-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSelected psychometric properties of the Czech Version of the FACT-B Scale (Version 4) for Measuring Quality of Life in Breast Cancer Patients
Vybrané psychometrické vlastnosti české verze dotazníku FACT-B (verze 4) měření kvality života u pacientek s karcinomem prsu
Zlepšování výsledků onkologické léčby přitáhlo větší pozornost k longitudinálnímu sledování kvality života (QoL). Funkční posouzení onkologické léčby (FACT) je standardním nástrojem měření QoL u onkologických pacientů, přičemž varianta FACT-B se používá pro pacientky s karcinomem prsu. Cílem této studie je informovat o výsledcích validizace překladu dotazníku FACT-B (verze 4) do češtiny: jde o vůbec první validizaci nástroje QoL pro pacientky s karcinomem prsu v České republice. Byla použita standardní popisná statistika a zkoušky. Spolehlivost dílčích škál skóre QoL byla měřena Cronbachovým alfa koeficientem. K analýze konstruktové validity nástroje byly použity explorační a konfirmační faktorová analýza, Pearsonův a Spearmanův koeficient korelace. Tato analýza byla zpracována s pomocí programů SPSS 23.0.0. a Stata 13. Výsledky validizace jsou obdobné jako další validizace přeložených národních verzí FACT-B, které po překladu vykazují uspokojivou spolehlivost a citlivost a spolehlivou vnitřní strukturu. Analýza jeho vnitřní struktury odhalila skryté faktory, které vyplývají z dílčích škál dotazníku FACT-B, a prokázala mezikulturní platnost českého překladu. Naše výsledky ukazují, že český překlad dotazníku FACT-B je spolehlivým a platným nástrojem měření QoL u pacientek s karcinomem prsu v České republice a může být využíván v budoucím výzkumu a /nebo klinické praxi.
Klíčová slova:
nástroj validizace QoL – překlad FACT-B do češtiny – analýza faktorů – karcinom prsu
Authors: Kateřina Skřivanová 1,2; Jiří Jarkovský 3; Klára Benešová 3; Jan Nedvěd 1; Dagmar Brančiková 4; Hana Peterková 1; Tomáš Svěrák 5; Ľubomíra Anderková 5; Nela Elfmarková 5; Marcela Bendová 6; Luboš Minář 6; Markéta Protivánková 4; Ladislav Dušek 3
Authors place of work: Department of Psychology and Psychosomatics, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Department of Clinical Psychology, Faculty Hospital, Brno, Czech Republic 2; Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Internal Medicine, Haematology and Oncology, Faculty of Medicine, Masaryk University and Faculty Hospital Brno, Czech Republic 4; Faculty of Medicine and Applied Neuroscience Research Group, Central European Institute of Technology, Masaryk University (CEITEC MU), Brno, Czech Republic 5; Department of Obstetrics and Gynaecology, Faculty of Medicine, Masaryk University and Faculty Hospital Brno, Czech Republic 6
Published in the journal: Prakt Gyn 2016; 20(3-4): 156-161
Category: Onkogynekologie
Předběžné výsledky byly formou posteru publikovány na Brněnských onkologických dnech v roce 2016.
Summary
Improvements in the outcomes of cancer treatment have drawn more attention to longitudinal monitoring of the quality of life (QoL). Functional assessment of cancer therapy (FACT) is a standard instrument for QoL measurement in cancer patients; a variant FACT-B is used for breast cancer patients. The aim of this study is to report the validation results of FACT-B (version 4) translation to the Czech language; it is the first validation ever of a QoL instrument for breast cancer patients in the Czech Republic. Standard descriptive statistics and tests were applied. The reliability of QoL scores subscales was measured by the Cronbach’s alpha coefficient. Pearson’s and Spearman’s correlation coefficient, exploratory and confirmative factor analysis were used to analyse the construct validity of the instrument. The analysis was computed using SPSS 23.0.0. and Stata 13. The validation results are similar to other validations of national FACT-B translations with a good reliability and sensitivity, and a reliable internal structure after translation. The analysis of its internal structure revealed the hidden factors attributable to subscales of FACT-B, and proved the cross-cultural relevance of the Czech translation. Our results suggest that the Czech translation of FACT-B is a reliable and valid instrument for QoL measurement for breast cancer patients in the Czech Republic, and that it can be used in future research and/or clinical practice.
Keywords:
breast cancer – FACT-B translation into Czech – factor analysis – QoL instrument validationIntroduction
Improvements in the outcomes of cancer treatment and the possibility of long-term survival have drawn more attention to quality of life (QoL) monitoring. The QoL definition is derived from a concept that describes four areas of a fulfilling life for an individual: physical, psychological, social status and spiritual aspects [1]. QoL in cancer patients is affected by consequences of the disease itself, and by side effects of treatment [2].
Breast cancer is the most common cancer type among Czech women, which is a phenomenon occurring in most economically developed countries. Its incidence has seen a moderate growth over the last few decades. Fortunately, due to an early detection of tumours and improved therapeutical strategies, mortality rates have stabilised and the number of breast cancer survivors has been growing [3].
The functional assessment of cancer therapy (FACT) scale is a standard instrument to measure QoL in cancer patients. The FACT-general (FACT-G) scale [4] is useful for the QoL analysis in various types of cancer; the FACT-B (Version 4) questionnaire is its variant, specifically designed for breast cancer [5]. FACT-B is a questionnaire used for self-assessment and includes the assessment of Physical, Social, Emotional and Functional Well-Being plus the breast cancer subscale (Additional Concerns). Each item is rated on a five-point scale (0 – not at all; 1 – a little bit; 2 – somewhat; 3 – quite a bit; 4 – very much) with a higher total score indicating better functional status.
Validation of the Czech translation is necessary for the usage in the population of Czech breast cancer survivors, as cross-cultural transfer of QoL measures [6] can bring some serious issues during its non-validated application. We focused on broad psychosocial and diseased-related factors that can be associated with QoL in a Czech sample of women after breast cancer treatment as part of a four-year, on-going, complex research project [7].
Research Questions
The aim of this study is to report the validation results of the translation of FACT-B scale Version 4 to the Czech language; it is the first validation ever of a QoL measure for Czech breast cancer patients.
Patients and methods
A total of 106 breast cancer patients were consecutively selected and recruited. Twenty-three subjects were followed prospectively for 2 years and available for sensitivity testing of FACT-B; remaining 83 were selected for psychometric analysis – a cross-sectional sample was used for FACT-B (Version 4) validation. Eligibility criteria included histologically confirmed breast cancer, age 30–90 years, and a sufficient knowledge of the Czech language. All patients underwent curative treatment at the Faculty Hospital Brno. All patients provided informed consent with their participation in the study; the study was approved by the Institutional Review Board of the Faculty Hospital Brno.
The basic clinical characteristics are given in tab. 1; no statistically significance differences between the groups were found. In the groups of retrospectively and prospectively followed patients, the median age was 63 and 64 years respectively; other characteristics included BMI (26.7 and 26.3), postmenopausal status (80 % and 71 %), marital status (54 % and 65 % were married), and stages at the time of diagnosis (25 % and 39 % for 0–I; 60 % and 57 % for II–III; 15 % and 4 % for IV).
Tab. 1. Basic characteristics 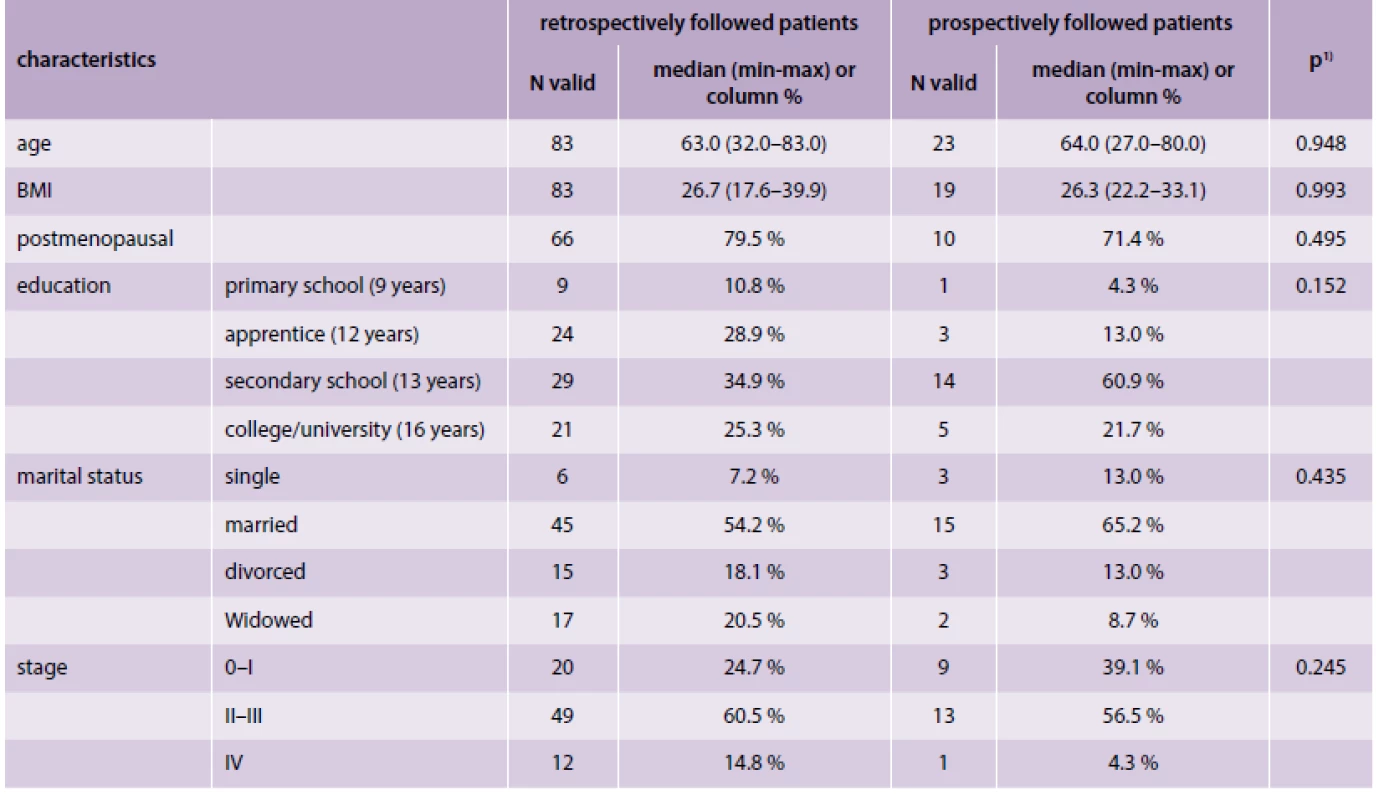
1) p-value of Mann-Whitney U test for continuous variables or p-value of Fisher’s exact test for categorical variables QoL was assessed using the Czech research version of FACT-B (Version 4) questionnaire, which was the main goal of the validation, and the Short Form [4,8] Health Survey (SF-36) questionnaire, which was adopted as a reference general measure of QoL. The SF-36 questionnaire was validated on the Czech population and was adopted as a reference questionnaire in the analysis [9]. SF-36 is a survey constructed to study health-related quality of life and consists of eight health concepts: Physical Functioning, Role Functioning – Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Functioning – Emotional, and Mental Health. Total scores can vary from 0 to 100, with higher scores representing a more favorable quality of life. Scores were calculated according to the original US version. Three qualified psychologists carried out semi-structured interviews with all patients. The FACT-B was administered and scored in accordance with manual for version 4 of the FACIT measurement system [10]. The Czech translation and the scoring manual of FACT-B were provided by FACIT (Functional Assessment of Chronic Illness Therapy).
Statistical methodology
Standard descriptive statistics were applied in the analysis; absolute and relative frequencies were used for categorical variables, and median with min–max range or mean with standard deviation were used for continuous variables. Statistical significance of differences between groups were assessed using the Mann-Whitney U test for continuous variables, and the Fisher’s exact test for categorical variables. The reliability of QoL scores subscales was measured by the Cronbach’s alpha coefficient. A repeated measures model was applied to compare differences in QoL over time in subgroups prospectively followed for sensitivity evaluation. Pearson’s correlation coefficient, exploratory and confirmatory factor analysis were used to analyse the construct validity of the instrument. The SF-36 instrument was used as a reference due of a lack of gold standard. The analysis was computed using SPSS 23.0.0. (IBM Corporation, 2015) and Stata 13 (StataCorp. 2013).
Results
The subgroup of 83 retrospectively followed patients was used as the main dataset for the validation of FACT-B score on the Czech population. The total FACT-B score in analysed patients was 94.2 (SD 18.3) with Cronbach’s alpha 0.770; two out of five FACT-B subscales (Physical well-being, Functional well-being) have Cronbach’s alpha > 0.8 (good), and the values were 0.778 (acceptable) for Social/family well-being, 0.692 (questionable) for Emotional well-being and 0.564 (poor) for Additional concerns. In comparison, Cronbach’s alpha was > 0.8 (good) for SF-36 in the total score and all subscores. Descriptive statistics and reliability of FACT-B and SF-36 are given in tab. 2. The consistency of FACT-B with already validated SF-36 in evaluating QoL was analysed using their correlations (criteria-related validity evaluation). With the exception of Social/family well-being, all other subscales and overall FACT-B score statistically significantly correlated with SF-36 subscales; statistically significant correlations were 0.3 to 0.8; FACT-B variability is explained by SF-36 from 9–64 %; the results are generally consistent in terms of FACT-B measuring the Qol.
Tab. 2. Descriptive statistics and reliability of FACT-B and SF-36 subscales 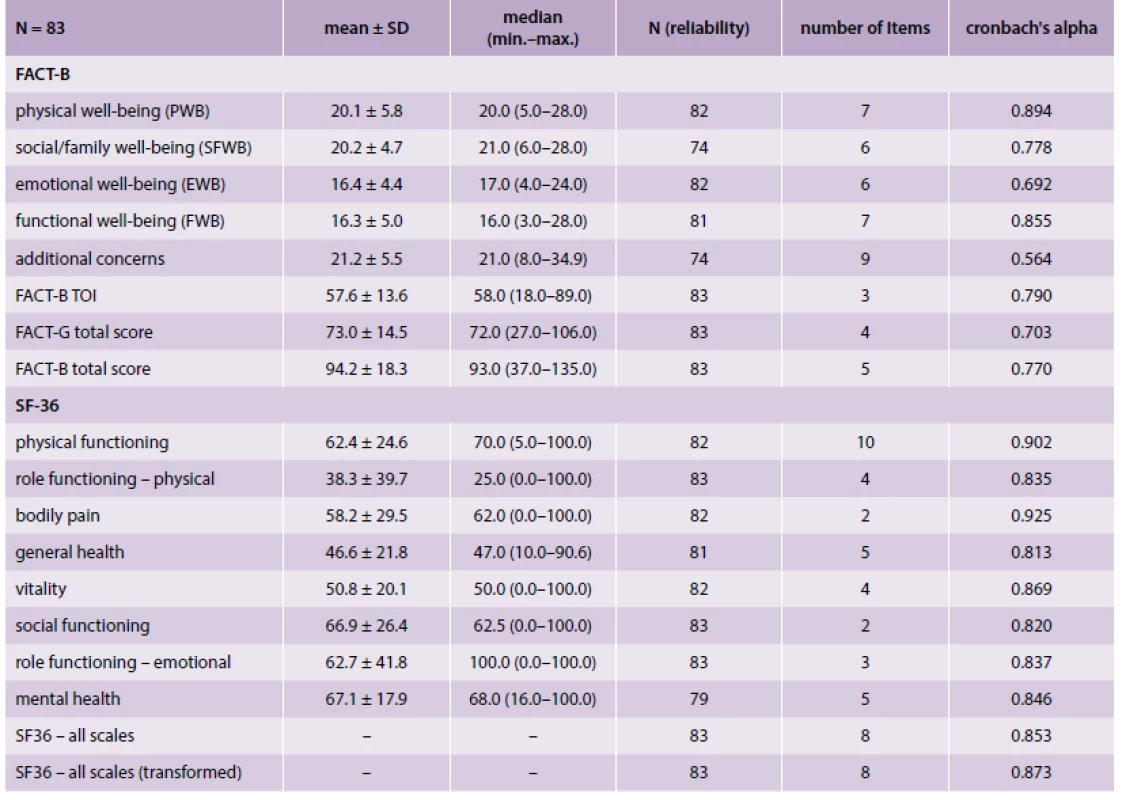
Further analysis of FACT-B was aimed at the internal correlation structure (construct validity evaluation). The correlations between the total FACT-B score and subscores were 0.4 to 0.8; the explained variability of FACT-B by subscores is 16–64 %; for individual subscores, the correlation is 0.0–0.9, and the explained variability is 0–81 %. Various levels of correlation of different subscores suggest an internal structure of subscores, mutual independence of some subscores, and potential existence of hidden factors. The first step was the factor analysis with principal components extraction and varimax rotation aimed on the best explanation of factor axes generating hypotheses about the number and definition of hidden factors, which was subsequently tested using exploratory factor analysis. The results in fig. 1 show three factor axes: the 1st explains 16 % of variability and is closely related to Physical well-being and Functional well-being; the 2nd explains 12 % of variability and is defined by Emotional well-being and Additional concerns; and the 3rd explains 9 % of variability and is defined by Social/family well-being; other factor axes explain a substantially lower portion of variability and have no clear relationship to FACT-B subscores. Based on the results, three hidden factors (physical status/complains, social support and psychological distress) were proposed and tested using confirmative factor analysis with maximum likelihood estimating method. The tested model had chi-square 55.6, RMSEA (Root Mean Square Error of Approximation) 0.065, CFI (Comparative Fit Index) 0.947 and TLI (Tucker Lewis Index) 0.929 (fig. 2) shows the structure of FACT-B by confirmative factor analysis, where defined hidden factors are closely and statistically significantly related to their defining items.
Fig. 2. Structural equation modelling 
The prospective subgroup of 23 patients was used for the analysis of consistency of QoL measures over time (the median time between measurements was 14 months), as documented in tab. 3. All FACT-B subscales were statistically significantly correlated over time; the same applies for the SF-36 subscales with the exception of Role functioning – physical.
Tab. 3. Correlation of subscales before treatment and after treatment in prospectively followed patients 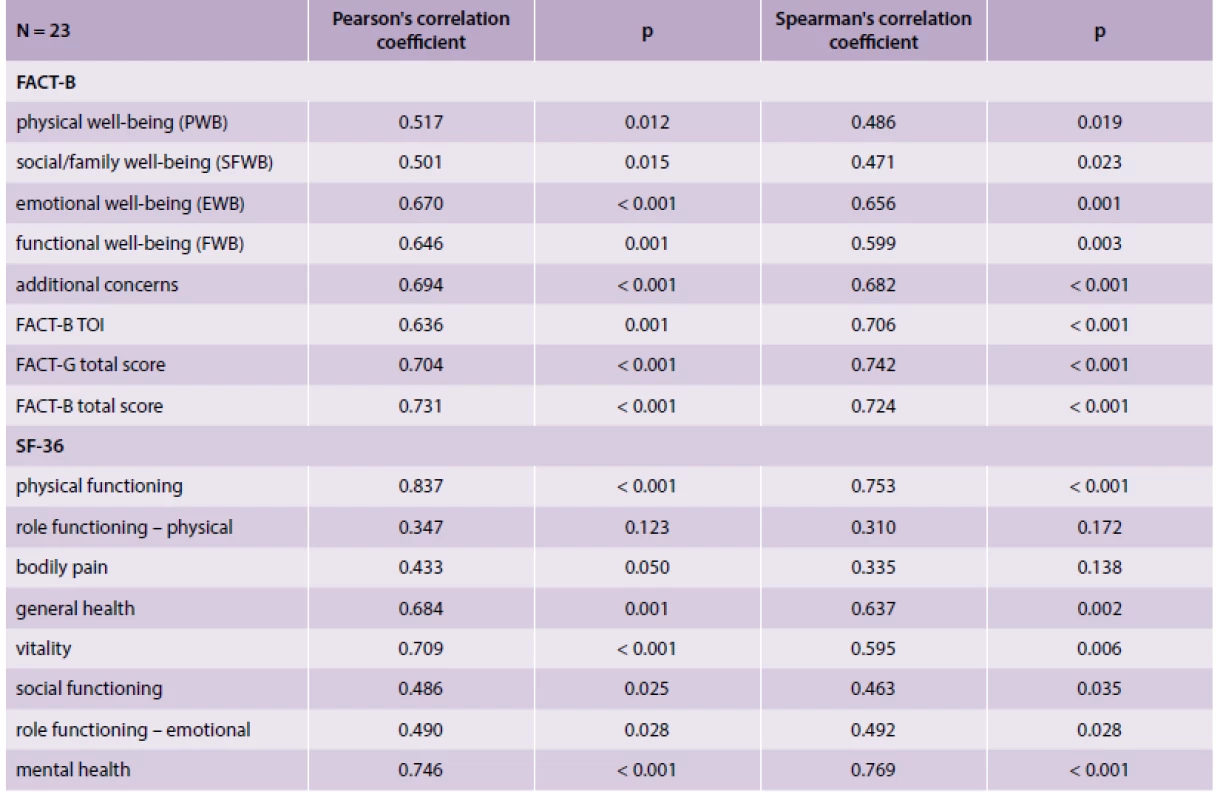
Discussion
Cronbach’s alpha in our analysis was 0.770, which is a lower value than in the original English version [11]; nevertheless, it is still considered as acceptable [12]. Correlation analysis, exploratory and confirmative factor analysis were used to confirm the construct validity in our study. The construct of the instrument turned out to be consistent with the original concept, and showed a reasonable overall construct validity. Results of our analyses confirmed the internal consistence of both primary items and subscales, and identified hidden factors with a close relation to subscales defined in FACT-B and providing independent dimensions of QoL; the RMSEA, CFI and TLI of tested model are considered as acceptable for an adequate fit of the model [13].
Compared to SF-36, which is a general measure of QoL, FACT-B as a specific measure of QoL showed a good criterion-related validity. The criteria-related validity of the Czech version of FACT-B was tested by its correlation with the already validated SF-36 QoL measure with highly significant results; the only exception was the Social/family well-being subscale, which has no direct counterpart in SF-36. The results of the validation are similar to other validations of national translations of FACT-B, which also show a good reliability, sensitivity and reliable internal structure after translation [14,15,16].
Limitations
Our study has some limitations. We did not evaluate the sensitivity to QoL changes in a long-term follow-up, and the sample size is relatively small. Future studies are needed to determine whether FACT-B is an efficient and sensitive tool for assessing QoL changes in Czech breast cancer patients.
Conclusion
The Czech translation of FACT-B score was found to be acceptable and reliable, with consistent but still sensitive results when applied over time. The analysis of its internal structure revealed hidden factors attributable to predefined subscales of original FACT-B, and proved the cross-cultural relevance of the Czech translation. Our results suggest that the Czech translation of FACT-B is a reliable and valid instrument for QoL measuring in Czech breast cancer patients.
Acknowledgement
We would like to thank Jason Bredle (FACIT) for providing the instrument, and to all study participants. The study was supported by the Czech Science Foundation (P407/12/0607).
Conflict of interest
We declare no conflict of interests.
Doručeno do redakce 30. 12. 2016
Přijato po recenzi 31. 1. 2017
Mgr. et Mgr. Kateřina Skřivanová, Ph.D.
kskrivan@med.muni.cz
Department of Clinical Psychology,
Faculty of Medicine,
Masaryk University and Faculty Hospital Brno,
Czech republic
www.med.muni.cz
Zdroje
1. Ferrell, B.R., Dow, K.H., Grant, N. (1995): Measurement of the quality o1. Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res 1995; 4(6): 523–531.
2. Scieszka M, Zielinski M, Machalski M. et al. Quality of life in cancer patients treated by chemotherapy. Neoplasma 2000; 47(6): 396–399.
3. Dusek L, Muzik J, Maluskova D et al. Cancer incidence and mortality in the Czech Republic. Klin Onkol 2014; 27(6): 406–423.
4. Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy (FACT) Scale: Development and validation of the general measure. J Clin Oncol 1993; 11(3): 570–579.
5. Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System Version 4. Dostupné z WWW: <http://www.facit.org/facitorg/questionnaires>.
6. Lent L, Hahn E, Eremenco S et al.Using cross-cultural input to adapt the Functional Assessment of Chronic Illness Therapy (FACIT) scales. Acta Oncol 1999; 38(6): 695–702.
7. Anderkova L, Elfmarkova N, Sverak T et al. Change in Quality of Life measured over Time in Czech Women with Breast Cancer. Klin Onkol 2016; 29(2): 113–121. Dostupné z DOI: <http://dx.doi.org/10.14735/amko2016113>.
8. Ware JE jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30(6): 473–483.
9. Sobotík Z. Zkušenosti s použitím předběžné verze amerického dotazníku o zdraví (sf-36). Zdravotnictví v České republice 1998; 1(1–2): 50–54.
10. Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System Version 4. CORE: Evanston 1997.
11. Brady MJ, Cella DF, Mo F et al. Reliability and validity of the Functional Assessment of Cancer Therapy – Breast (FACT-B) quality of life instrument. J Clin Oncol 1997; 15(3): 974–986.
12. DeVellis RF. Scale development: Theory and Applications (Applied Social Research Methods). 3rd ed. Sage: Los Angeles 2011 : 109–110. ISBN 978–1412980449.
13. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 1999; 6(1): 1–55. Dostupné z DOI: <http://dx.doi.org/10.1080/10705519909540118>.
14. Pandey M, Thomas BC, Ramdas K et al. Quality of life in breast cancer patients: Validation of a FACT-B Malayalam version. Qual Life Res 2002; 11(2): 87–90.
15. Wan C, Zhang D, Yang Z et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat 2007; 106(3): 413–418.
16. Yoo HJ, Ahn SH, Eremenco S et al. Korean translation and validation of the functional assessment of cancer therapy-breast (FACT-B) scale version. Qual Life Res 2005; 14(6): 1627–1632.
Štítky
Dětská gynekologie Gynekologie a porodnictví Reprodukční medicína
Článek Pain-free childbirth in 2016Článek Fear of pregnant womenČlánek Veřejné slyšení v Senátu Parlamentu ČR k problematice sexuálního násilí, Praha, 4. října 2016Článek Z odborné literaturyČlánek Z odborné literaturyČlánek Z odborné literaturyČlánek Editorial
Článek vyšel v časopisePraktická gynekologie
Nejčtenější tento týden
2016 Číslo 3-4- Alergie na antibiotika u žen s infekcemi močových cest − poznatky z průřezové studie z USA
- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Magnosolv a jeho využití v neurologii
- Isoprinosin je bezpečný a účinný v léčbě pacientů s akutní respirační virovou infekcí
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
-
Všechny články tohoto čísla
- Editorial
- Preeclampsia today and tomorrow
- Conservative therapy of interstitial pregnancy with the use of methotrexate – case study
- Pain-free childbirth in 2016
- Can semi-quantitative analysis of amniotic fluid glucose be useful in gynecology and obstetrics? A pilot study.
-
Cardiovascular changes in pregnancy II.
Preeclampsia and its long-term consequences for mother and offspring - Fear of pregnant women
- Physiotherapy after gynecologic abdominal surgery
- Selected psychometric properties of the Czech Version of the FACT-B Scale (Version 4) for Measuring Quality of Life in Breast Cancer Patients
- Oběti sexuálního násilí v gynekologické ordinaci II – co je dobré znát
- Veřejné slyšení v Senátu Parlamentu ČR k problematice sexuálního násilí, Praha, 4. října 2016
-
13th Congress ISIR and ESRI
22.–25. 6. 2016, Erfurt, Germany - Z odborné literatury
- Z odborné literatury
- Z odborné literatury
- Praktická gynekologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Physiotherapy after gynecologic abdominal surgery
- Conservative therapy of interstitial pregnancy with the use of methotrexate – case study
- Preeclampsia today and tomorrow
- Pain-free childbirth in 2016
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




