-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe efficiency of micro-vascular decompression versus micro-vascular decompression with partial sensory rhizotomy for classical trigeminal neuralgia – a retrospective analysis of 58 patients
Účinnost mikrovaskulární dekomprese v porovnání s mikrovaskulární dekompresí s parciální senzorickou rhizotomií při klasické neuralgii trojklanného nervu – retrospektivní analýza 58 pacientů
Cíl: Cílem této studie bylo zhodnotit účinnost mikrovaskulární dekomprese (MVD) s parciální senzorickou rhizotomií (PSR) nebo bez ní při léčbě neuralgie trojklanného nervu, která je refrakterní vůči medikamentózní nebo funkční léčbě.
Materiál a metody: Do studie bylo zařazeno 58 pacientů, u kterých byla diagnostikována klasická neuralgie trojklanného nervu a v letech 2010–2016 podstoupili operační dekompresi zadní jámy lební provedenou stejným chirurgem. Podle typu operace byly ve studii dvě skupiny, a to s 30 (51,7 %) pacienty léčenými pomocí MVD a 28 (48,2 %) pacienty léčenými kombinací MVD + PSR. Zdravotní záznamy byly analyzovány retrospektivně z hlediska intraoperačních nálezů, skóre intenzity bolesti, rekurence bolesti, pooperačních výsledků a komplikací.
Výsledky: V časném pooperačním období byla okamžitá úleva od bolesti v obou skupinách podobná. Podíl rekurence po 36 měsících od operace byl 23,3 % ve skupině léčené pomocí MVD a 7,1 % ve skupině léčené kombinací MVD + PSR. Doba přežívání bez bolesti po 36 měsících od operace byla ve skupině MVD 31,83 ± 1,9 (95% CI 28,3–35,63) měsíce, což bylo méně než 35,14 ± 1,1 (95% CI 32,81–37,48) měsíců ve skupině MVD + PSR (p = 0,086). Co se týče pooperačních komplikací, jeden pacient (1,7 %) měl akutní subdurální krvácení a u dalšího (1,7 %) došlo k faciální paréze; hypestezie byla zaznamenána u 18 (31 %) pacientů. V žádné ze skupin nedošlo k úmrtí.
Závěr: MVD a MVD + PSR jsou bezpečné a účinné metody při klasické neuralgii trojklanného nervu.
Klíčová slova:
mikrovaskuární dekomprese – parciální senzorická rhizotomie – neuralgie trojklanného nervu
Authors: Y. Gezercan 1; V. Acik 1; B. Arslan 2; S. K. Olguner 1; İ. İstemen 1; E. Bilgin 1; H. Millet 1; A. Arslan 1; A. İ. Okten 1
Authors place of work: Department of Neurosurgery, Adana, City Training and Research Hospital, Adana, Turkey 1; Department of Anesthesia and Intensive Care, Adana City Training and, Research Hospital, Adana, Turkey 2
Published in the journal: Cesk Slov Neurol N 2020; 83/116(5): 544-549
Category: Původní práce
doi: https://doi.org/10.14735/amcsnn2020544Summary
Aim: This study aimed to assess the effectiveness of micro-vascular decompression (MVD) with or without partial sensory rhizotomy (PSR) in the treatment of trigeminal neuralgia which is refractory to medical and functional treatments.
Materials and methods: Fifty-eight patients who were diagnosed with classical trigeminal neuralgia and underwent posterior fossa decompression surgery from 2010 to 2016 by the same surgeon were included in the study. There were two groups of patients, according to operation type, with 30 (51.7%) patients treated with MVD and 28 (48.2%) treated with MVD + PSR. Records were analysed retrospectively for intraoperative findings, pain intensity scores, recurrence of pain, postoperative results and complications.
Results: In the early postoperative period, immediate pain relief was similar between the groups. The recurrence rate 36 months after surgery in the MVD and MVD + PSR groups was 23.3 and 7.1%, respectively. The pain-free survival time in the MVD group was 31.83 ± 1.9 (95% CI: 28.3–35.63) months, which was shorter than the 35.14 ± 1.1 (95% CI: 32.81–37.48) months in the MVD + PSR group at 36 months after surgery (P = 0.086). Concerning postoperative complications, one patient had an acute subdural haemorrhage (1.7%) and another had facial paralysis (1.7%), while hypoesthesia was experienced in 18 (31%) patients. There were no deaths in either group.
Conclusion: MVD and MVD + PSR are safe and effective procedures for classical trigeminal neuralgia.
Keywords:
micro-vascular decompression – partial sensory rhizotomy – trigeminal neuralgia
Introduction
Classical trigeminal neuralgia (TN) is characterised by shock-like, severe, transient, repetitive attacks of pain in the trigeminal nerve distribution area of the face [1,2]. The attacks can occur spontaneously or typically, can be provoked by talking, chewing, shaving, teeth brushing or even a light touch [1,2]. The disease occurs more frequently in women and older people. The precise mechanisms underlying TN remain unclear, but likely involve vascular compression of the trigeminal nerve at the brain stem root entry zone [3]. Pharmacotherapy is the preferred initial management approach in TN, but in most patients, it fails or causes adverse effects in the long-term, which may result in a relapse. Various minimally invasive methods including percutaneous balloon compression, radiofrequency thermal rhizotomy and glycerol rhizotomy, or radiological procedures such as gamma knife or linear accelerator, are recommended for patients who are refractory to pharmacological treatments [4,5]. If all these methods fail to control the pain, then surgical micro-vascular decompression (MVD) or MVD with partial sensory rhizotomy (PSR) may be considered [6,7]. Recently, several studies have reported successful results with MVD + PSR, compared to MVD alone as the first-line surgical option for TN [8,9]. In this study, we compared the effectiveness of MVD and MVD + PSR in 58 patients with TN with pain refractory to medical, minimally invasive, and radiosurgical treatments.
Materials and methods
The clinical records of 58 patients diagnosed with classical TN who underwent surgical treatment at the Adana City Training and Research Hospital, Turkey from January 2010 to December 2016 were retrospectively examined.
The diagnostic criteria for classical TN were based on a history and clinical presentation fulfilling the International Headache Society criteria for classical TN [1]. The inclusion criteria were: medical history more than 6 months; pharmacotherapy for more than 3 months before surgery with recurrence or no efficacy; at least 2 attempts with minimally invasive procedures with recurrence or no efficacy; no history of other surgical treatment; and postoperative follow-up data available for at least 3 years. The exclusion criteria were: pregnant or nursing mothers; pathologies such as multiple sclerosis plaques, tumours and abnormalities of the skull base; and severe liver, kidney or cardio-pulmonary dysfunction.
Each patient was informed of the risks and benefits of the two surgical techniques. Besides MVD, consent was obtained for PSR in all cases. PSR was performed in addition to MVD where there was: 1. no clear vascular contact with the trigeminal nerve root; 2. significant adhesion of the arachnoid to the nerve roots. In total, 58 patients were included in the study, 30 of whom underwent MVD and 28 MVD + PSR.
The following details were available for analysis: patients’ age, gender, pain lateralisation, distribution zones of the pain, pain intensities at presentation and at the time of the study, duration of symptoms before our centre evaluation and before the study, treatment modalities received, intraoperative findings, side effects (facial numbness, dysesthesia, keratitis, trigeminal motor dysfunction, haemorrhage, infarction mortality), and neurosensory status at final evaluation. The pain intensity was rated using the Barrow Neurological Institute’s (BNI) Pain Intensity Scale [10]. The BNI pain intensity scoring criteria are:
- no pain;
- occasional pain, not requiring medication;
- some pain, controlled with medication;
- some pain, not controlled with medication;
- severe pain/no pain relief.
All patients were followed-up periodicallyafter surgery for at least 3 years, with the BNI Pain Intensity Scale repeated at each visit. For analysis, all patients were categorised into three outcome categories by their BNI Pain Intensity Scale score. BNI-I and II were considered an excellent outcome, BNI-III was a good outcome and BNI-IV and V were taken as recurrence. All procedures were performed by the author (Y. G.) using the technique described below.
Surgical Technique
Under general anaesthesia, patients were immobilised in the sitting position on the Mayfield headrest, with the head at a 15° angle on the lesion side. A 3 × 3 cm craniotomy was performed with a standard suboccipital retro-sigmoid approach. The dura was opened as a semilunar opening over the sigmoid sinus. The lateral cerebello-medullary cistern was opened to achieve cerebrospinal fluid (CSF) drainage and minimal cerebellar retraction was performed. The arachnoid membrane was opened from the path from Meckel’s cave to the brain stem to allow the mobilisation of arteries superiorly. The trigeminal nerve was inspected for compression using a microscope and an angled endoscope (45 degrees), allowing a view of 360° to reveal the vascular structures creating pressure. After the vascular structure creating pressure on the nerve was identified, it was reserved along the artery’s proximal and distal line. After the compressing artery was repositioned, it was kept separate from the nerve by placing a Teflon patch between the nerves and compressing artery (Fig. 1). Gel foam or fibrin adhesive was used to fix the Teflon patch when required.
Fig. 1. Placement of Tefl on between the trigeminal nerve and vascular structure.
Obr. 1. Umístění tefl onu mezi trojklanný nerv a cévní strukturu.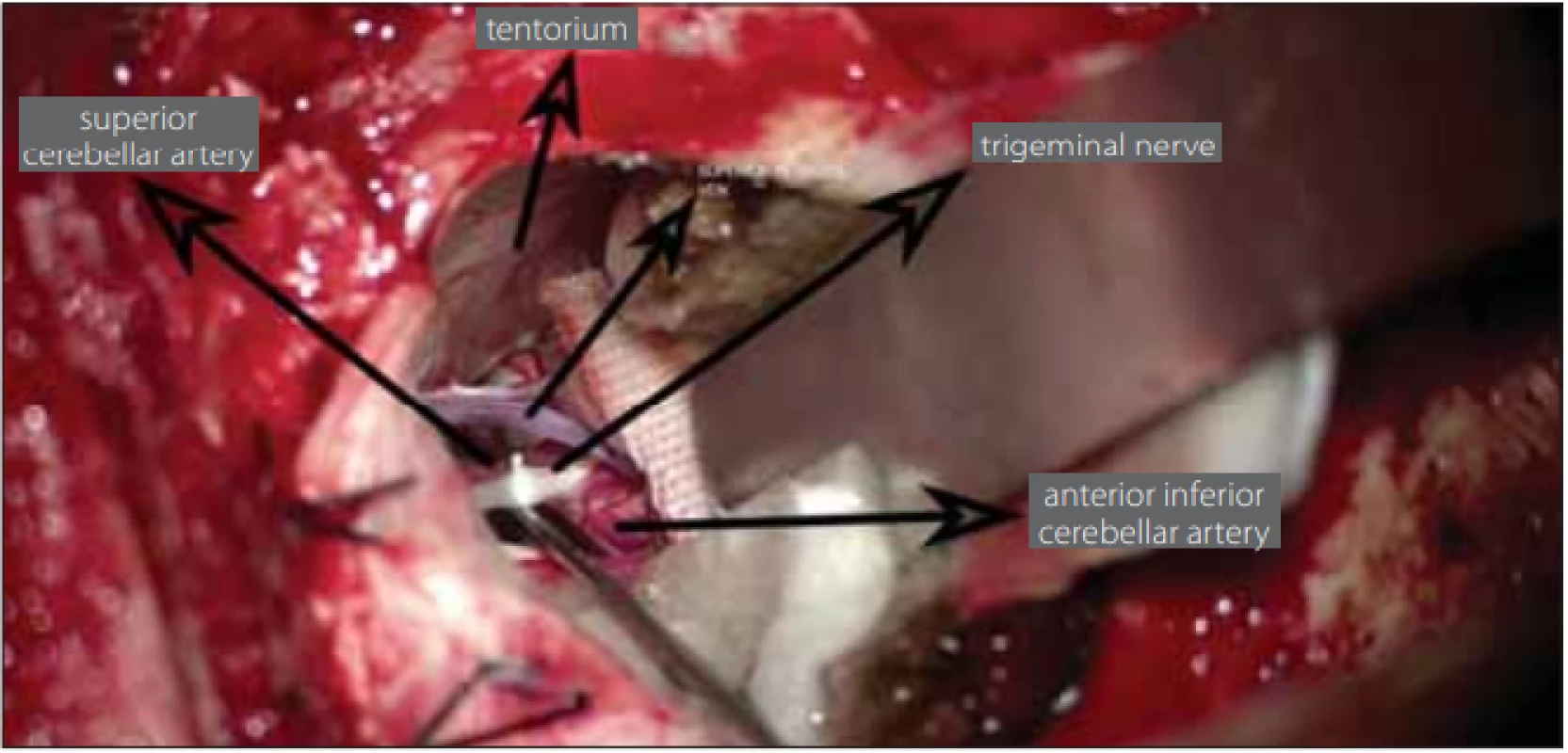
In patients undergoing PSR after MVD, approximately one-third of the inferolateral part of the sensory branch of the trigeminal nerve was cut with micro-scissors at a distance of 2–5 mm from the pons exit without coagulation (Fig. 2).
Fig. 2. Partial sensory rhizotomy on the trigeminal nerve using micro-scissors.
Obr. 2. Parciální senzorická rhizotomie trojklanného nervu pomocí mikronůžek.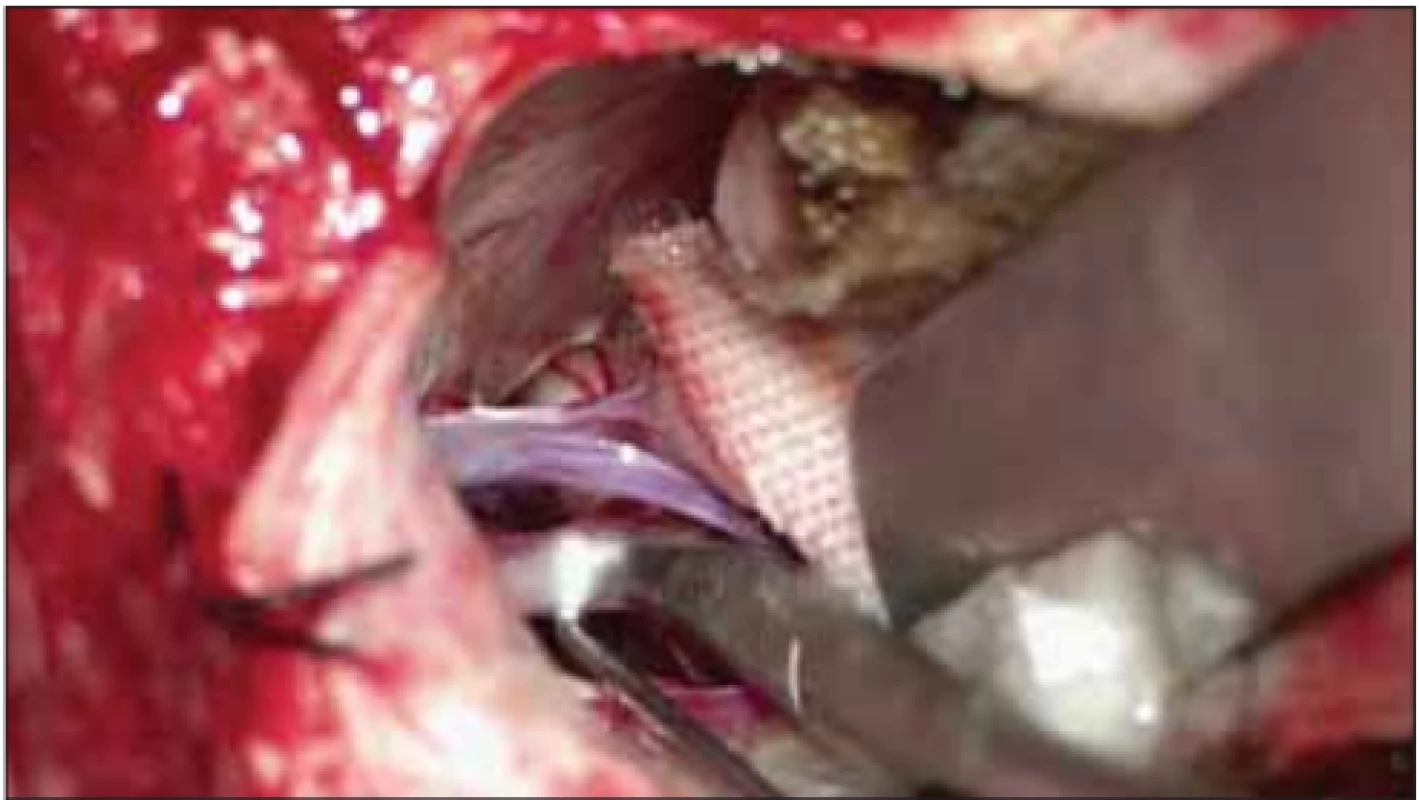
Statistical Analysis
SPSS 21.0 software (IBM, Armonk, NY, USA) was used to conduct the data analysis. The qualitative variables are presented as frequency and percentage distribution and the quantitative variables are presented as mean ± standard deviation or range of minimum to maximum values. The Student’s t-test was used for comparing means of age. Chi-square analysis or Fisher’s exact test was applied to the categorical data. The Kaplan–Meier test was used to draw relapse-free survival curves and the Log-Rank test was used to compare relapse-free survival time. P < 0.05 was considered as statistically significant.
Results
Micro-vascular decompression was performed in 30 (51.7%) cases and MVD + PSR was performed in 28 (48.2%) cases. The mean age was 53 ± 5.8 years and 32 (55.1%) patients were female. As shown in Tab. 1, gender, age, duration of disease, pain distribution and pain lateralisation were similar between the two groups. All of the 58 patients had previously received at least one type of pharmaceutical management of TN and 31 (53.4%) patients had previously been treated with radiofrequency, 12 (20.6%) with alcohol or glycerol injection, and 4 (6%) with a gamma knife. The pain distributions were maxillary in 17 (29.3%) patients, mandibular in 10 (17.2%) patients, and both mandibular and maxillary in 26 (44.8%) patients. In both groups, the pain was slightly more often on the right side; the MVD group had 56.6% (17/30) and the MVD + PSR group had 57.1% (16/28).
Tab. 1. General characteristics of the patients with classical trigeminal neuralgia between the MVD and MVD + PSR groups. 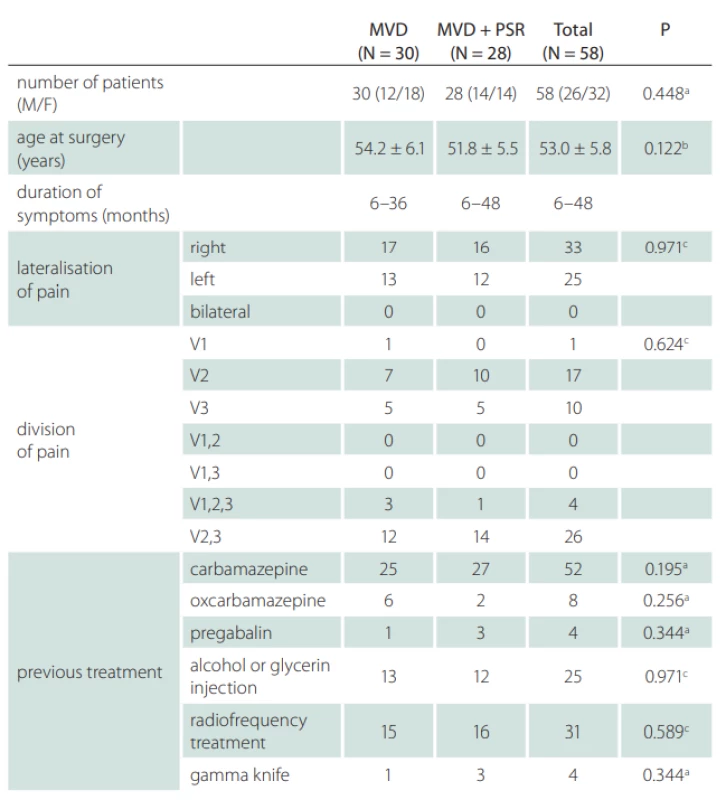
a Fisher’s Exact test; b Student’s t-test; c Chi-Squared test means are given ± standard deviation of the means F – female; M – male; MVD – micro-vascular decompression; N – number; PSR – partial sensory rhizotomy; V1 – ophthalmic division of trigeminal nerve; V2 – maxillary division of trigeminal nerve; V3 – mandibular division of trigeminal nerve, During the exploration of the trigeminal nerve, the cause of neuralgia was determined to be vascular pressure in 54 (93.2%) patients and due to arachnoid adherence in 4 (6.8%) patients. The vascular structure, most often creating pressure, was the superior cerebellar artery (Fig. 3). The superior petrosal vein exerted pressure in 8 (13.7%) patients (Tab. 2).
Fig. 3. The superior petrosal vein, trigeminal nerve and superior cerebellar artery, which create pressure on the trigeminal nerve, are seen in the dissection during surgery.
Obr. 3. Během operace je na řezu vidět vena petrosa superior, trojklanný nerv a arteria cerebelli superior, která tlačí na trojklanný nerv.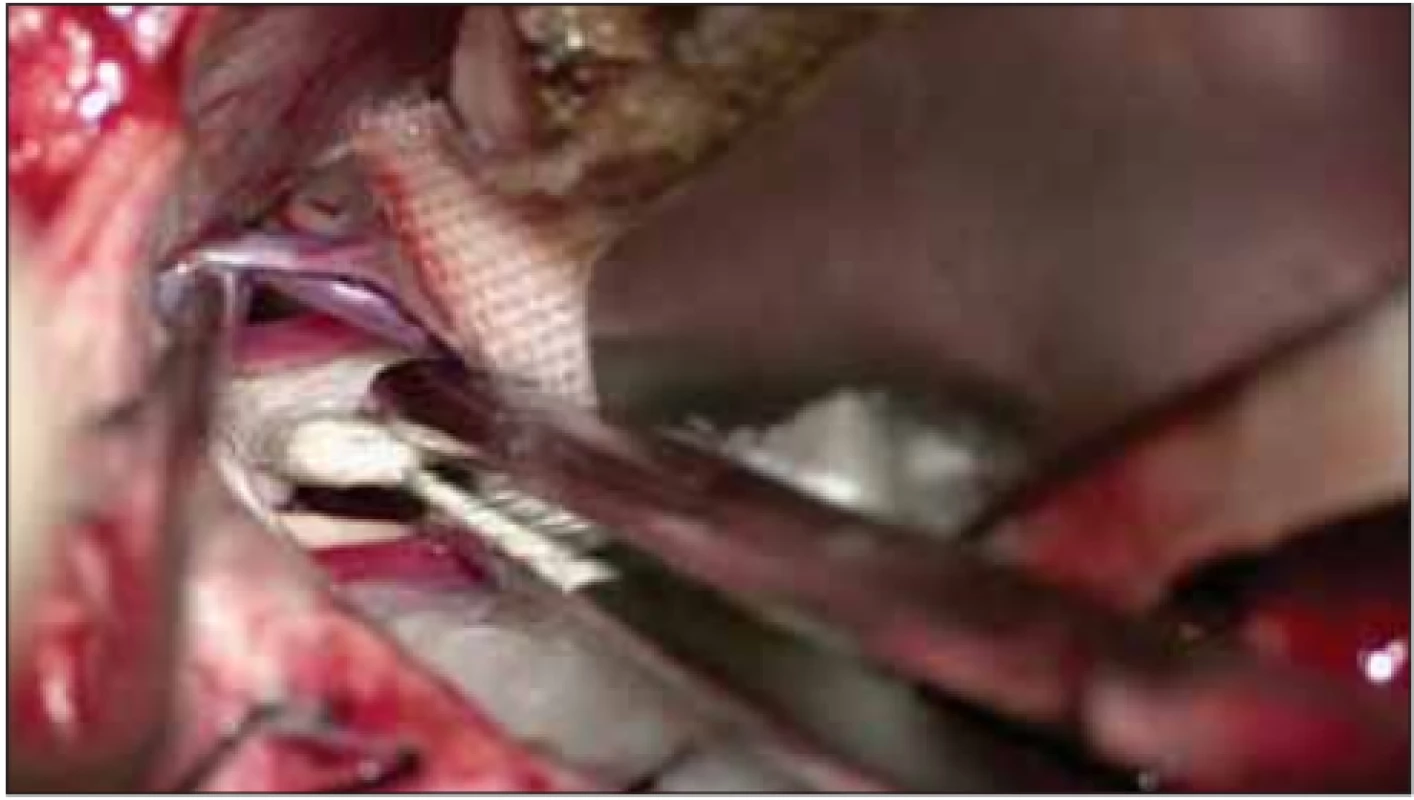
Tab. 2. Responsible artery and abnormal fi ndings in surgery 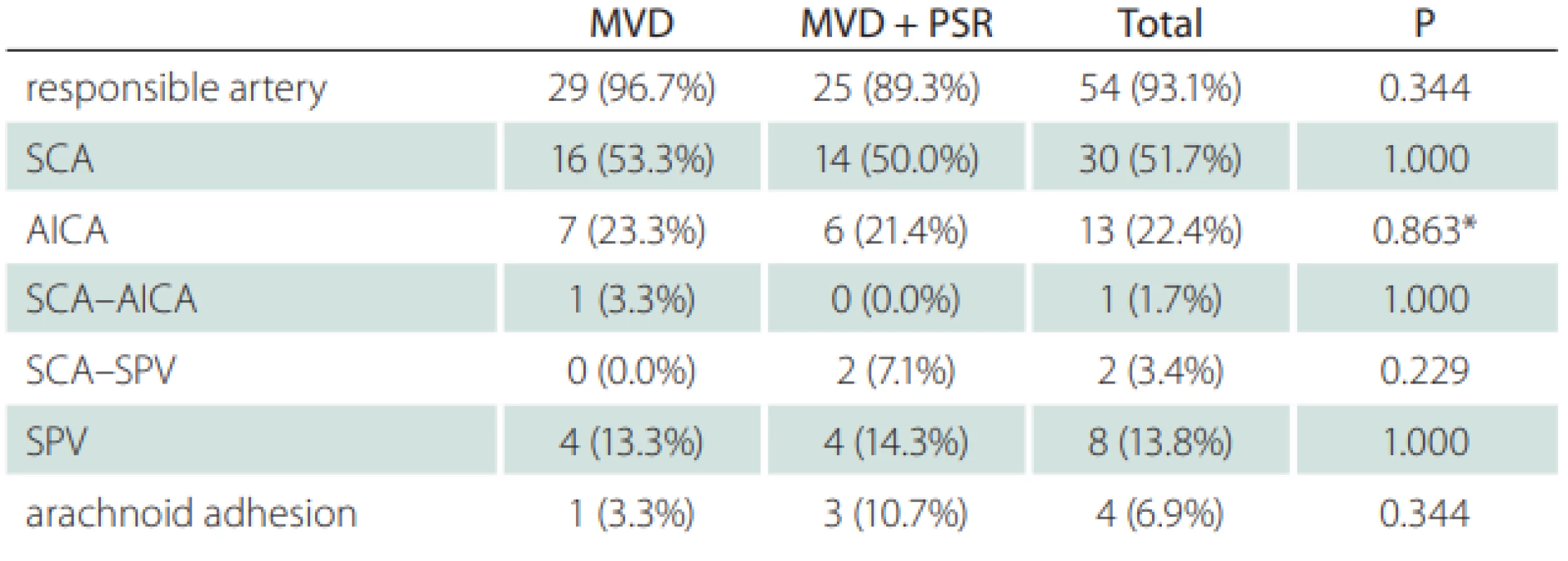
*chi-squared test
AICA – anterior inferior cerebellar artery; BA – basilar artery; MVD – micro-vascular decompression; PSR – partial sensory rhizotomy; SCA – superior cerebellar artery; SPV – superior petrosal veinIn the early postoperative period, the Barrow score of 29 in 30 patients (96.6%) in the MVD group was grade I, while one patient was grade IV. In the MVD + PSR group, 27 patients (96.4%) had a Barrow score of grade I, while one patient had a score of grade II. In the early postoperative period, there was no significant difference in the BNI pain intensity score between the two groups. At the end of the 3rd year, the number of patients with Barrow score grade I was 24 (85.7 %) for the MVD + PSR group and 20 (66.6%) for the MVD group (P = 0.127). At the end of the first--year follow-up, the proportion of patients with an excellent outcome in the MVD + PSR group (96.4%) was higher compared to the MVD group (86.6%) (P = 0.354). All patients were followed-up for 36-months. At 12 and 36 months after surgery, the recurrence rates in the MVD group were 13.3 % and 23.3%, respectively and in the MVD + PSR group were 3.6 and 7.1%, respectively (both P > 0.05) (Tab. 3, Fig. 4). At 36 months after surgery, the recurrence rate in the MVD group was higher than that of the MVD + PSR group, but the difference was not statistically significant (P = 0.147). The pain-free survival time in the MVD group was 31.83 ± 1.9 (95% CI: 28.3–35.63) months, which was shorter than the 35.14 ± 1.1 (95% CI: 32.81–37.48) months in the MVD + PSR group at 36 months after surgery (P = 0.086).
Tab. 3. Pain assessment of the patients after surgery. 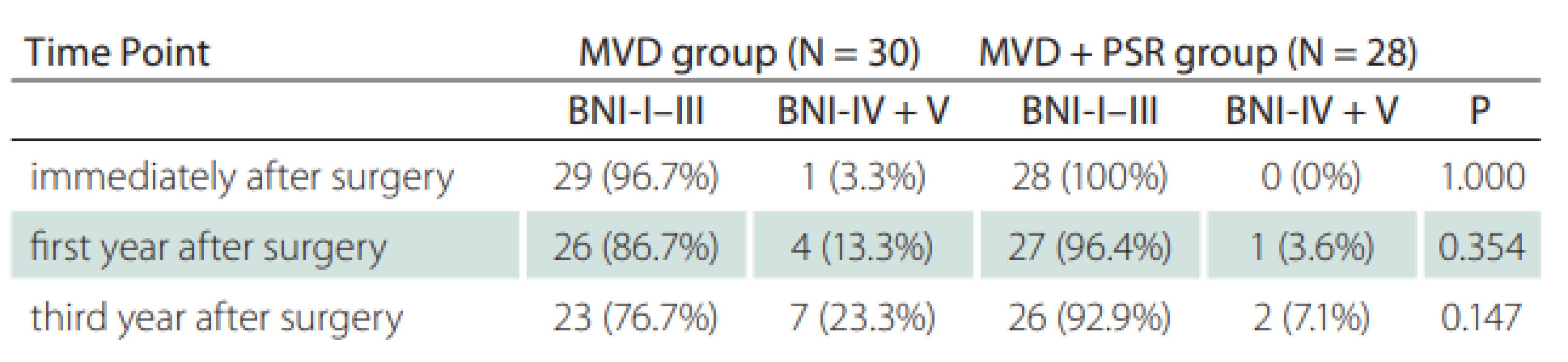
BNI – Barrow Neurological Institute’s Pain Intensity Scale; BNI-I–III – no recurrence; BNI-IV + V – recurrence; MVD – micro-vascular decompression; PSR – partial sensory rhizotomy Fig. 4. Kaplan-Meier curves for pain-free survival after posterior fossa exploration.
Obr. 4. Kaplan-Meierovy křivky pro přežívání bez bolesti po průzkumu zadní jámy lební.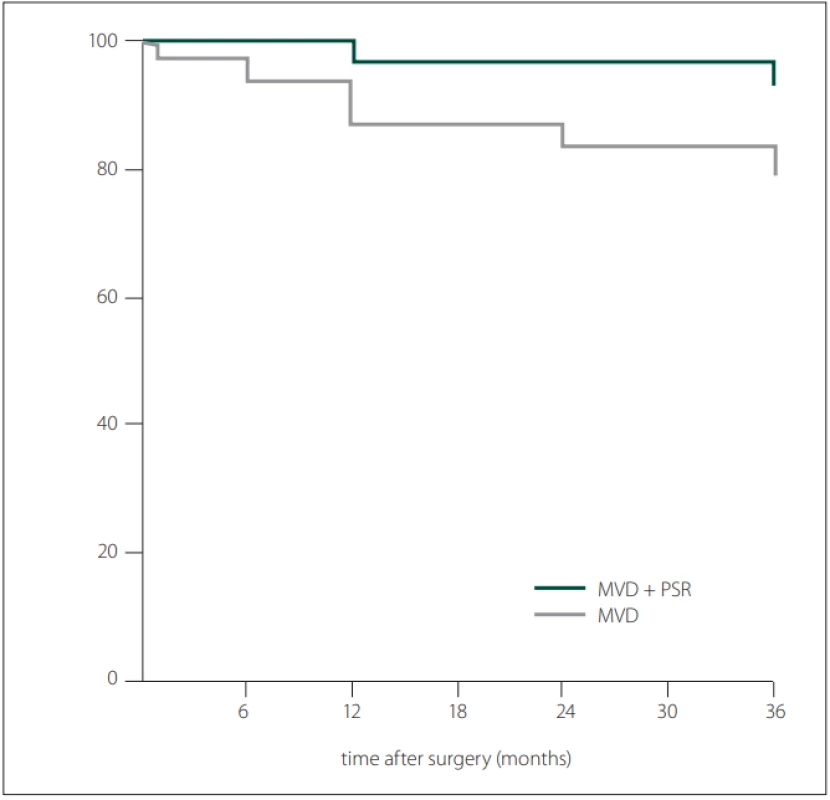
MVD – micro-vascular decompression; PSR – partial sensory rhizotomy
MVD – mikrovaskulární dekomprese; PSR – parciální senzorická rhizotomieNo patient died during our study. In our series, pneumocephalus of varying degrees was observed in patients depending on the sitting position, but it resolved within the first 7 days. As a severe complication, acute subdural haemorrhage of the spinning type developed in one patient, who was treated conservatively. Temporal vertigo attacks were observed in 3 (5.1%) patients. Hypoesthesia or topognosis was experienced around the mouth or on the face in 2 (6.6%) patients after MVD and in 12 (42.8%) patients who underwent MVD + PSR. Significant relief was achieved with medical treatment. Facial paralysis (Brackman-Hause 3) developed in one patient in the MVD + PSR group; however, it recovered after 3 months. CSF leakage occurred in another patient and was treated with lumbar drainage.
Discussion
Several studies reported the efficiency of PSR in reducing recurrence in patients with TN undergoing posterior fossa craniotomies [6,9,11]. Adding the PSR procedure to MVD surgery complicates the operation, raising the concern that the combination will result in increased early morbidity and complications. Other studies compared outcomes of the combined procedure with isolated MVD surgery to evaluate the risks and benefits [6]. In a study of 252 patients, Bederson et al reported more favourable outcomes with MVD + PSR compared to MVD or PSR alone [12]. Recently, Zhang et al reported better pain control with MVD + PSR surgery than MVD alone [6]. In our study, we performed MVD + PSR on 28 patients, irrespective of vascular compression. PSR was focused on one-third of the inferolateral portion of the trigeminal nerve closest to the pons. The Barrow score was I in 96.4% of patients in the early postoperative period, 89.2% at the end of the first year, and 85.7% at the end of the third year. In the present study, the average recurrence rate in patients with MVD + PSR surgery was about 2.3% annually.
Micro-vascular decompression is still the gold standard in TN treatment. Jannetta, a leading surgeon using MVD in the surgical treatment of TN, reported that this procedure protected the nerves, provided immediate relief in most cases (90%), and avoided recurrence of pain for up to 1 year [13]. In a series of 1,204 cases followed-up for an average of 6.2 years, Barker et al reported the pain-free rate to be 70% without the need for any medications [14]. Zhang et al reported that pain was eliminated in 128 out of 137 patients (90.1%) who underwent MVD and there was no change in 5 patients. Pain was not reported in 74 out of 82 cases (90.2%) at the 2-year follow-up period [6]. In our series, immediate pain relief was achieved in 96.6% of the patients in the MVD group. However, the proportion of patients who were completely pain-free at the end of the third year dropped to 66.6%.
The recurrence of TN symptoms remains a challenge for neurosurgeons. There are studies reporting recurrence rates ranging from 25 to 37% [13–16]. The highest recurrence rate in the literature was reported by Revuelta-Gutierrez et al, who observed a recurrence rate of 14.8% during the first 3 years and 43.2% after the fourth year [17]. In the present study, the average recurrence rate in patients after MVD surgery was about 7.6% annually. Some studies attempted to uncover the cause of recurrences seen after MVD. Chen et al found that Teflon granulomas caused the recurrence in 50% of cases, new arterial loops in 30%, and venous compression in 10%. No pathology was found in 10% of patients [18]. Kureshiet et al reported new incidences of vascular pressure in 30% of 23 patients that needed to be re-explored [19]. Although Ugwuanyi et al observed arachnoid adherences in 83.3% of the cases, recurrence was found to be due to new vascular loops, arachnoid cysts, and veins [20]. Upon re-exploration of 51 patients with recurrence, Jannetta et al found new vascular loops in 42 cases [21]. Although TN is rarely caused by vascular structures formed by veins when it does occur, the recurrence rates are higher [6]. In one study of MVD performed in 393 patients with TN due to pressure caused by veins, the recurrence rate was reported to be 31%. New veins were found to have developed around the trigeminal root [22].
In PSR, the transmission ducts on the trigeminal nerve or demyelinated zones on the trigeminal root are more severely damaged than in MVD and the procedure is more complex than simple neural decompression [6]. The low efficacy of MVD may be due to a change in the function of ganglion neurons by decompression alone without causing damage to the ganglion or trigeminal root. However, Baechli and Gratzi stated that the authorities who preferred PSR could not base this on any scientific evidence [23].
Partial sensory rhizotomy involves cutting the infero-caudal-lateral part of the trigeminal nerve using different techniques. A review of the literature suggests that PSR significantly alleviates pain in patients with partial sensory losses only on the face or on the side of the mouth. Bederson et al recommend PSR to be performed by cutting up to two-thirds of the inferior half of the sensory branch as close as possible to the brain stem in most of the cases in which MVD will be performed for TN without vascular touch or deformities in the nerve [12]. Klun et al recommend cutting one-third of the major portion of the nerve close to the brain stem [11]. Zhang et al, in their series of 210 cases, performed MVD + PSR in 68 patients [6]. When applying PSR, they cut the sensory branch of the trigeminal nerve with scissors as close as possible to the brain stem, one-third and one–fifth of the area without coagulation. They reported failure in only one patient in the early period.
The complications emerging after surgical treatment of TN include corneal reflex loss, masseter muscle paralysis or weakness, anaesthesia dolorosa, keratitis, third and sixth nerve lesion, and postoperative CSF leakage [6,9,12,24]. Cerebellar injury, hearing loss, and facial paralysis may also occur. Wei et al reported permanent cranial nerve damage in 4.7% of cases [15]. The death rates reported in the literature vary between 0.2–1.2% [6,13,24–26]. The cause of death is generally related to cerebellar or brain stem infarction. Facial hypoesthesia or numbness was more frequently reported in combined MVD + PSR surgery than MVD alone [6,25]. Zhang et al found that these complications occurred in 1.3% of patients in the MVD group and 33.8% of patients in the MVD + PSR group [6]. Similarly, we found a higher percentage of patients with hypoesthesia after surgery in the MVD + PSR group than in the MVD group. In the study performed by Daniyal et al, quality of life and postsurgical complications were compared at the end of the fifth year in TN patients treated with MVD or PSR. They stated that although complications such as burning, pain, and paraesthesia were more common in PSR, they did not affect the quality of life score [27].
There are some limitations of this study. First, it is a retrospective analysis. Second, the study had a relatively small sample size. Larger series with long-term follow-up are needed to confirm the effectiveness of the MVD + PSR approach and to establish whether MVD + PSR is a suitable alternative for selected patients who require surgery.
Conclusion
Our results showed that MVD and MVD + PSR are effective in the surgical treatment of classical TN. Compared to MVD, the MVD + PSR
procedure had a lower rate of pain recurrence and a longer pain-free survival time during the 3-year follow-up. However, this difference was not statistically significant. Our sample size was too small for us to conduct a precise analysis, so more comprehensive and precise research is needed.
Ethical principles
The study was approved by the Adana City Training and Research Hospital Clinical Researches Ethics Board (February 2018, ref: 12; 173) and has been performed according to the ethical standards of the 1964 Declaration of Helsinki.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Ali Arslan, MD
Adana Sehir Egitim ve Arastirma Hastanesi
Kisla Mah. Dr. Mithat Ozsan Blv. No:1 010650
Yüregir/Adana
Turkey
e-mail: aliarslan26062006@hotmail.com
Accepted for review: 4. 4. 2020
Accepted for print: 1. 10. 2020
Zdroje
1. Olesen J. The international classification of headache disorders: 2nd edition. Cephalalgia 2004; 24 (Suppl 1): 9–160. doi: 10.1111/j.1468-2982.2003.00824.x.
2. Scholz J, Finnerup NB, Attal N et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 2019; 160 (1): 53–59. doi: 10.1097/j.pain.00000 00000001365.
3. Mistry AM, Niesner KJ, Lake WB et al. Neurovascular compression at the root entry zone correlates with trigeminal neuralgia and early microvascular decompression outcome. World Neurosurg 2016; 95 : 208–213. doi: 10.1016/j.wneu.2016.08.040.
4. Jha A. Trigeminal neuralgia: therapeutic options. Neurol India 2015; 63 (6): 840. doi: 10.4103/0028-3886.170066.
5. Urgosik D, Rulseh AM, Keller J et al. Trigeminal nerve asymmetry in classic trigeminal neuralgia – evaluation by magnetic resonance imaging. Cesk Slov Neurol N 2014; 77/110 (5): 582–585. doi: 10.14735/amcsnn2014582.
6. Zhang L, Zhang Y, Li C, Zhu S. Surgical treatment of primary trigeminal neuralgia: comparison of the effectiveness between MVD and MVD + PSR in a series of 210 patients. Turk Neurosurg 2012; 22 (1): 32–38. doi: 10.5137/1019-5149.JTN.4447-11.2.
7. Zakrzewska JM, Lopez BC, Kim SE et al. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery 2005; 56 (6): 1311. doi: 10.1227/01.neu.0000159883.35957.e0.
8. Tan M, Lu Y, Jiang H et al. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: a systematic review and meta-analysis. J Cell Biochem 2019; 120 (4): 5852–5859. doi: 10.1002/jcb.27870.
9. Kang IH, Park BJ, Park CK et al. A clinical analysis of secondary surgery in trigeminal neuralgia patients who failed prior treatment. J Korean Neurosurg Soc 2016; 59 (6): 637–642. doi: 10.3340/jkns.2016.59.6.637.
10. Chen HI, Lee JYK. The measurement of pain in patients with trigeminal neuralgia. Clin Neurosurg 2010; 57 : 129–133.
11. Klun B. Microvascular decompression and partial sensory rhizotomy in the treatment of trigeminal neuralgia. Neurosurgery 1992; 30 (1): 49–52. doi: 10.1227/00006123-199201000-00009.
12. Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg 1989; 71 (3): 359–367. doi: 10.3171/jns.1989.71.3.0359.
13. McLaughlin MR, Jannetta PJ, Clyde BL et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg 1999; 90 (1): 1–8. doi: 10.3171/jns.1999.90.1.0001.
14. Barker FG, Jannetta PJ, Bissonette DJ et al. The long--term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 1996; 334 (17): 1077–1083. doi: 10.1056/NEJM199604253341701.
15. Wei Y, Pu C, Li N et al. Long-term therapeutic effect of microvascular decompression for trigeminal neuralgia: Kaplan-Meier analysis in a consecutive series of 425 patients. Turk Neurosurg 2018; 28 (1): 88–93. doi: 10.5137/1019-5149.JTN.18322-16.1.
16. Tyler-Kabara EC, Kassam AB, Horowitz MH et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg 2002; 96 (3): 527–531. doi: 10.3171/jns.2002.96.3.0527.
17. Revuelta-Gutiérrez R, López-González MA, Soto--Hernández JL. Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surg Neurol 2006; 66 (1): 32–36. doi: 10.1016/j.surneu.2005.10.018.
18. Chen JF, Lee ST, Lui TN et al. Teflon granuloma after microvascular decompression for trigeminal neuralgia. Surg Neurol 2000; 53 (3): 281–287. doi: 10.1016/S0090-3019 (00) 00169-5.
19. Kureshi SA, Wilkins RH. Posterior fossa reexploration for persistent or recurrent trigeminal neuralgia or hemifacial spasm: surgical findings and therapeutic implications. Neurosurgery 1998; 43 (5): 1111–1116. doi: 10.1097/00006123-199811000-00061.
20. Ugwuanyi UC, Kitchen ND. The operative findings in re-do microvascular decompression for recurrent trigeminal neuralgia. Br J Neurosurg 2010; 24 (1): 26–30. doi: 10.3109/02688690903507489.
21. Jannetta PJ, Bissonette DJ. Management of the failed patient with trigeminal neuralgia. Clin Neurosurg 1985; 32 : 334–347.
22. Lee SH, Levy EI, Scarrow AM et al. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery 2000; 46 (2): 356–362. doi: 10.1097/00006123-200002000-00019.
23. Baechli H, Gratzl O. Microvascular decompression in trigeminal neuralgia with no vascular compression. Eur Surg Res 2007; 39 (1): 51–57. doi: 10.1159/000098436.
24. Kabatas S, Albayrak SB, Cansever T et al. Microvascular decompression as a surgical management for trigeminal neuralgia: a critical review of the literature. Neurol India 2009; 57 (2): 134-138. doi: 10.4103/0028-3886.51279.
25. Zakrzewska JM, Lopez BC, Kim SE et al. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery 2005; 56 (6): 1304–1311. doi: 10.1227/01.NEU.0000159883.35957.E0.
26. Amagasaki K, Watanabe S, Naemura K et al. Safety of microvascular decompression for elderly patients with trigeminal neuralgia. Clin Neurol Neurosurg 2016; 141 : 77–81. doi: 10.1016/j.clineuro.2015.12.019.
27. Jafree DJ, Williams AC, Zakrzewska JM. Impact of pain and postoperative complications on patient-reported outcome measures 5 years after microvascular decompression or partial sensory rhizotomy for trigeminal neuralgia. Acta Neurochir (Wien) 2018; 160 (1): 125–134. doi: 10.1007/s00701-017-3350-6.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2020 Číslo 5- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Diffuse low grade gliomas
- Respiratory rehabilitation in patients with amyotrophic lateral sclerosis
- Socio-economic impact of headache disorders –reasons and improvement possibilities
- Neurorehabilitation in patients with amyotrophic lateral sclerosis
- Surgical treatment of degenerative scoliosis
- Functional and structural cortical changes in patients with non-specific low back pain
- Cognitive-motor interference after stroke
- Sonographic evaluation of sciatic nerve in individuals with S1 radicular symptoms
- Taste strips – the method of a self-administered taste function test
- Amnesia Light and Brief Assessment (ALBA) test – the second version and repeated examinations
- The efficiency of micro-vascular decompression versus micro-vascular decompression with partial sensory rhizotomy for classical trigeminal neuralgia – a retrospective analysis of 58 patients
- Patient with Parkinson‘s disease in data sources of the National Health Information System
- Treatment with intravenous trombolysis out of stroke center
- Decompressive craniectomy in malignant middle cerebral artery infarction – monoinstitutional retrospective analysis of a set of 33 patients
- Consecutive low-flow and high-flow EC-IC bypass in ischemia prevention during internal carotid artery sacrifice in intracavernous aneurysm
- Venous sinus stenting in idiopathic intracranial hypertension
- Posterior reversible encephalopathy syndrome associated with Neisseria elongata meningitis
- Hyperbarická oxygenoterapie & mozek; přehled z výroční konference EUBS 2019
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Diffuse low grade gliomas
- Respiratory rehabilitation in patients with amyotrophic lateral sclerosis
- Amnesia Light and Brief Assessment (ALBA) test – the second version and repeated examinations
- Neurorehabilitation in patients with amyotrophic lateral sclerosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



