-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The International Classification of Diseases for Oncology Integrated with the Melanoma Histogenetic Model
Mezinárodní klasifikace nemocí pro onkologii integrovaná s histogenetickým modelem melanomu
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Obdrženo: 24. 12. 2018
Authors: L. Roncati; F. Piscioli
Published in the journal: Klin Onkol 2019; 32(2): 155-156
Category: Dopis redakci
doi: https://doi.org/10.14735/amko2019155Summary
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Obdrženo: 24. 12. 2018
Malignant melanoma can be defined, quite simply, as a malignant neoplasm derived from melanocytes; however, there is great histological and, consequently, clinical variability from case to case [1]. In order to try to overcome this intrinsic difficulty, various classification systems have been proposed over the years; in this regard, the World Health Organization (WHO) introduced its notorious classification about half a century ago [2]. Currently, the International Classification of Diseases for Oncology (ICD-O), provided by the WHO International Agency for Research on Cancer (IARC), distinguishes the in situ forms from invasive ones, recognising among these four main morphological subtypes – nodular melanoma, superficial spreading melanoma, lentigo maligna melanoma and acral lentiginous melanoma [3]. The ICD-O classification includes further morphological codes, such as balloon cell melanoma, regressing melanoma, amelanotic melanoma, melanoma in junctional nevus, melanoma in precancerous melanosis, desmoplastic melanoma, neurotropic melanoma, mucosal lentiginous melanoma, melanoma in giant pigmented nevus / congenital melanocytic nevus, mixed epithelioid and spindle cell melanoma, epithelioid cell melanoma, spindle cell melanoma (not otherwise specified), spindle cell melanoma (type A), spindle cell melanoma (type B) and malignant blue nevus [3]. Alongside a strictly morphological classification, a histogenetic model, based on the concept of tumour progression, is regaining ground [4,5]. In fact, at the onset, a melanoma is characterised by a non-tumorigenic radial growth phase (RGP), inside the epidermis (intraepidermal) or within the papillary dermis (microinvasive), devoid of metastatic potential, which may be followed, early or late, by a tumorigenic vertical growth phase (VGP), with deeper extension in the dermis or beyond, nodular confluence, mitotic activity and metastatic capacity (tab. 1). The unique exception to this is represented by nodular melanoma, in which either RGP is rapidly overrun by VGP or the tumour arises directly from dermal melanocytes [6]. Today, the Breslow depth remains the single most important prognostic factor for clinically localised primary melanoma – it allows the identification of melanoma as ultra-thin (≤ 0.5 mm), thin (≤ 1 mm), thick (> 1 mm) or ultra-thick (> 6 mm) [7,8]. The systematic application of the histogenetic model to the Breslow depth explains the debated reason why some thin melanoma behave aggressively, because they possess an early tumorigenic VGP inside them [9]. Moreover, any diagnostic report should also be accompanied by further well-known microstaging attributes, such as Clark level, mitotic count, lymphovascular invasion, perineural infiltration, ulceration, satellitosis, tumour infiltrating lymphocytes and, if available, sentinel lymph node status [10,11]. In conclusion, we believe that a renewed histogenetic approach to melanoma diagnosis deserves a wide scientific dissemination, for a better clinical management of individual cases in the era of personalised medicine.
Tab. 1. Melanoma progression model. 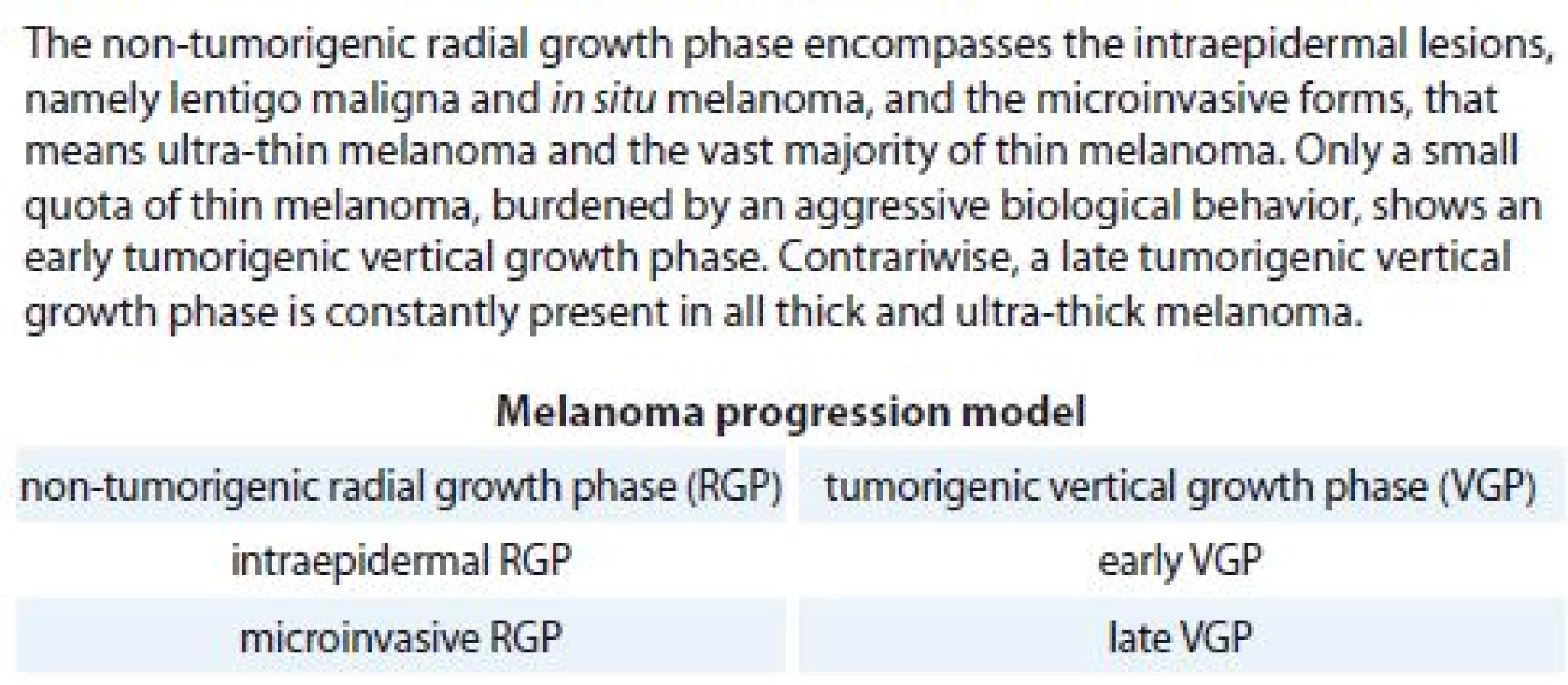
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manu script met the ICMJE recommendation for biomedical papers.
Dr. Luca Roncati, M.D., PhD
Department of Medical and Surgical Sciences
Institute of Pathology
University Hospital of Modena
Policlinico via del Pozzo, 71
I-41124 Modena, Italy
e-mail: emailmedical@gmail.com
Submitted: 24. 12. 2018
Zdroje
1. Roncati L, Piscioli F, Pusiol T. Current controversies on sentinel node biopsy in thin and thick cutaneous melanoma. Eur J Surg Oncol 2017; 43 (2): 506–507. doi: 10.1016/j.ejso.2016.09.014.
2. Duncan LM. The classification of cutaneous melanoma. Hematol Oncol Clin North Am 2009; 23 (3): 501–513. doi: 10.1016/j.hoc.2009.03.013.
3. Fritz A, Percy C, Jack A et al (eds.). International classification of diseases for oncology (ICD-O). 3rd ed. Geneva: WHO Press 2013 : 70–71.
4. Piscioli F, Pusiol T, Roncati L. Thin melanoma subtyping fits well with the American Joint Committee on Cancer staging system. Melanoma Res 2016; 26 (6): 636. doi: 10.1097/CMR.0000000000000301.
5. Roncati L, Piscioli F, Pusiol T. Surgical outcomes reflect the histological types of cutaneous malignant melanoma. J Eur Acad Dermatol Venereol 2017; 31 (6): e279–e280. doi: 10.1111/jdv.14023.
6. Greenwald HS, Friedman EB, Osman I. Superficial spreading and nodular melanoma are distinct biological entities: a challenge to the linear progression model. Melanoma Res 2012; 22 (1): 1–8. doi: 10.1097/CMR.0b013e 32834e6aa0.
7. Roncati L, Piscioli F, Pusiol T. Sentinel lymph node in thin and thick melanoma. Klin Onkol 2016; 29 (5): 393–394.
8. Meguerditchian AN, Asubonteng K, Young C, et al. Thick primary melanoma has a heterogeneous tumor biology: an institutional series. World J Surg Oncol 2011; 9 : 40. doi: 10.1186/1477-7819-9-40.
9. Piscioli F, Pusiol T, Roncati L. Critical points of T1 stage in primary melanoma. Melanoma Res 2017; 27 (4): 399. doi: 10.1097/CMR.0000000000000357.
10. Roncati L, Barbolini G, Piacentini F et al. Prognostic factors for breast cancer: an immunomorphological update. Pathol Oncol Res 2016; 22 (3): 449–452. doi: 10.1007/s12253-015-0024-7.
11. Piscioli F, Pusiol T, Roncati L. Higher predictive value of sentinel lymph node biopsy in patients with histological subcategorization of thin melanoma. Int J Dermatol 2017; 56 (5): e93–e94. doi: 10.1111/ijd.13548.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2019 Číslo 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- Chromothripsis – Extensive Chromosomal Rearrangements and Their Significance in Cancer
- High Level of Circulating Microparticles in Patients with BCR/ABL Negative Myeloproliferative Neoplasm – a Pilot Study
- Gastrointestinal Stromal Tumours of the Rectum – Evaluating the National Registry Data with Respect to its Use in Clinical Practice
- Gorlin-Goltz syndrome
- Clinical Management and Outcome in Extreme Retroperitoneal Growing Teratoma Syndrome of Testicular Origin – Clinical Management and Effect of the Treatment
- Hepatic Injury Induced by a Single Dose of Nivolumab – a Case Report and Literature Review
- Leptomeningeal Metastasis in a Breast Cancer Treated with Two Lines of Intrathecal Chemotherapy – a Case Report
- IgG4 Sclerosing Cholangitis – an Inflammation Imitating Tumour of the Pancreas and Biliary Tract
- Aktuality z odborného tisku
- The International Classification of Diseases for Oncology Integrated with the Melanoma Histogenetic Model
- Tailoring Nutritional Interventions with Molecular Pathophysiology of Cancer Cachexia – a Possible Solution to an Old Problem
- Onkologie v obrazech
- Když pomáháme imunitnímu systému
- Therapy of Relapsed/Refractory Acute Lymphoblastic Leukemia Today and Tomorrow
- Oligometastatic Prostate Cancer
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromothripsis – Extensive Chromosomal Rearrangements and Their Significance in Cancer
- Gorlin-Goltz syndrome
- Oligometastatic Prostate Cancer
- Hepatic Injury Induced by a Single Dose of Nivolumab – a Case Report and Literature Review
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



