-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
HCV genotype shift occurred over the 15 years in PWIDs in the Czech Republic
Vývoj v zastoupení genotypů HCV u injekčních uživatelů drog v České republice během 15 let
Cíl:
Pokračovat ve sledování vývoje epidemie HCV infekce, zvláště genotypového zastoupení u injekčních uživatelů drog (PWID) v Praze a středních Čechách.
Metody:
V letech 2010–2012 bylo zařazeno 546 pacientů, bývalých či současných injekčních uživatelů drog, kteří byli vstupně testováni na přítomnost anti-HCV protilátky, u pozitivních byla pomocí Real-time PCR provedena kvantifikace a genotypizace viru. Získaná data z let 2010–2012 byla následně porovnána s kontrolními skupinami z období let 1998–2000 a 2005–2007.
Výsledky:
Z 546 zařazených a testovaných pacientů bylo 393 (72 %) anti-HCV pozitivních; z nich 269 (68,4 %) mělo detekovatelnou HCV PCR RNA. Nejčastější subtyp HCV byl 3a u 97 pacientů (36,1 %), 1a u 85 pacientů (31,6 %) a 1b u 57 pacientů (21,2 %). Tyto tři subtypy byly zodpovědné za téměř 89 % infekcí ve zkoumané skupině.
Závěry:
Byl potvrzen statisticky významný nárůst v zastoupení genotypů 1a a 3a se současným poklesem frekvence genotypu 1b. U genotypu 1b a genotypu 3a narůstala statistická významnost změn v závislosti na roce záchytu. Popsaný genotypový posun odráží vývoj epidemie HCV a odpovídá aktuálně převažujícímu způsobu přenosu HCV infekce.
Klíčová slova:
HCV – genotypy HCV – injekční uživatelé drog (PWID) – Česká republika
Authors: L. Krekulová; V. Řehák; Z. Oktábec; J. Vacek
Authors place of work: 4th Department of Internal Medicine, 1st Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic 1; Remedis, s. r. o., Prague, Czech Republic 2; Department of Addictology, 1st Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic 3
Published in the journal: Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 1, s. 3-8
Category: Původní práce
Summary
Objectives:
To follow on the epidemiology of HCV, especially genotypes spreading among people who inject drugs (PWID) in Prague and surrounding Central Bohemia, Czech Republic.
Methods:
546 patients who reported past and/or recent injecting of drugs were recruited in the years 2010–2012. They were initially tested for anti-HCV. Real-time PCR was used for quantification and genotyping of hepatitis C virus. Obtained data from the years 2010–2012 were compared with historical controls from periods of 1998–2000 and 2005–2007.
Results:
Of 546 initially recruited and tested patients were 393 (72%) anti-HCV seropositive and of them 269 (68.4%) had detectable HCV PCR RNA. The most prevalent subtype was 3a in 97 patients (36.1%), 1a was detected in 85 patients (31.6%) and 1b in 57 patients (21.2%). These three genotypes were responsible for nearly 89% of infections.
Conclusion:
Significant increase in both genotypes 1a and 3a over the 15 years was apparent and significant, followed by the decrease in genotype 1b. In the genotype 1b and genotype 3a the significance has risen with the years of data collection. Described genotypic shift reflects the evolution of HCV epidemics and corresponds with the mode of transmission.
Keywords:
Czech Republic – HCV – HCV genotypes – people who inject drugs
INTRODUCTION
Hepatitis C remains the most prevalent blood borne disease, affecting predominantly people who inject drugs (PWID) in the Czech Republic (CR). In contrast with common population in CR where the historical HCV prevalence used to be very low [1], the transmission in PWIDs in CR [2] is still ongoing in relatively constant fashion. The introduction of broad variety of preventative and awareness programs in early nineties of the last century has apparently had a favorable influence on the control of HIV transmission in PWID group and the number of reported cases of HIV remains low and stable [3]. Such interventions were only partially effective in the case of HCV because of virus higher infectivity and complex nature of HCV transmission [4, 5].
PWIDs are generally a group which is difficult to access and manage [6]. Only part of the users is aware of their infection status, thus low-threshold harm reduction interventions, such as needle-syringe exchange programs (NSP) and opioid substitution treatment (OST), including testing for HCV and treatment of HCV, are primary prerequisities for HCV control [7].
HCV genotype distribution possesses distinct epidemiological patterns in different countries and regions. There is increasing evidence that the mode of transmission could favor propagation of certain genotypes [8].
Particular genotypes are directly related to clinical outcomes and also determine treatment schedules [9]. The importance of HCV genotypes and subtypes has gained even higher significance with the latest directly acting antivirals (DAA) [10, 11], where the treatment must be tailored precisely according to the subtype, disease stage and resistance scenario. The upcoming pangenotypic medication combinations might resolve this complicated situation.
HCV genotype distribution evolves in specific populations in particular locations over time, as was reported on many occasions [8, 9]. Changes in the routes of transmission are considered as one of the major determinants of HCV genotypic shift. Genotypes 1a and 3a have recently been spread mainly by the risky activities such as injecting drugs or risky tattooing in certain groups.
Tab. 1. Characteristics of patients in 2010–2012 (N/I: not investigated) 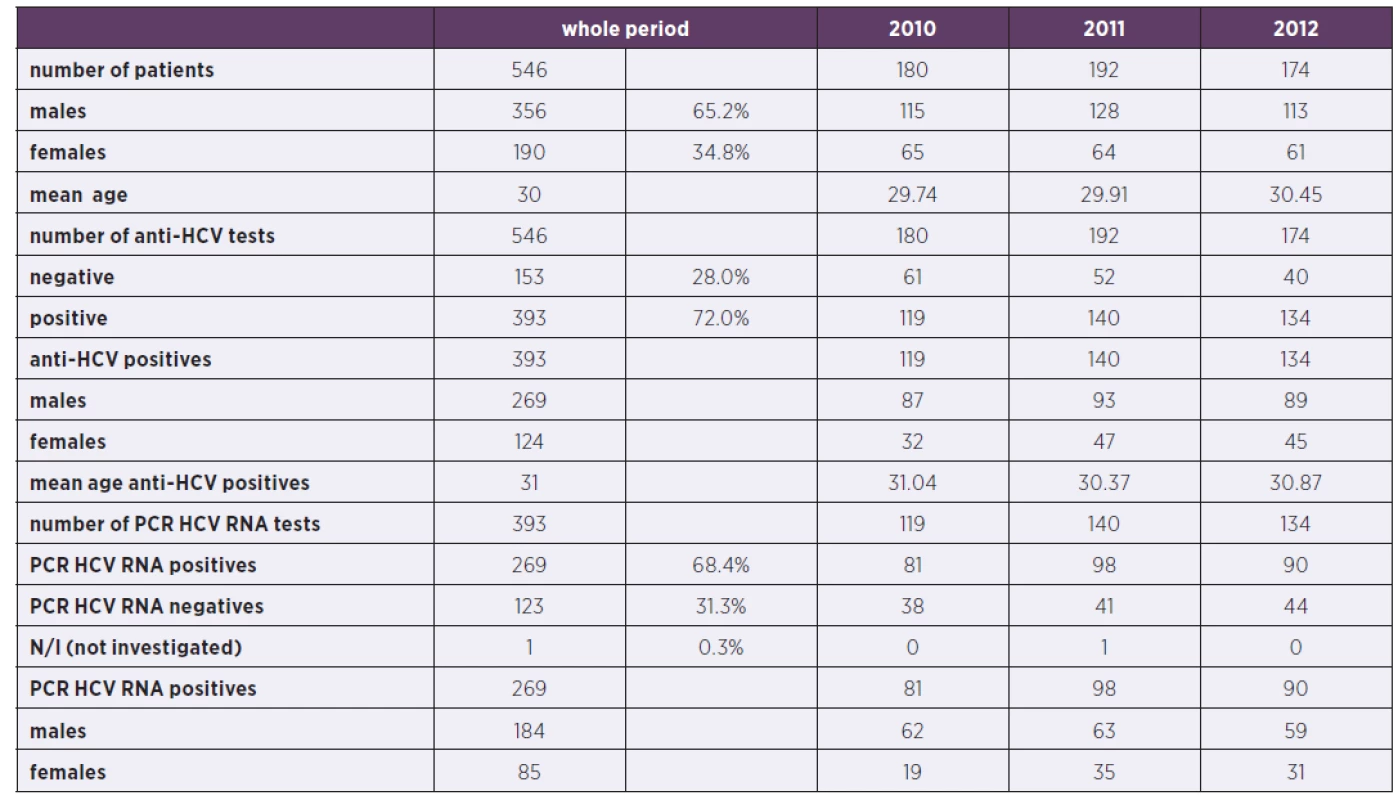
In our previous work we looked at the genotypes in two papers. In the first publication which was a pilot study, we described 1b as dominating genotype in CR. It was a reflection that 1b used to be a baseline genotype at the beginning of the epidemic [12]. With the ongoing widespread habit of injecting drugs in CR we further noticed the changes in HCV epidemiology and genotypic shift in favor of 1a and 3a genotypes as we described in the second paper [13]. The comparison with the 2010–2012 periods will follow in Table 2. Based on the literature, our previous and current experience we expect continuous changes in HCV epidemiology. In this work we further follow on the development in the distribution of genotypes in the population of CR.
Tab. 2. Comparison of patients in the three periods (N/I: not investigated) 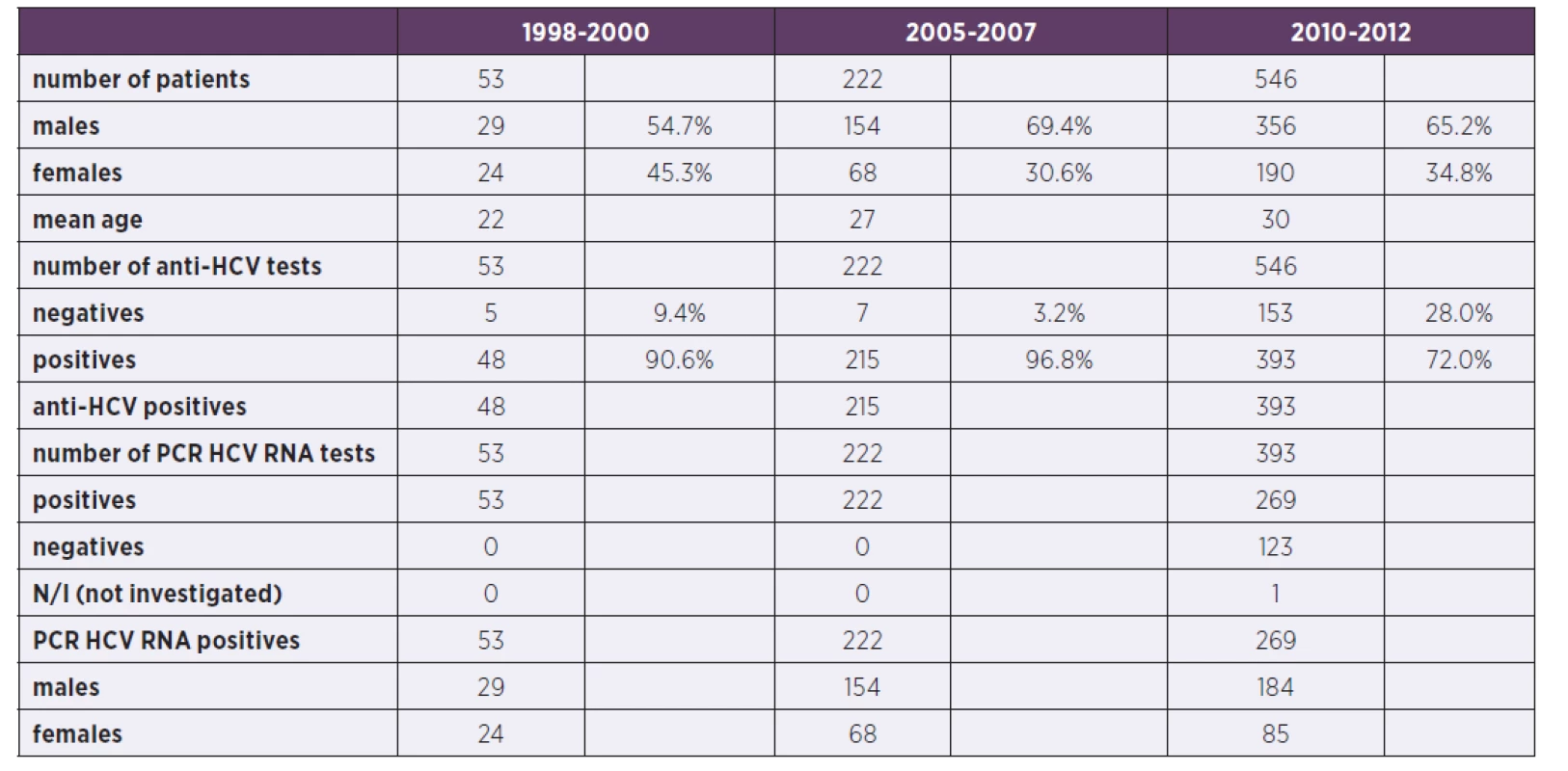
Remedis, where all work has been done, is multidisciplinary outpatient clinic. It provides various services for drug users in one place together with regular medical care and non-medical services, e.g. OST, social programs, etc. Program of Comprehensive Care was established in 1997 with the goal to reach drug users and to offer timely and targeted specific health care services for drug users including the individually tailored HCV therapy. The “under-one-roof concept” was established at that time and subsequently became a current good practice. With this approach Remedis evolved in the largest HCV treatment center in the country, which further enhances the impact of the treatment-as-prevention approach applied within this risk group in this clinic.
CR belonged and still belongs to countries with low HCV prevalence. Unfortunately, data available on HCV prevalence in CR are scarce. The last published serological survey was performed in 2001, where the general anti-HCV seroprevalence was 0.2% [1]. More recent data are based on estimates only, where the viremic prevalence was estimated to be 0,4% and seroprevalence was back calculated as 0,6% in 2012 [14]. We believe that the prevalence in general, especially older population in CR was low and remains so also at present. This is witnessed by the limited number of liver transplants secondary to HCV infection [15], low numbers of HCV captures in general laboratories and transfusion facilities [16].
The only published multicentric study described the average country HCV seroprevalence in PWID within the range of 30%, although there were found broad variations among regions and the highest prevalence was detected in large cities [17]. Substantial drop in the HCV incidence in CR could be achieved in reasonably brief period of time, provided the focused prevention, identification of those infected and fully accessible DAA treatment will be available without restrictive limits.
Objectives
The aim of the work was to monitor the epidemiological situation and the spread of viral hepatitis C in the population of PWID in Prague and Central Bohemia, in particular to monitor the representation of individual HCV genotypes in this subgroup and to compare the results with our data published in the past. The genotypes found in the cohort of patients from the years 2010–2012 were compared with the pilot groups investigated in 1998–2000 and 2005–2007.
PATIENTS AND METHODS
Patients for current work (the last group) were recruited over the years 2010–2012. The basic demographic data and epidemiological and clinical characteristics were obtained from each patient on admission to the study.
The study was designed as prospective monocentric study among all PWIDs who were for the first time registered for HCV infection screening (or diagnosis confirmation) without any relation to further individual’s cooperation or participation in any subsequent treatment programs. None of the recruited subjects in this study were included in our previous papers.
Study population
All subjects enrolled in the study over the years 2010–2012 were residents of the Czech Republic. All enrolled subjects admitted past or current injecting habit (PWID). They were consecutively referred from cooperating street-work programs or low-threshold facilities or self-referred to Remedis. Informed consent with testing and participation in the study was obtained from all subjects prior to initial interview.
Laboratory methods
The anti-HCV tests and HCV RNA detection, genotype and subtype identification were performed in certified laboratories (ISO 15198) with standard, validated and certified methods. AntiHCV was tested by Monolisa anti-HCV version 2, BioRad, SA. Anti-HCV positive samples (or those suspected from acute HCV) were tested by RT-PCR.
The assessment of HCV RNA in blood samples was done by standardized PCR method (COBAS® AmpliPrep/COBAS® TaqMan HCV Test, Roche Molecular Systems, Inc., Branchburg, NJ, 08876 USA) with limit of detection 15 IU/ml and linearity 43–6,9 × 107 IU/ml and genotyping was performed in samples with detectable viremia (COBAS® HCV GT, Roche Molecular Systems, Inc., Branchburg, NJ, 08876 USA).
In the two previous historical cohorts the direct sequencing of NS5B locus was used as the most reliable method at that time [12, 13].
The statistical data were processed with SPSS Pearson Chi-Square Tests and Z-tests with Bonferroni correction were used for comparison of the three above described periods.
RESULTS
546 new patients, meeting above mentioned criteria were newly registered in Remedis in Prague in the years 2010–2012. Majority of them were males (65%), average age of the whole group was 30 years. The basic demographic data and epidemiological and clinical characteristics are described in the Table 1.
All study participants were tested for HIV with negative result.
393 patients (72%) were anti-HCV seropositive and of them 269 were viremic (HCV RNA positive). Amongst HCV replicating patients, there were 184 males and 85 females. Genotype composition is shown in the Graph 1. The most prevalent subtype was 3a in 97 patients (36.1%), 1a was detected in 85 patients (31.6%) and 1b in 57 patients (21.2%). These three genotypes were responsible for nearly 89% of infections, the rest (4.2%) consisted of mixed genotypes or undistinguishable genotype 1, 1 patient had genotype 2. 7.1% of samples were not typeable for different reasons.
Graph 1. Percentage representation of main genotypes in three periods 
Genotype representation in 2010–2012 is summarized in Table 3 and Graph 1.
Tab. 3. Comparison of particular genotypes in three periods 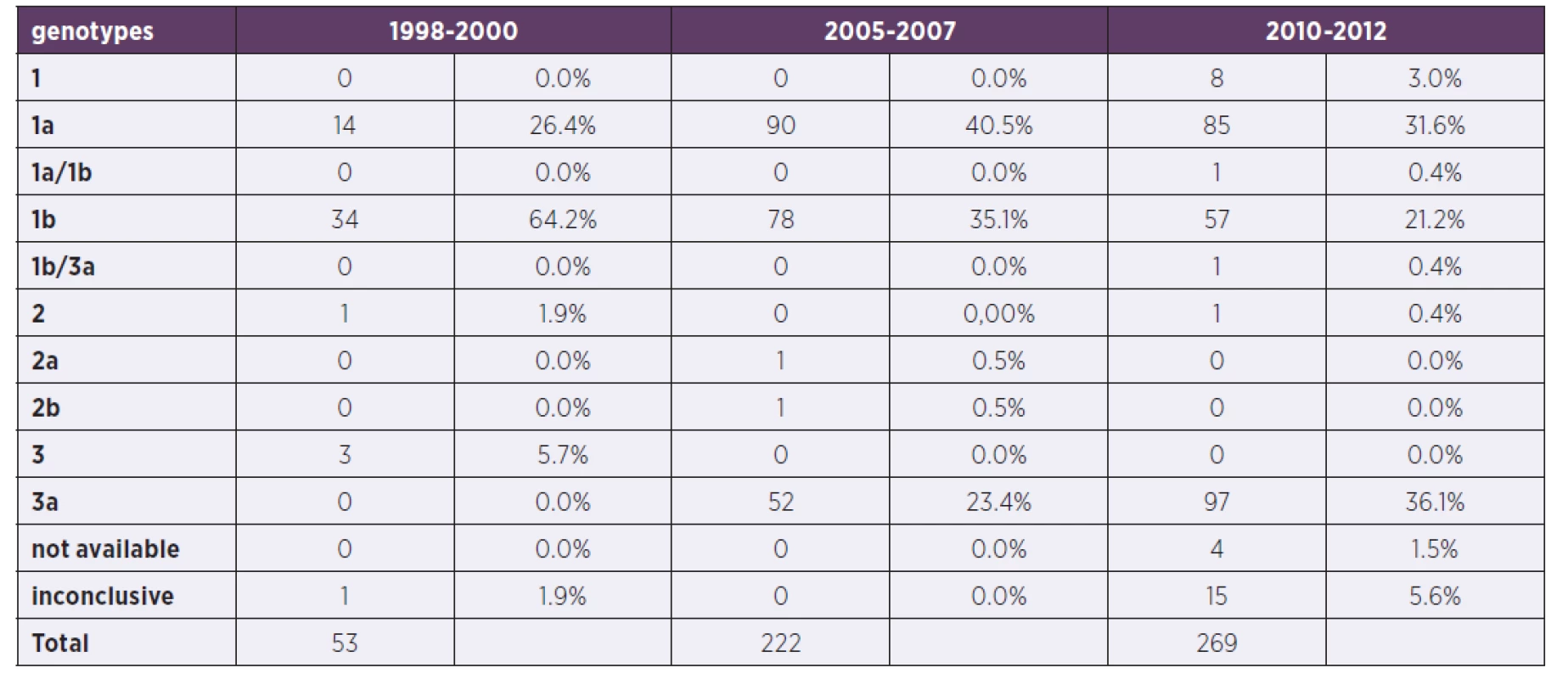
For the comparison with the two previous time periods (1998–2000 and 2005–2007) see the Table 2 where the characteristics of patients are shown and Table 3 where the genotype comparison is shown.
The development (genotype shift) of main genotypes over these three periods is depicted in Graph 2.
Graph 2. Genotype composition in 2010-2012 
Genotype 1–8 patients (3.0%), genotype 1a – 85 patients (31.6%), genotype 1a/1b: 1 patient (0.4%), genotype 1b – 57 patients (21.2%), genotype 1b/3a – 1 patient (0.4%), genotype 2 – 1 patient (0.4%), genotype 3a – 97 patients (36.1%), in 15 patients genotyping was inconclusive (5.6%) and in 4 patients (1.5%) wasn’t available. Increase in both genotypes 1a and 3 over the years was apparent and significant, followed by the decrease in genotype 1b.
We have performed the Pearson Chi-Square Tests and Z-tests with Bonferroni correction for comparison of the three periods. As the results indicate there were statistically significant differences between the groups in the meaning of Pearson Chi-Square Tests (p < 0.001). When the Z-tests with Bonferroni correction were applied, the results showed that in the three genotypes tested (1a, 1b and 3a) there were significant statistical differences. In the genotype 1b and genotype 3a the significance of differences of relative prevalence has risen with the years of data collection (p < 0.001 in the genotype 1b for period 1998–2000 vs. 2005–2007 comparison and p = 0.019 for period 2005–2007 vs. 2010–2012 comparison, in the genotype 3a p = 0.014 and p < 0.001 respectively). The prevalence of genotype 1a in period 2005–2007 was significantly higher in comparison with the genotype 3a prevalence between all periods (p = 0.038).
DISCUSSION AND CONCLUSION
In the three subsequent three-year periods we compared our observations and genotyping data of unique newly registered patients. Although ageing, the HCV infected population in the CR is still relatively young, males remain predominant. The most significant change observed was the steady decrease of initially dominant HCV subtype 1b. Observed directions in genotypic shift testify rather for ongoing HCV epidemic than for stabilized prevalence in the population. The described trends in the evolution of HCV genotypes distribution possess some similarities with other countries. We might assume backwards, that the onset of HCV epidemic among PWID in CR occurred in the times of political isolation and that the initial source was similar like in common population infected from blood transfusions and other medical interventions before 1992. Other subtypes might have been introduced in subsequent years, after the political release from the sources abroad where the 1a and 3a are widely prevalent in high-risk populations. Unlike some countries though, where the incidence of one of the genotypes dominates (either 1a or 3a) [18], in CR we can see the increase in both 1a and 3a genotypes. It might be interesting to follow on the further development and result of the “race” of the two competing genotypes. Detailed and updated knowledge of HCV epidemiological characteristics in particular regions can help in precise targeting of eradication campaigns. With the WHO and other authorities goal of HCV elimination in the context of current highly efficacious antiviral treatment [19] the urgent need to precisely document the epidemiological situation and to track all risk groups, excluded communities and another possible reservoirs of HCV possess the greatest challenge.
Do redakce došlo dne 5. 4. 2018.
Adresy pro korespondenci:
MUDr. Laura Krekulová, Ph.D.
REMEDIS, s. r. o
Vladimírova 10
140 00 Praha 4
Mgr. PharmDr. Zbyněk Oktábec, Ph.D.
e-mail: zbynek.oktabec@lf1.cuni.cz
Zdroje
1. Němeček V, Částková J, Fritz P, et al. The 2001 serological survey in the Czech Republic – viral hepatitis. Central European Journal of Public Health, 2003; 11, suppl.:S54–61.
2. Mravčík V (Ed.), Chomynová P, Grohmannová K, et al. Výroční zpráva o stavu ve věcech drog v České republice v roce 2014 [Annual Report on Drug Situation 2014 – Czech Republic]. Praha: Úřad vlády České republiky, 2015.
3. Malý M, Němeček V, Zákoucká H. Výskyt a šíření HIV/AIDS v České republice v roce 2016 (The prevalence and spread of HIV/AIDS in the Czech Republic in 2016). Zprávy z centra epidemiologie a mikrobiologie (SZÚ, Praha), 2017; 26 : 6–7.
4. The Negative Impact Of The War On Drugs On Public Health: The Hidden Hepatitis C Epidemic: Report of the global commission on drug policy. The global commission on drug policy [online]. 2013, May [cit. 2018-03-24]. Dostupné na www: http://www.globalcommissionondrugs.org/hepatitis/gcdp_hepatitis_english.pdf.
5. Nečas V, Mravčík V (Eds.). Prevence a kontrola infekčních nemocí u injekčních uživatelů drog: metodický pokyn ECDC a EMCDDA. Praha: Úřad vlády České republiky, 2012. ISBN 978-80-7440-064-3.
6. Bruggmann P. Accessing Hepatitis C patients who are difficult to reach: it is time to overcome barriers. Journal of Viral Hepatitis, 2012;19(12):829–835. DOI: 10.1111/jvh.12008. ISSN 13520504. Dostupné na www: http://doi.wiley.com/10.1111/jvh.12008.
7. Greberly J, Dore GJ, Morin S, et al. Elimination of HCV as a public health concern among people who inject drugs by 2030 – What will it take to get there? Journal of the International AIDS Society, 2017;20(1):22146. DOI: 10.7448/IAS.20.1.22146. ISSN 17582652. Dostupné na www: http://doi.wiley.com/10.7448/IAS.20.1.22146.
8. Pawlotsky J-M, Tsakiris L, Roudot-Thoraval F, et al. Relationship between Hepatitis C Virus Genotypes and Sources of Infection in Patients with Chronic Hepatitis C. Journal of Infectious Diseases, 1995;171(6):1607–1610. DOI: 10.1093/infdis/171.6.1607. ISSN 0022-1899. Dostupné na www: https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/171.6.1607.
9. Backmund M, Meyer K, Von Zielonka M, et al. Treatment of hepatitis C infection in injection drug users. Hepatology, 2001;34(1):188–193. DOI: 10.1053/jhep.2001.25882. ISSN 02709139. Dostupné na www: http://doi.wiley.com/10.1053/jhep.2001.25882.
10. Pawlotsky J-M. New Hepatitis C Therapies: The Toolbox, Strategies, and Challenges. Gastroenterology, 2014;146(5):1176–1192. DOI: 10.1053/j.gastro.2014.03.003. ISSN 00165085. Dostupné na www: http://linkinghub.elsevier.com/retrieve/pii/S0016508514003047.
11. Persico M, Coppola N, Rosato V, et al. HCV antiviral therapy in injection drug users: difficult to treat or easy to cure? Annals Of Hepatology, 2015;14(3):325–332. Dostupné na www: ttp://www.annalsofhepatology.com/revista/numeros/2015/HP153-06-HCV%20(Antiviral)%20(FF_310315m)_PROTEGIDO.pdf.
12. Krekulova L, Rehak V, Madrigal N, et al. Genotypic and Epidemiologic Characteristics of Hepatitis C Virus Infections among Recent Injection Drug User and Nonuser Populations. Clinical Infectious Diseases, 2001;33(8):1435–1438. DOI: 10.1086/323199. ISSN 1058-4838. Dostupné na www: https://academic.oup.com/cid/article-lookup/doi/10.1086/323199
13. Krekulová L, Řehák V, Strunecký O, et al. Situace a trendy v zastoupení genotypů viru hepatitidy C v populaci injekčních uživatelů drog. Epidemiologie, Mikrobiologie a Imunologie, 2009;58(2):84–89.
14. Bruggmann P, Berg T, Øvrehus A, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. Journal Of Viral Hepatitis, 2014;21 : 5–33. DOI:10.1111/jvh.12247. ISSN 13520504. Dostupné na www: http://doi.wiley.com/10.1111/jvh.12247.
15. Šperl J, Fraňková S, Trunečka P. Transplantace jater pro chronickou hepatitidu C: význam protivirové léčby. Gastroenterol. Hepatol., 2013;67(5):407–412.
16. Němeček V, Strunecký O. Genotypová heterogenita viru hepatitidy C (HCV) u dárců krve v ČR [Genotypic heterogeneity of hepatitis C virus (HCV) from blood donors in the Czech Republic]. Epidemiologie, Mikrobiologie a Imunologie, 2009;58(2):63–72.
17. Zábranský T, Mravčík V, Korčišová B, et al. Hepatitis C Virus Infection among Injecting Drug Users in the Czech Republic – Prevalence and Associated Factors. European Addiction Research, 2006;12 : 151–160.
18. Robayes G, Bielen R, Azar D, et al. Global genotype distribution of hepatitis C viral infection among people who inject drugs. Journal of Hepatology;65(6):1094–1103. Dostupné na www: https://www.journal-of-hepatology.eu/article/S0168-8278(16)30418-4/fulltext.
19. Greberly J, Dalgard O, Conway B, et al. Efficacy and safety of sofosbuvir/velpatasvir in people with chronic hepatitis C virus infec-
tion and recent injecting drug use: the SIMPLIFY study. Journal of Hepatology;66(1), Supplement:S513. Dostupné na www: https://www.journal-of-hepatology.eu/article/S0168-8278(17)31428-9/fulltext.Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2019 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Clinical and microbiological characteristics of Clostridium difficile infection in children hospitalized at the Departement of Paediatric Infectious Diseases in Brno between 2013 and 2017
- Progressive multifocal leukoencephalopathy – epidemiology, immune response, clinical differences, treatment
- HCV genotype shift occurred over the 15 years in PWIDs in the Czech Republic
- Botulism – a rare but still present, life-threatening disease
- Decontamination of CBRN units contaminated by highly contagious biological agents
- Typhoid fever in the Czech Republic and an imported case after return from the Rainbow Gathering in Italy
- Zemřel prof. MUDr. Jan Šejda, DrSc.
- Zemřela RNDr. Eva Aldová, CSc. (*21. 11. 1922 – †20. 12. 2018)
- Prevalence of hepatitis C virus infection in adults with the risk factors
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Botulism – a rare but still present, life-threatening disease
- Progressive multifocal leukoencephalopathy – epidemiology, immune response, clinical differences, treatment
- Typhoid fever in the Czech Republic and an imported case after return from the Rainbow Gathering in Italy
- Decontamination of CBRN units contaminated by highly contagious biological agents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



