-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Advanced Paternal Age Is Associated with Impaired Neurocognitive Outcomes during Infancy and Childhood
Background:
Advanced paternal age (APA) is associated with an increased risk of neurodevelopmental disorders such as autism and schizophrenia, as well as with dyslexia and reduced intelligence. The aim of this study was to examine the relationship between paternal age and performance on neurocognitive measures during infancy and childhood.Methods and Findings:
A sample of singleton children (n = 33,437) was drawn from the US Collaborative Perinatal Project. The outcome measures were assessed at 8 mo, 4 y, and 7 y (Bayley scales, Stanford Binet Intelligence Scale, Graham-Ernhart Block Sort Test, Wechsler Intelligence Scale for Children, Wide Range Achievement Test). The main analyses examined the relationship between neurocognitive measures and paternal or maternal age when adjusted for potential confounding factors. Advanced paternal age showed significant associations with poorer scores on all of the neurocognitive measures apart from the Bayley Motor score. The findings were broadly consistent in direction and effect size at all three ages. In contrast, advanced maternal age was generally associated with better scores on these same measures.Conclusions:
The offspring of older fathers show subtle impairments on tests of neurocognitive ability during infancy and childhood. In light of secular trends related to delayed fatherhood, the clinical implications and the mechanisms underlying these findings warrant closer scrutiny.
Published in the journal: . PLoS Med 6(3): e32767. doi:10.1371/journal.pmed.1000040
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000040Summary
Background:
Advanced paternal age (APA) is associated with an increased risk of neurodevelopmental disorders such as autism and schizophrenia, as well as with dyslexia and reduced intelligence. The aim of this study was to examine the relationship between paternal age and performance on neurocognitive measures during infancy and childhood.Methods and Findings:
A sample of singleton children (n = 33,437) was drawn from the US Collaborative Perinatal Project. The outcome measures were assessed at 8 mo, 4 y, and 7 y (Bayley scales, Stanford Binet Intelligence Scale, Graham-Ernhart Block Sort Test, Wechsler Intelligence Scale for Children, Wide Range Achievement Test). The main analyses examined the relationship between neurocognitive measures and paternal or maternal age when adjusted for potential confounding factors. Advanced paternal age showed significant associations with poorer scores on all of the neurocognitive measures apart from the Bayley Motor score. The findings were broadly consistent in direction and effect size at all three ages. In contrast, advanced maternal age was generally associated with better scores on these same measures.Conclusions:
The offspring of older fathers show subtle impairments on tests of neurocognitive ability during infancy and childhood. In light of secular trends related to delayed fatherhood, the clinical implications and the mechanisms underlying these findings warrant closer scrutiny.Introduction
In recent decades there has been increased attention to health outcomes in the offspring of older fathers. Evidence shows that advanced paternal age (APA) is associated with an increased risk of a wide range of disorders [1]. While not discounting the influence of various age-related psychosocial factors that may translate to differential health outcomes for the offspring of older fathers (e.g., higher socioeconomic status, better education), advances in genomics have refocused attention on the vulnerability of sperm from older fathers to carrying de novo mutations. The development of the germ cell differs between human males and females—there are many more germline cell divisions in the life history of a sperm relative to that of an oocyte [2]. In the female there are 22 mitotic cell divisions that occur in utero. In contrast, after puberty, progenitor sperm stem cells undergo mitotic cell division once every 16 d. By age 20 the progenitor sperm cells have undergone approximately 150 cell divisions. By age 50 this number is 840. Thus, the chance of copy error mutations increases with age in males more dramatically than for females.
Advanced paternal age is associated with increased fetal deaths [3,4] and certain rare congenital syndromes (e.g., achondroplasia) [1,5]. In recent years evidence has accumulated linking APA with a wide range of neurological and neuropsychiatric conditions including Alzheimer's disease [6,7], bipolar disorder [8], dyslexia [9], neural tube defects [10], and epilepsy [11]. A sizeable body of evidence has accumulated linking APA with an increased risk of schizophrenia [12–18]. A recent meta-analysis based on eight studies found that paternal age above 35 was associated with an increased risk of schizophrenia [19]. There is also evidence linking APA to autism spectrum disorders [20–24].
The associations between APA and outcomes such as autism and schizophrenia are of particular interest, as these disorders have recently been associated with genomic structural variation [25–30]. It is feasible that APA-related mechanisms may contribute to genomic structural variation (e.g., copy number variants, microdeletions) [2]. Thus, within the fields of schizophrenia and autism research, there has been an unexpected convergence between epidemiology and molecular biology.
While there is good evidence linking paternal age with several clinically distinct neurodevelopmental disorders, the evidence linking paternal age and other neurocognitive outcomes such as general intelligence is less robust. Earlier studies noted an association between APA and poorer performance on neurocognitive tests [31–34]. This issue has been addressed specifically in a recent study based on male and female Israeli conscripts (age 16–17 y, n = 44,175) [35]. The study found independent effects of paternal age on offspring intelligence with the lowest scores associated with both younger and older fathers (inverted “U”-shaped association). This finding is in contrast to the association between maternal age and offspring intelligence, where most studies have reported a linear association between older maternal age and superior neurocognitive ability [36–39].
The aim of the present study was to explore the association between paternal age and a range of neurocognitive measures using a large, prospective birth cohort: the US-based Collaborative Perinatal Project (CPP). Based on the literature linking increased paternal age with a range of developmental anomalies and neuropsychiatric disorders, we hypothesized that the children of older fathers would have lower scores on various tests used to measure neurocognitive ability when assessed at 8 mo, 4 y, and 7 y. While a study based on this same cohort had previously identified that the offspring of older mothers had superior performance on neurocognitive functioning [36], we also took the opportunity to re-examine this hypothesis in the current analyses.
Methods
Sample Selection
The Collaborative Perinatal Project (CPP) recruited pregnant women from 12 university-affiliated hospital clinics in the United States of America from 1959 to 1965. The selection method varied from centre to centre, with between 14% and 100% of the registered pregnant women being invited to participate. At centres with less than 100% sampling, women were selected according to various quasi-random rules (e.g., every nth woman). Of 132,560 eligible pregnancies, 55,908 pregnancies were included, which was a proportion representative of the original sampling frame [40,41].
In order to reduce the impact of prematurity on the neurocognitive outcome measures, we restricted the sample to offspring born after 37 wk gestation. In order to minimize statistical complexities arising from dependent data, we restricted the sample to (a) singleton pregnancies, and (b) one randomly chosen pregnancy for each woman enrolled in the study.
Measures of Neurocognitive Function
Study offspring were assessed at regular intervals until age 7 y. Detailed descriptions of the methods used for cognitive assessments have been published elsewhere [36,42]. At 8 mo of age the Bayley Scales for Infant Development were administered [43,44]. Two scores were available: (a) Mental Scale, which assesses aspects of development including sensory discrimination and eye-hand coordination, and (b) Motor Scale, which assessed various aspects of fine and gross motor coordination. At age 4 y the children were administered (a) the Stanford Binet Intelligence Scale, Form L-M (a measure of general intelligence in young children) [45,46], and (b) the Graham-Ernhart Block Sort Test, which assesses conceptual and perceptual motor ability. This test involves increasingly difficult tasks that range from matching simple like-shaped blocks, to sorting blocks according to one or two dimensions (e.g., colour, shape, size) [47]. At age 7 y the children were administered the widely used Wechsler Intelligence Scale for Children (WISC) [48]. Scores for Full Scale, Verbal, and Performance were available for this study. However, the two WISC subscales (Verbal and Performance) were strongly correlated with WISC Full Scale IQ (Pearson correlation = 0.90 and 0.89, respectively), thus only the WISC Full Scale IQ results are presented. The Wide Range Achievement Test (WRAT) scale was also used at the age 7 y follow-up in order to evaluate academic achievements (e.g., the ability to read words, comprehend sentences, spell, and compute solutions to math problems) [49]. Scores for WRAT Arithmetic, Reading, and Spelling were available in this study. Because the WRAT Reading, Spelling, and Arithmetic scores were all strongly correlated (Pearson correlations of 0.65 to 0.89), only the WRAT Reading is presented.
Statistical Methods
For the primary analyses, we modelled nonlinear associations between parental age and neurocognitive outcomes using a generalized additive model [50]. We used the generalized cross-validation algorithm to select the degree of nonlinearity. To verify the assumptions of the models, we examined the residuals to check (a) their normality and (b) their homoscedasticity (constant variance) against paternal age.
Each parent's age (at the birth of the child) was adjusted for the other parent's age. For the primary analyses, we examined a simple model (Model 1) adjusted for offspring sex, other parent's age, mother's race, weeks of gestation, and child's age at testing (which varied slightly at the 8 mo, 4 y, and 7 y follow-ups). In order to explore if various socioeconomic variables influenced the strength of the association, a second model (Model 2) also included additional adjustments related to maternal marital status, family socioeconomic status and parental mental health. Socioeconomic status was measured by a composite index that averaged centiles derived from maternal and paternal education and occupation, as well as family income [51].
Because maternal and paternal age were strongly correlated (Pearson correlation = 0.80), we checked the models for colinearity using the variance inflation factor [52]. The variance inflation factors were roughly three for paternal and maternal age in all models. This value is well below the suggested threshold of ten [52], and hence we modelled both ages together.
The results of the primary analyses are displayed graphically, with the nonlinear model fitted for both maternal and paternal age (and 95% confidence intervals [CIs]). The variance explained (adjusted R-squared) and the p-values for each of the primary analyses are also shown in tabular form. Nonlinear models do not lend themselves to simple quantitative descriptions (e.g., statements such as “the outcome variable falls by a certain number of units for every additional 5 years of paternal age” cannot be made for nonlinear relationships). In order to facilitate interpretation of the primary analyses, we also provided estimates (and 95% CIs) for each outcome variable at two paternal ages (20 and 50 y).
As secondary analyses, we examined the association between paternal age and offspring neurocognition according to various strata of maternal age. This removes widely diverse effects due to maternal age on neurocognitive outcomes by estimating the effect of paternal age in subgroups where mother's ages were highly comparable. We identified cohort members where maternal ages fell within roughly 5 y age strata: <20, 20–24, 25–29, 30–34, 35–39, 40+. For these secondary analyses, we also chose a more stringent test of the association between the variables of interest. For each of the neurocognitive variables, we stratified the sample by sex, age, and race and then dichotomized the sample into a low-achievers group, defined as the lowest 10% of scores in each sex, age, and race group, versus the remaining 90% of the group. We calculated the adjusted odds ratio for being in the low achievers group for a 5 y increase in paternal age using conditional logistic regression.
All p-values were two-sided and statistical significance was set at 0.05. We used the mgcv library in R to fit the generalized additive models [53] and SAS PROC PHREG for the conditional logistic regression [54].
Results
There were 55,740 singleton pregnancies. Of these, 12,297 children were excluded because of (a) missing maternal and/or paternal age (1,542), (b) having indeterminate or unspecified sex (1,050), or (c) gestational age that was missing or less than 37 wk (9,705). After randomly selecting one live-born offspring per study mother, this left a total of 33,437 study offspring (17,148 males) available for the main analyses. Of these, 51% of the mothers were white, 39% black, and the remaining 10% were Asian and other racial groups. Finally, 6,355 children were missing information about age at testing at 8 mo, while 9,930 were missing age at testing at 4 y, and 9,109 were missing age at testing at 7 y. Those with missing paternal age were significantly more likely to have missing outcome variables at 8 mo, 4 y, and 7 y (each p < 0.001).
Table 1 shows descriptive statistics for paternal and maternal age and differences in parental age. On average, fathers were 3 to 4 y older than mothers, but the differences in parental age varied widely. Concerning the primary analyses, there was a statistically significant association between advanced paternal age and inferior performance on all neurocognitive tests (all p < 0.001) except for Bayley Motor score (Model 2, p = 0.104) (see Table 2). Concerning the influence of maternal age, there were statistically significant associations between advanced maternal age and superior performance on all measures. Figures 1 and 2 show the mean adjusted score for paternal and maternal age for the outcome variables based on Models 1 and 2 respectively. Apart from the direction of the association between maternal and paternal age, the association between maternal age and the outcome variables at ages 4 and 7 y was curvilinear (generally steep at younger ages, then less steep at older ages), in contrast to the near-linear association with paternal age. Post-hoc analyses examining the goodness-of-fit of nonlinear versus linear models indicated that two of the variables were adequately capture by simple linear models (Bayley Mental score and Graham Ernhart Block Sort Test), but that nonlinear models were best suited for all other variables (unpublished data). Table 3 shows the estimated scores (and 95% CIs) for two paternal ages (20 and 50 y) based on the nonlinear modelling used in the primary analyses. For Model 2, the adjusted R-squared ranged from 2.4% (Bayley Motor) to 29.5% (WISC Full Scale IQ).
Tab. 1. 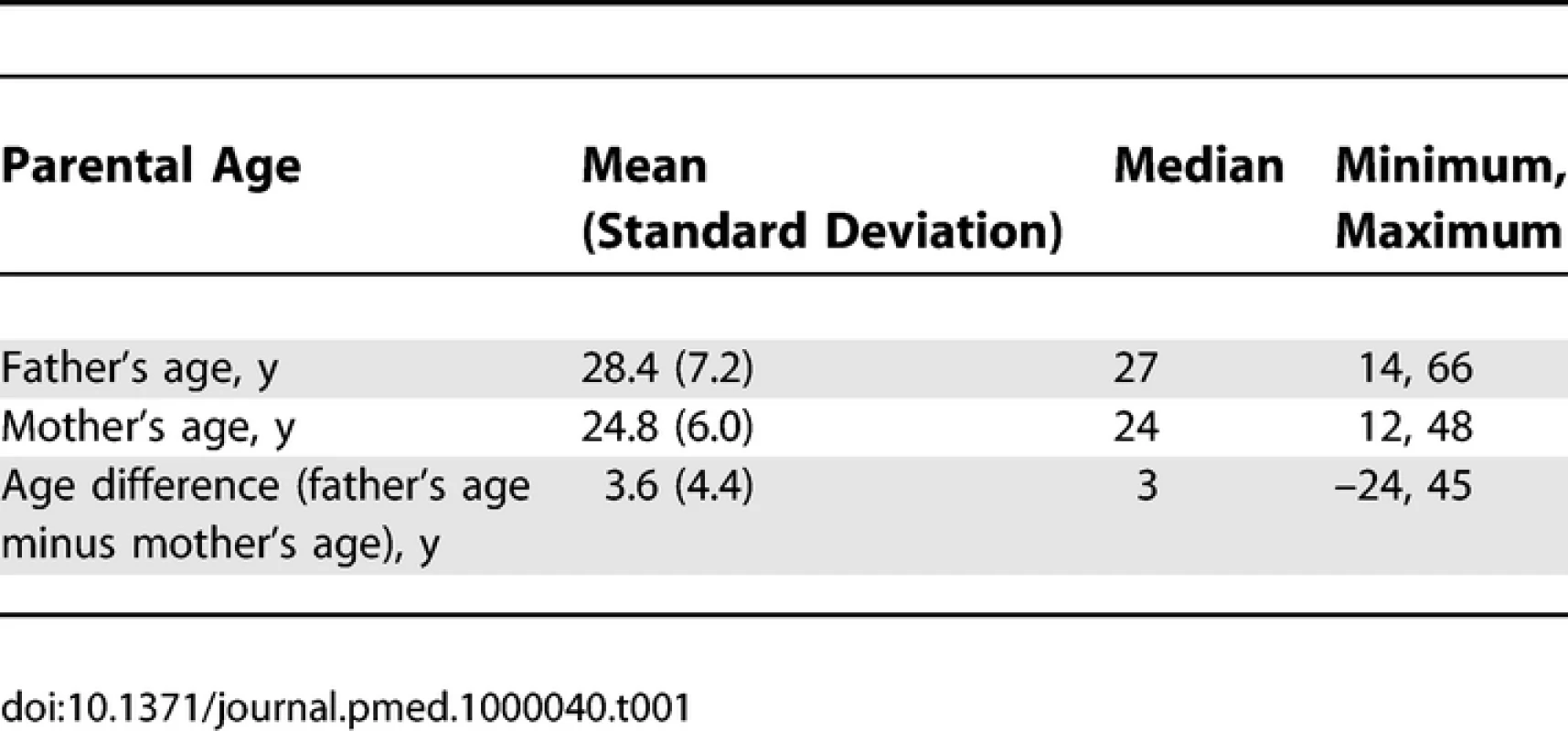
Descriptive Statistics of Maternal and Paternal Age, and Parental Age Difference (<i>n</i> = 33,437) Tab. 2. 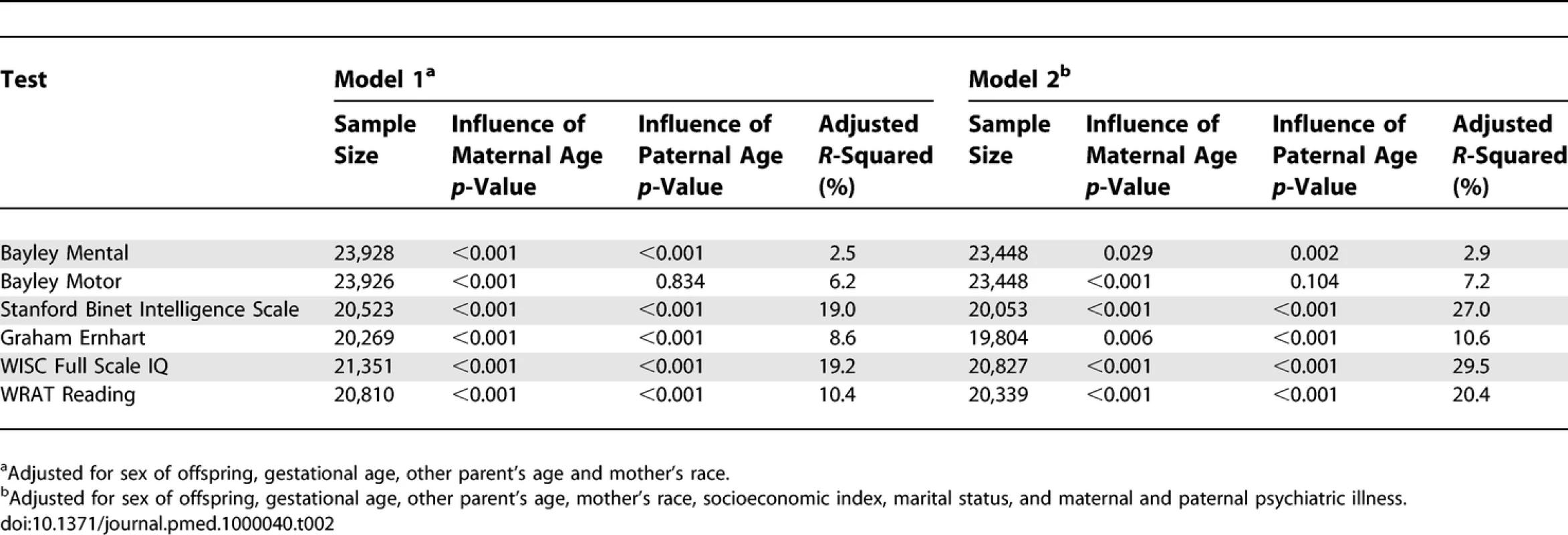
Primary Analyses: Summary Table for the Nonlinear Model Fits for Models 1 and 2 Fig. 1. Primary Analyses: Model 1—Adjusted for Other Parent's Age, Mother's Race, Gestational Age, and Child Gender 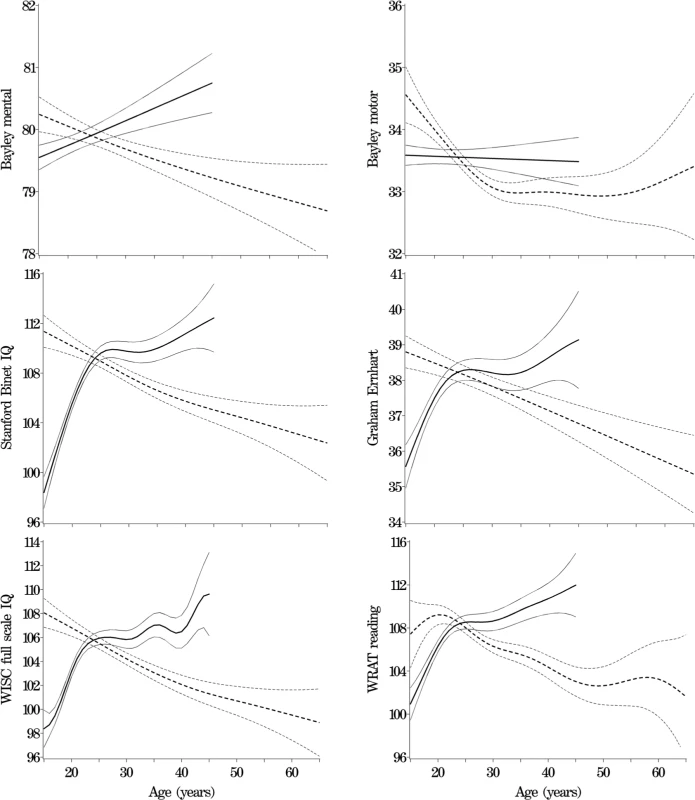
Solid lines ranging from 15 to 45 y for maternal age, dotted lines ranging from 15 to 65 y for paternal age. Nonlinear model fit with 95% CIs. Fig. 2. Primary Analyses: Model 2—Adjusted for Other Parent's age, Mother's Race, Gestational Age, and Child Gender, Socioeconomic Index, Marital Status, and Maternal and Paternal Mental Illness 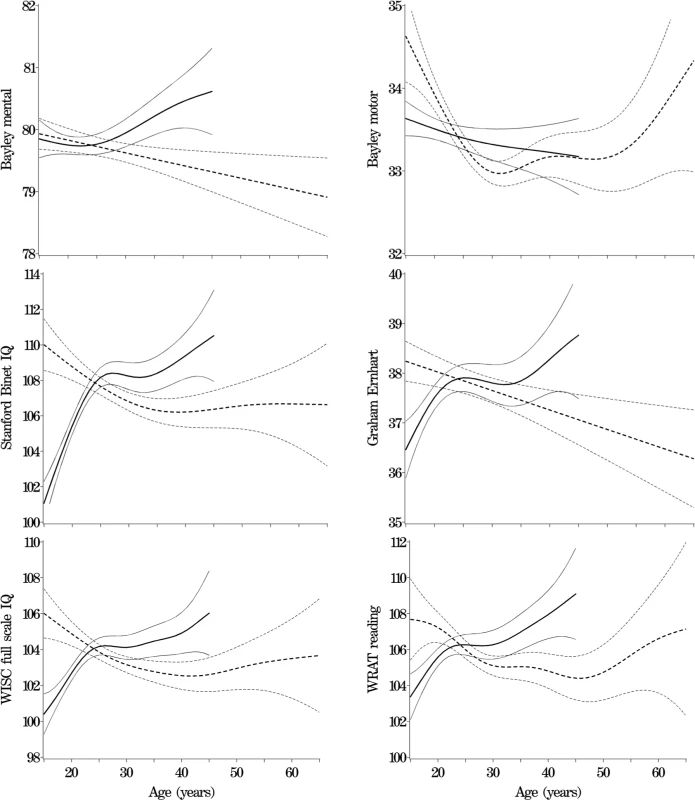
Solid lines ranging from 15 to 45 y for maternal age, dotted lines ranging from 15 to 65 y for paternal age. Nonlinear model fit with 95% CIs. Tab. 3. 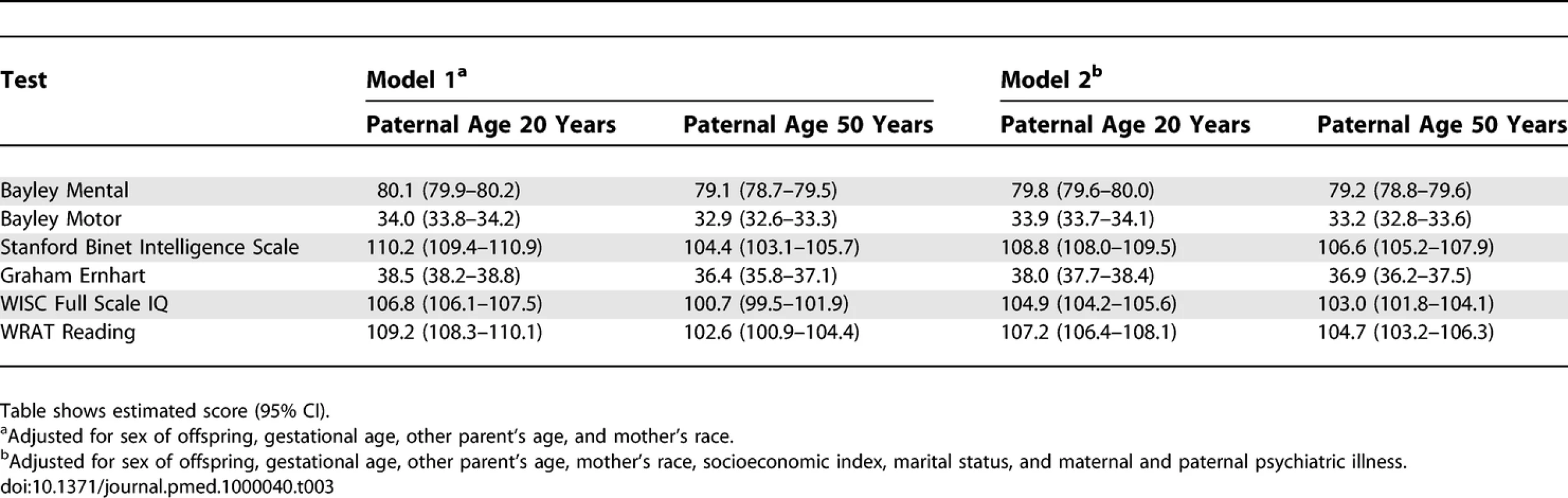
Primary Analyses: Estimates for Two Paternal Ages Based on the Nonlinear Model Fits for Models 1 and 2 Concerning the secondary analyses, the odds ratio (Model 2) for being in the lowest decile for each neurocognitive variable was significantly associated with elevated paternal age for three of the neurocognitive measures (Bayley Motor, Graham Ernhart Block Sort Test, WISC Full Scale IQ), with trend level association identified for the other three measures (Bayley Mental, Stanford Binet Intelligence Scale, WRAT Reading) (Table 4).
Tab. 4. 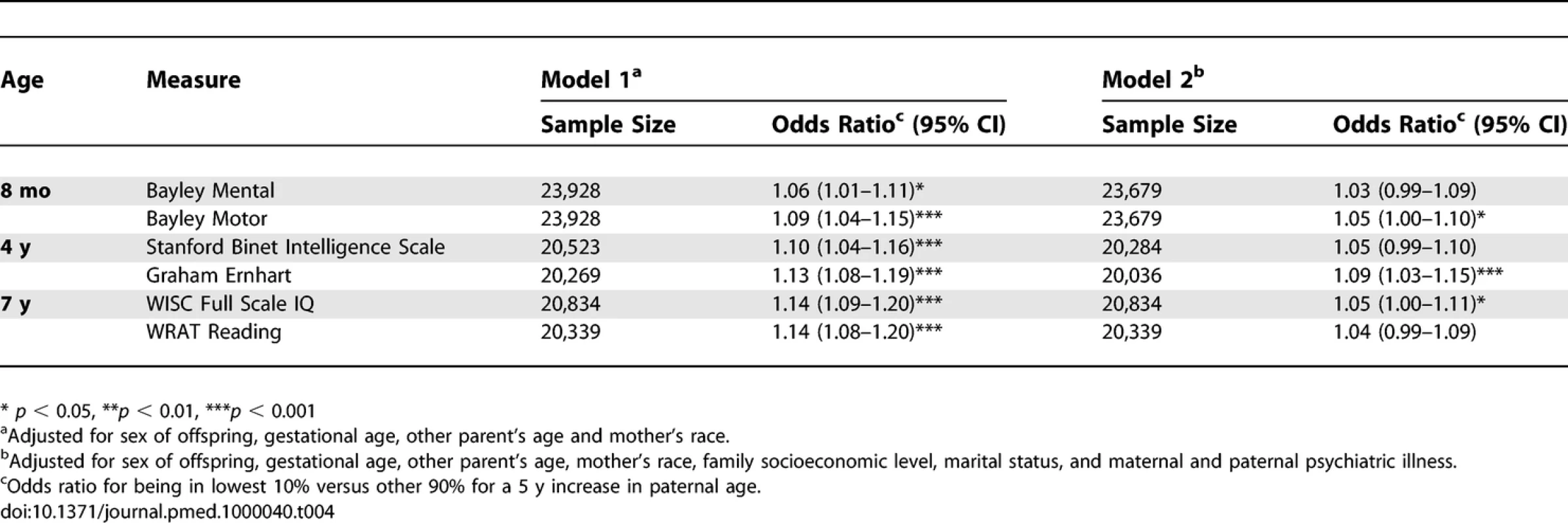
Secondary Analyses: Associations between Increasing Paternal Age and Neurocognitive Measures in Children, Results from Logistic Regression Analyses Using Subgroups of Women with Similar Ages Discussion
We report, to our knowledge for the first time, that the offspring of older fathers show impairments on a range of neurocognitive tasks during infancy and childhood. The pattern of findings was relatively consistent across ages and across neurocognitive domains, with near-linear declines found in most of the measures. When the data were examined with a more stringent definition of cognitive impairment (scores in the lowest 10%), a significant relationship between APA and impaired neurocognition was found for three of the six outcome variables, with trend level associations found for the remaining three variables. These findings persisted after adjustment for a range of socioeconomic variables and for parental mental health. In striking contrast to the findings for APA, the association between advanced maternal age and performance on neurocognitive tasks was in the opposite direction.
The findings differ somewhat from those reported by Malaspina et al. [35], who reported on four different measures related to cognitive ability in teenagers (age 16–17y). In that study the offspring of both younger (<20 y) and older fathers (>40 y) had impaired neurocognitive performance compared to those with fathers in the other age strata. However, differences between the Malaspina et al. study and the current study with respect to the psychometric measures and the age of the offspring make direct comparisons difficult. As expected, the current study also identified an association between advanced maternal age and superior performance on the neurocognitive tests, in keeping with some [36–39] but not all studies [35].
The association between APA and reduced neurocognitive ability may have important implications for clinical outcomes previously linked to APA. While not all individuals with autistic spectrum disorders have impaired intelligence, many have specific learning disabilities and/or intellectual handicap [55]. With respect to schizophrenia, systematic reviews and meta-analyses have shown a reliable, medium-sized impairment in premorbid intelligence associated with this disorder [56,57]. For example, Woodberry et al. [57] reported that years before the onset of psychotic symptoms, individuals who later developed schizophrenia had IQ scores that, on average, were approximately one-half of a standard deviation below that of healthy comparison participants. Consistent with these findings, a systematic review of the antecedents of schizophrenia based on prospective birth cohorts [58] provided robust evidence that individuals who later develop schizophrenia show deviation during childhood on a range of cognitive measures related to intelligence, motor development, speech and language, and educational outcomes. In particular, cohort members who later developed schizophrenia, as a group, achieved lower scores on intelligence tests in childhood and adolescence than their peers [59–61].
The findings from this study linking APA and impaired cognition may be best conceptualized within the notion of impaired cognitive reserve [62,63]. Just as superior cognitive capacity appears to provide a buffer against dementia [64,65], subtle APA-related impairments in neurocognitive ability may contribute to an increased risk of a diverse range of adverse neurological and neuropsychiatric health outcomes.
The study has several caveats. Nonrandom sample attrition and missing data may influence the generalisability of the findings [41]. Those with missing data on paternal age were more likely to be lost to follow-up. It will be important to examine the variables of interest in cohorts with optimal participant retention and minimal missing data. More importantly, the cohort members were born in the United States during the 1960s, thus the generalisability of the findings with respect to more contemporary cohorts needs to be examined. While it is feasible that various economic and psychosocial factors that can influence childhood developmental trajectories may have changed in recent decades, there is no reason to suspect that the putative biological processes linking APA and adverse health outcome would have varied over this time frame. Finally, it is important to note that these analyses investigated neurocognitive outcomes only until age 7 y, and it is feasible that the offspring of older fathers “catch up” during later childhood. How the subtle neurocognitive features associated with APA translate into later educational and mental health outcomes across the lifespan remains to be determined.
With respect to the mechanism of action underpinning these findings, several hypotheses warrant further scrutiny. While twin studies have demonstrated that cognitive ability and brain structure are heritable [66,67], studies based on sibships within the CPP have also confirmed that socioeconomic factors play a role in mediating the heritable aspects of intelligence [68]. With respect to paternal age, a broad range of socioeconomic factors improve with increasing age, thus most commentators believe that the offspring of older parents would have better access to health and educational services compared to the offspring of younger parents (who tend to have lower education and poorer income) [69]. For example, Fergusson and Lynsky [38] found that offspring of younger mothers tended to be born into relatively poorly educated and socially disadvantaged families. These authors commented that children born to young mothers were exposed to less nurturing and more changeable home environments. One would expect that such mechanisms would also operate with respect to paternal age. Clearly, our findings linking APA with impaired neurocognitive development cannot be readily explained by these social mechanisms.
Mechanisms related to the development of the male germline warrant consideration [70]. Each time the cell divides, the replication of the genome introduces the possibility of copy error mutations. In humans it has been confirmed that sperm from older men have significantly more mutations [2,71,72]. Levels of DNA proofreading and repair enzymes also decline as a function of APA [16] and DNA fragmentation increases [73], further compromising the integrity of gene replication. Apart from genetic changes (i.e., changes in DNA basepair sequence), APA may also involve abnormal epigenetic mechanisms [74–76].
Unravelling the molecular mechanisms underlying the association between APA and adverse health outcomes will be a substantial task for the biomedical research community. The precise location and nature of these mechanisms will probably vary substantially from offspring to offspring. It is unlikely that they will “map” neatly to a few loci, nor probably to one mechanism (e.g. genetic, epigenetic). With respect to genetic mechanisms, these may include single nucleotide mutations, or various types of genomic rearrangements (e.g., microdeletions, tandem and trinucleotide repeat expansions, microduplication or higher order expansions, aneuploidy). Animal experiments based on inbred rodent strains may provide the most efficient way to explore genetic and epigenetic factors mediating APA and brain development. Comparable to “forward genetics” platforms based on chemical mutagens [77,78], rodent-based APA models could provide an age-related mutagenesis experiment that has epidemiological face validity [79].
The observation linking APA with risk of schizophrenia has led to the hypothesis that APA-related mechanisms are contributing de novo mutations, which could explain the persistence of schizophrenia in the population in spite of reduced fertility and/or fecundity associated with this disorder [80]. APA-related mechanisms could accumulate over several generations, with the full clinical phenotype “breaking through” only after a critical threshold of certain mutations have accumulated [81,82]. In light of secular trends related to delayed parenthood [83], and in light of the potential for APA-related mechanisms to accumulate over several generations, the association between APA and subtle deficits in neurocognitive outcomes warrants closer scrutiny. While most of the neurocognitive differences were small at the individual level, these could have important implications from a public health perspective [84].
Zdroje
1. TorielloHVMeckJM
2008
Statement on guidance for genetic counseling in advanced paternal age.
Genet Med
10
457
460
2. CrowJF
2000
The origins, patterns and implications of human spontaneous mutation.
Nat Rev Genet
1
40
47
3. Nybo AndersenAMHansenKDAndersenPKDavey SmithG
2004
Advanced paternal age and risk of fetal death: a cohort study.
Am J Epidemiol
160
1214
1222
4. KleinhausKPerrinMFriedlanderYPaltielOMalaspinaD
2006
Paternal age and spontaneous abortion.
Obstet Gynecol
108
369
377
5. RousseauFBonaventureJLegeai-MalletLPeletARozetJM
1994
Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia.
Nature
371
252
254
6. BertramLBuschRSpieglMLautenschlagerNTMullerU
1998
Paternal age is a risk factor for Alzheimer disease in the absence of a major gene.
Neurogenetics
1
277
280
7. WhalleyLJThomasBMStarrJM
1995
Epidemiology of presenile Alzheimer's disease in Scotland (1974–88) II. Exposures to possible risk factors.
Br J Psychiatry
167
732
738
8. FransEMSandinSReichenbergALichtensteinPLangstromN
2008
Advancing paternal age and bipolar disorder.
Arch Gen Psychiatry
65
1034
1040
9. JayasekaraRStreetJ
1978
Parental age and parity in dyslexic boys.
J Biosoc Sci
10
255
261
10. McIntoshGCOlshanAFBairdPA
1995
Paternal age and the risk of birth defects in offspring.
Epidemiology
6
282
288
11. VestergaardMMorkAMadsenKMOlsenJ
2005
Paternal age and epilepsy in the offspring.
Eur J Epidemiol
20
1003
1005
12. BrownASSchaeferCAWyattRJBeggMDGoetzR
2002
Paternal age and risk of schizophrenia in adult offspring.
Am J Psychiatry
159
1528
1533
13. ByrneMAgerboEEwaldHEatonWWMortensenPB
2003
Parental age and risk of schizophrenia: a case-control study.
Arch Gen Psychiatry
60
673
678
14. DalmanCAllebeckP
2002
Paternal age and schizophrenia: further support for an association.
Am J Psychiatry
159
1591
1592
15. El-SaadiOPedersenCBMcNeilTFSahaSWelhamJ
2004
Paternal and maternal age as risk factors for psychosis: findings from Denmark, Sweden and Australia.
Schizophr Res
67
227
236
16. MalaspinaDHarlapSFennigSHeimanDNahonD
2001
Advancing paternal age and the risk of schizophrenia.
Arch Gen Psychiatry
58
361
367
17. SiposARasmussenFHarrisonGTyneliusPLewisG
2004
Paternal age and schizophrenia: a population based cohort study.
Bmj
329
1070
18. ZammitSAllebeckPDalmanCLundbergIHemmingsonT
2003
Paternal age and risk for schizophrenia.
Br J Psychiatry
183
405
408
19. WohlMGorwoodP
2007
Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring.
Eur Psychiatry
22
22
26
20. LauritsenMBPedersenCBMortensenPB
2005
Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study.
J Child Psychol Psychiatry
46
963
971
21. GillbergC
1980
Maternal age and infantile autism.
J Autism Dev Disord
10
293
297
22. ReichenbergAGrossRWeiserMBresnahanMSilvermanJ
2006
Advancing paternal age and autism.
Arch Gen Psychiatry
63
1026
1032
23. CantorRMYoonJLFurrJLajonchereCM
2007
Paternal age and autism are associated in a family-based sample.
Mol Psychiatry
12
419
421
24. CroenLANajjarDVFiremanBGretherJK
2007
Maternal and paternal age and risk of autism spectrum disorders.
Arch Pediatr Adolesc Med
161
334
340
25. BergJSBrunetti-PierriNPetersSUKangSHFongCT
2007
Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region.
Genet Med
9
427
441
26. SebatJLakshmiBMalhotraDTrogeJLese-MartinC
2007
Strong association of de novo copy number mutations with autism.
Science
316
445
449
27. WeissLAShenYKornJMArkingDE
2008
Association between microdeletion and microduplication at 16p11.2 and autism.
N Engl J Med
358
667
675
28. StefanssonHRujescuDCichonSPietilainenOPIngasonA
2008
Large recurrent microdeletions associated with schizophrenia.
Nature
455
232
236
29. StoneJLO'DonovanMCGurlingHKirovGKBlackwoodDH
2008
Rare chromosomal deletions and duplications increase risk of schizophrenia.
Nature
455
237
241
30. XuBRoosJLLevySvan RensburgEJGogosJA
2008
Strong association of de novo copy number mutations with sporadic schizophrenia.
Nat Genet
40
880
885
31. AurouxMRMayauxMJGuihard-MoscatoMLFromantinMBartheJ
1989
Paternal age and mental functions of progeny in man.
Hum Reprod
4
794
797
32. Dietz-HelmersA
1974
On correlation between the generation age of the fathers and grandfathers and the intelligence of the descendants.
Experientia
30
567
570
33. NewcombeHBTavendaleOG
1965
Effects of father's age on the risk of child handicap or death.
Am J Hum Genet
17
163
178
34. RobertsJEngelA
1974
Family background, early development and intelligence of children 6–11 years.
Vital Health Statistics
11/142
42
35. MalaspinaDReichenbergAWeiserMFennigSDavidsonM
2005
Paternal age and intelligence: implications for age-related genomic changes in male germ cells.
Psychiatr Genet
15
117
125
36. BromanSHNicholsPLKennedyWA
1975
Preschool IQ. Prenatal and early developmental correlates
Hillsdale, New Jersey
Lawrence Erlbaum Associates
37. LoblMWelcherDWMellitsED
1971
Maternal age and intellectual functioning of offspring.
Johns Hopkins Med J
128
347
361
38. FergussonDMLynskeyMT
1993
Maternal age and cognitive and behavioural outcomes in middle childhood.
Paediatr Perinat Epidemiol
7
77
91
39. ZybertPSteinZBelmontL
1978
Maternal age and children's ability.
Percept Mot Skills
47
815
818
40. NiswanderKRGordonM
1972
The women and their pregnancies
Philadephia
Saunders
41. HardyJB
2003
The Collaborative Perinatal Project: lessons and legacy.
Ann Epidemiol
13
303
311
42. BromanSBienEShaughnessyP
1985
Low achieving children: the first seven years
Hillsdale, New Jersey
Lawrence Erlbaum Associates
43. BayleyN
1969
Bayley scales of infant development
San Antonio, Texas
Psychological Corporation
44. BayleyN
1969
Manual for the Bayley Scales of Infant Development
New York
The Psychological Corporation
45. BeckerKA
2003
History of the Stanford-Binet intelligence scales: content and psychometrics. Stanford-Binet Intelligence Scales, Fifth Edition Assessment Service Bulletin No 1
Itasca, Illinois
RIverside Publishing
46. TermanLMMerrillMA
1960
Stanford-Binet Intelligence Scale
Boston
Houghton Mifflin
47. GrahamFKErnhartCBBermanPW
1963
Brain injury in the preschool child: some developmental considerations: 1. Performance of normal children.
Psychol Monogr
77
1
16
48. WechslerD
1949
Manual for the Wechsler Intelligence Scale for Children
New York
The Psychological Corporation
49. JastakSWilkinsonGSJastakJ
1936
Wide Range Achievement Test. 6th ed
Jastak Associates Inc
50. RuppertDWandMPCarrollRJ
2003
Semiparametric Regression
New York
Cambridge University Press
51. MyrianthopoulosNCFrenchKS
1968
An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population.
Soc Sci Med
2
283
299
52. NeterJKunterMHWassermanWNachtsheimCJ
2004
Applied Linear Statistical Models
Homewood
McGraw-Hill/Irwin
53. WoodSN
2006
Generalized Additive Models: An introduction with R
Boca Raton, Florida
Chapman and Hall/CRC
54. SAS Institute
2001
SAS 9.1.3
Cary, NC
SAS Institute
55. O'BrienGPearsonJ
2004
Autism and learning disability.
Autism
8
125
140
56. AylwardEWalkerEBettesB
1984
Intelligence in schizophrenia: meta-analysis of the research.
Schizophr Bull
10
430
459
57. WoodberryKAGiulianoAJSeidmanLJ
2008
Premorbid IQ in schizophrenia: a meta-analytic review.
Am J Psychiatry
165
579
587
58. WelhamJIsohanniMJonesPMcGrathJ
2008
The Antecedents of Schizophrenia: A Review of Birth Cohort Studies.
Schizophr Bull
E-pub ahead of print. doi:10.1093/schbul/sbn084
59. JonesPRodgersBMurrayRMarmotM
1994
Child development risk factors for adult schizophrenia in the British 1946 birth cohort.
Lancet
344
1398
1402
60. KremenWSBukaSLSeidmanLJGoldsteinJMKorenD
1998
IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study.
Am J Psychiatry
155
672
677
61. CannonTDBeardenCEHollisterJMRossoIMSanchezLE
2000
Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study.
Schizophr Bull
26
379
393
62. BarnettJHSalmondCHJonesPBSahakianBJ
2006
Cognitive reserve in neuropsychiatry.
Psychol Med
36
1053
1064
63. O'DonovanMCKirovGOwenMJ
2008
Phenotypic variations on the theme of CNVs.
Nat Genet
40
1392
1393
64. ValenzuelaMJSachdevP
2006
Brain reserve and cognitive decline: a non-parametric systematic review.
Psychol Med
36
1065
1073
65. SternY
2006
Cognitive reserve and Alzheimer disease.
Alzheimer Dis Assoc Disord
20
112
117
66. Hulshoff PolHESchnackHGPosthumaDMandlRCBaareWF
2006
Genetic contributions to human brain morphology and intelligence.
J Neurosci
26
10235
10242
67. PosthumaDDe GeusEJBaareWFHulshoff PolHEKahnRS
2002
The association between brain volume and intelligence is of genetic origin.
Nat Neurosci
5
83
84
68. TurkheimerEHaleyAWaldronMD'OnofrioBGottesmanII
2003
Socioeconomic status modifies heritability of IQ in young children.
Psychol Sci
14
623
628
69. SteinZSusserM
2000
The risks of having children in later life. Social advantage may make up for biological disadvantage.
BMJ
320
1681
1682
70. PearsonCENichol EdamuraKClearyJD
2005
Repeat instability: mechanisms of dynamic mutations.
Nat Rev Genet
6
729
742
71. BoschMRajmilOEgozcueJTempladoC
2003
Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age.
Eur J Hum Genet
11
754
759
72. GlaserRLBromanKWSchulmanRLEskenaziBWyrobekAJ
2003
The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm.
Am J Hum Genet
73
939
947
73. WyrobekAJEskenaziBYoungSArnheimNTiemann-BoegeI
2006
Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm.
Proc Natl Acad Sci U S A
103
9601
9606
74. PerrinMCBrownASMalaspinaD
2007
Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia.
Schizophr Bull
33
1270
1273
75. OakesCCLa SalleSSmiragliaDJRobaireBTraslerJM
2007
Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells.
Dev Biol
307
368
379
76. OakesCCSmiragliaDJPlassCTraslerJMRobaireB
2003
Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats.
Proc Natl Acad Sci U S A
100
1775
1780
77. KileBTHiltonDJ
2005
The art and design of genetic screens: mouse.
Nat Rev Genet
6
557
567
78. CasparyTAndersonKV
2006
Uncovering the uncharacterized and unexpected: unbiased phenotype-driven screens in the mouse.
Dev Dyn
235
2412
2423
79. McGrathJJ
2007
The surprisingly rich contours of schizophrenia epidemiology.
Arch Gen Psychiatry
64
14
16
80. McGrathJJHearleJJennerLPlantKDrummondA
1999
The fertility and fecundity of patients with psychosis.
Acta Psychiatrica Scandinavica
99
441
446
81. KellerMCMillerG
2006
Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best.
Behav Brain Sci
29
385
404
82. McGrathJJ
2006
The romance of balancing selection versus the sober alternatives: let the data rule (Commentary on Keller and Miller).
Behav Brain Sci
29
417
418
83. BrayIGunnellDDavey SmithG
2006
Advanced paternal age: how old is too old.
J Epidemiol Community Health
60
851
853
84. RoseG
1992
The strategy of preventive medicine
Oxford
Oxford University Press
Štítky
Interní lékařství
Článek Media Portrayals of Suicide
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2009 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Contrasting Effects of Maternal and Paternal Age on Offspring Intelligence
- Systematic Reviews of Genetic Association Studies
- Suicide after Leaving the UK Armed Forces —A Cohort Study
- Media Portrayals of Suicide
- The Need for Outreach in Preventing Suicide among Young Veterans
- Setting Research Priorities To Reduce Global Mortality from Childhood Diarrhoea by 2015
- Advanced Paternal Age Is Associated with Impaired Neurocognitive Outcomes during Infancy and Childhood
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Setting Research Priorities To Reduce Global Mortality from Childhood Diarrhoea by 2015
- Advanced Paternal Age Is Associated with Impaired Neurocognitive Outcomes during Infancy and Childhood
- Suicide after Leaving the UK Armed Forces —A Cohort Study
- Contrasting Effects of Maternal and Paternal Age on Offspring Intelligence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



