) while other studies used a different cut-off (such as 50) or they used different statistical methods since they had larger cohorts than our research group. In contrast to previously published reports [24], we found that patients in stage I had the highest score of psychological distress. These results could be distorted because of different time from diagnosis, when stage I is an early stage of the cancer and patients in this stage were diagnosed recently compared to patients in other stages. Some other studies also found that patients in early stages of cancer suffer moderate to severe psychological distress, even if they have relatively good prognosis [3,25].
However, major depressive disorders, anxiety, depressive symptoms and higher psychological distress in breast cancer patients are often underestimated and untreated [26,27]. One reason may be that doctors treat depressive and anxiety symptoms as a natural reaction to a serious illness or neuro-vegetative symptoms (weight loss, sleep disturbances, fatigue). Cognitive and emotional symptoms could also be interpreted as manifestations of the disease itself and not the depression [28]. High psychological distress, anxiety and depression are associated with reduction in quality of life, disruption of compliance and shorter survival time [2,29]. It also affects the function of the endocrine and immune systems, which affects the resistance of the body during tumor progression [28].
This is, however, only a basic screening which could not generate all important information about patients, such as an objective assessment of their psychological condition. As such, it would be useful to do a prospective study focused on the experience of psychological distress in patients with breast cancer directly following diagnosis.
In conclusion, we did not observe any significant differences in psychological distress in breast cancer patients compared to healthy controls after an average of 4.5 years after diagnosis, nor did we find any significant factor that could directly affect psychological distress. We can say that the level of psychological distress could be reduced over time following diagnosis.
We deeply thank the participants for their commitment to our research project.
This study was supported by a grant P407/ /12/0607 Czech Science Foundation.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Mgr. Katerina Skrivanova, PhD
Department of Psychology
and Psychosomatics
Faculty of Medicine
Masaryk University
Kamenice 126/3
625 00 Brno
Czech Republic
e-mail: kskrivan@med.muni.cz
Submitted: 6. 9. 2015
Accepted: 5. 10. 2015
Sources
1. Mužík J, Dušek L, Abrahámová J et al. Stručný přehled epidemiologie zhoubného novotvaru prsu v České republice. Onkologie 2009; 3(1): 7 – 11.
2. Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat 2008; 110(1): 9 – 17.
3. Bleiker EM, Pouwer F, van der Ploeg HM et al. Psychological distress two years after diagnosis of breast cancer: frequency and prediction. Patient Educ Couns 2000; 40(3): 209 – 217.
4. Hegel MT, Moore CP, Collins ED et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer 2006; 107(12): 2924 – 2931.
5. Bloom JR, Steward SL, Chang S et al. Then and now: quality of life of young breast cancer survivors. Psychooncology 2004; 13(3): 147 – 160.
6. Grabsch B, Clarke BM, Love A et al. Psychological morbidity and quality of life in women with advanced breast cancer: a cross-sectional survey. Palliat Support Care 2006; 4(1): 47 – 56.
7. Mols F, Vingerhoets AJ, Coebergh JW et al. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer 2005; 41(17): 2613 – 2619.
8. Burgess C, Cornelius V, Love S et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 2005; 330(7493): 702.
9. Bardwell WA, Natarajan L, Dimsdale JE et al. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol 2006; 24(16): 2420 – 2427.
10. Simpson JS, Carlson LE, Beck CA et al. Effects of a brief intervention on social support and psychiatric morbidity in breast cancer patients. Psychooncology 2002; 11(4): 282 – 294.
11. Hinz A, Krauss O, Hauss JP et al. Anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care (Engl) 2010; 19(4): 522 – 529. doi: 10.1111/ j.1365-2354.2009.01088.x.
12. Ando N, Iwamitsu Y, Kuranami M et al. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics 2011; 52(1): 56 – 64. doi: 10.1016/ j.psym.2010.11.012.
13. Cohen L, Hack TF, de Moor C et al. The effects of type of surgery and time on psychological adjustment in women after breast cancer treatment. Ann Surg Oncol 2000; 7(6): 427 – 434.
14. Maunsell E, Brisson J, Descheněs L. Psychological distress after initial treatment of breast cancer. Assessment of potential risk factors. Cancer 1992; 70(1): 120 – 125.
15. Kornblith AB, Herndon JE, Zuckerman E et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer 2001; 91(2): 443 – 454.
16. Fallowfield LJ, Hall A, Maguire GP et al. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ 1990; 301(6752): 575 – 580.
17. Gotay CC, Muraoka MY. Quality of life in long-term survivors of adult-onset cancers. J Natl Cancer Inst 1998; 90(9): 656 – 667.
18. Degoratis LR, Savitz KL. The SCL-90-R and Brief Symptom Inventory (BSI) in primary care. In Handbook of psychological assessment in primary care settings. Mahwah, NJ: U.S. Lawrence Erlbaum Associates Publishers 2000 : 848.
19. Hardt J, Gerbershagen HU, Franke P. The symptom check-list, SCL-90-R: its use and characteristics in chronic pain patients. Eur J Pain 2000; 4(2): 137 – 148.
20. Filip V et al. Praktický manuál psychiatrických posuzovacích stupnic. Praha: Psychiatrické centrum 1997 : 213.
21. Bieščad M, Szeliga P. Overenie konštruktovej validity sebaposudzovacej škály Symptom Checklist-90 (SCL-90). Československá psychologie 2005; 49(4): 342 – 356.
22. Burgess C, Cornelius V, Love S et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 2005; 330(7493): 702 – 706.
23. McCaul KD, Sandgren AK, King B et al. Coping and adjustment to breast cancer. Psychooncology 1999; 8(3): 230 – 236.
24. Iwatani T, Matsuda A, Kawabata H et al. Predictive factors for psychological distress related to diagnosis of breast cancer. Psychooncology 2013; 22(3): 523 – 529.
25. Culver JL, Arena PL, Antoni MH et al. Coping and distress among women under treatment for early stage breast cancer: comparing african americans, hispanics and non-hispanic whites. Psychooncology 2002; 11(6): 495 – 504.
26. Fann JR, Thomas-Rich AM, Katon WJ et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry 2008; 30(2): 112 – 126. doi: 10.1016/ j.genhosppsych.2007.10.008.
27. Ashbury FD, Madlensky L, Raich P et al. Antidepressant prescribing in community cancer care. Support Care Cancer 2003; 11(5): 278 – 285.
28. Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003; 54(3): 269 – 282.
29. Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc 2004; 52(1): 106 – 111.
30. Dorval M, Maunsell E, Deschênes L et al. Type of mastectomy and quality of life for long term breast carcinoma survivors. Cancer 1998; 83(10): 2130 – 2138.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2016 Issue 3-
All articles in this issue
- History of Oncology in Slovakia
- Lynch Syndrome – the Pathologist’s Diagnosis
- Current Possibilities for Predicting Responses to EGFR Blockade in Metastatic Colorectal Cancer
- Changes in the CD8+ Density of Tumor Infiltrating Lymphocytes after Neoadjuvant Radiochemotherapy in Patients with Rectal Adenocarcinom
- Localized Amyloidosis Involving the Nasal Cavity
- Carcinoid of the Appendix Goblet Cells Metastasize to the Orbit – a Clinical Case Report and Review of the Literature
- Evaluation of Dietary Habits in the Study of Pancreatic Cancer
- Screening of Psychological Distress 4.5 Years after Diagnosis in Breast Cancer Patients Compared to Healthy Population
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- History of Oncology in Slovakia
- Lynch Syndrome – the Pathologist’s Diagnosis
- Localized Amyloidosis Involving the Nasal Cavity
- Evaluation of Dietary Habits in the Study of Pancreatic Cancer
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

