-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Calcifying Pseudoneoplasm of the Neural Axis – a Case Report
Mozkový kámen – kazuistika
Cíl:
Cílem práce bylo shromáždit dostupná klinická, radiologická a patologická data o kalcifikujícím pseudotumoru nervové osy (CAPNON).Soubor:
Soubor je tvořen kazuistikou a současně přehledem všech pacientů publikovaných v anglické literatuře, vyhledaných v databázi PubMed pomocí klíčových slov: calcifying pseudoneoplasm, calcifying pseudotumour, brain stone, cerebral calculi a fibro‑osseous lesion. Zahrnuty jsou taktéž všechny citace případů v nalezených publikacích.Výsledky:
Včetně naší pacientky jsme v literatuře nalezli celkem 72 pacientů. Intrakraniálně bylo lokalizováno 62 % těchto lézí, v páteřním kanále 31 % a v kranio ‑ cervikálním přechodu zbylých 7 %. Průměrný věk výskytu je 45,6 (rozmezí 2 – 83) let. U intrakraniální lokalizace byla tato léze nalezena supratentoriálně v 74 % a infratentoriálně v 26 %. Z nejčastějším projevů intrakraniálních CAPNON jsme nalezli epilepsii v 33,3 %, bolesti hlavy v 24 %, nebo některý z příznaků zadní jámy v 16,7 %. U pacientů s epilepsii byl CAPNON nalezen v temporálním laloku v 50 %. U CAPNON nalezených v páteřním kanále byla dominantním projevem bolest v 82 %, následovaná poruchou chůze v 27 %. V kranio ‑ cervikálním přechodu byla dominantním příznakem bolest v 80 %.Závěr:
CAPNON je velmi vzácnou, benigní, pomalu rostoucí lézí centrální nervové soustavy. Ve většině případů ji nalezneme intrakraniálně s lehkou převahou u mužského pohlaví. Původ této léze není zcela jasný. Diagnóza CAPNON by měla být zvažována setkáme‑li se s nálezem solidně kalcifikované léze při vyšetření CT mozku a hypodenzní léze v T1 a T2 obraze při vyšetření MR. Léčebnou modalitou je radikální odstranění této léze. Incidentální léze musí být monitorovány, jelikož mohou růst a stát se symptomatickými.Klíčová slova:
kalcifikující pseudotumor – mozkový kámen – fibro-oseozní léze
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: T. Krejčí 1; P. Buzrla 2; Z. Večeřa 1; L. Křen 3; S. Potičný 1; O. Krejčí 1; T. Paleček 1; R. Lipina 1
Authors place of work: Neurosurgery Department, Faculty of Medicine and University Hospital of Ostrava, Czech Republic 1; Institute of Pathology, University Hospital of Ostrava, Czech Republic 2; Institute of Pathology, University Hospital of Brno, Czech Republic 3
Published in the journal: Cesk Slov Neurol N 2015; 78/111(5): 568-575
Category: Kazuistika
Summary
Aim:
The aim of this study was to summarize clinical, radiological and pathological data on calcifying pseudoneoplasm of the neural axis (CAPNON).Methods:
A case report of a female patient with CAPNON is presented. In addition, all CAPNON patient case histories published in English language are included. Articles were identified via PubMed searches using the following key words: calcifying pseudoneoplasm, calcifying pseudotumour, brain stone, cerebral calculi and fibro‑osseous lesion. All cases referenced within the identified publications are also included in this review.Results:
Seventy two cases were identified, including our case report. Of these, 62% were intracranial, 31% spinal and 7% were located in the cranio ‑ cervical junction (CCJ). The mean age of the CAPNON patients was 45.6 (range 2 – 83) years. 74% of intracranial CAPNON were supratentorial and 26% were infratentorial. Symptoms of intracranial CAPNON included epilepsy in 33.3%, headaches in 24% and posterior fossa symptoms in 16.7%. The majority of patients with epileptic seizures had lesions in the temporal lobe (50%). Pain was the dominant symptom in 82% and gait disorder in 27% of spinal CAPNON cases. Pain was the dominant symptom (80%) in CCJ CAPNON.Conclusion:
CAPNON is a rare, benign, slowly ‑ growing lesion of the central nervous system. In the majority of cases, location is intracranial and there is a slightly higher prevalence in men. The origin of CAPNON remains unclear. CAPNON should be considered whenever a CT scan reveals a calcified lesion combined with hypointensity on T1 and T2 - weighted MRI. Radical removal is the treatment of choice. Incidental lesions must be monitored, as they may grow and become symptomatic.Key words:
calcifying pseudotumour – brain stone – fibro-osseous lesionIntroduction
Calcifying pseudoneoplasm of the neural axis (CAPNON) is a very rare, benign and slowly‑ - growing lesion that may occur anywhere along the entire neural axis. Literature also refers to CAPNON as a ‘brain stone’, ‘cerebral calculi’ or a ‘fibro‑osseous lesion’ [1,2]. CAPNON may be extra ‑ axial or intra ‑ axial in the brain or in the spinal canal, both in extra ‑ and intradural locations. This lesion was first described by Miller in 1922 [1]; Bertoni et al. were the first to use the term ‘calcifying pseudoneoplasm of the neural axis’ in 1990 [3]. The aim of this study was to provide an overview of clinical, radiological and pathological data on CAPNON. We focused on epidemiology, location, radiological characteristics, clinical course and histopathologic characteristics; these are discussed together with recommendations for the management of these lesions.
Methods
One case report of a female patient with CAPNON is presented. In addition, all CAPNON patient case ‑ histories published in the literature since the first report of this lesion in 1922 were included. These were identified by searching the abstracts and titles of PubMed database articles for the following keywords: calcifying pseudoneoplasm, calcifying pseudotumour, brain stone, cerebral calculi and fibro‑osseous lesion. All cases referenced in the identified articles are also presented in this review.
Results
Case report
History and preoperative examination
In March 2012, a 38‑year ‑ old woman with no prior medical history was admitted to a local neurology department due to sudden onset of loss of consciousness with convulsions. Initial brain CT showed an atypical calcified lesion in the central right region (Fig. 1), also visible on an X‑ray image (Fig. 2). Brain MRI was subsequently performed, confirming the CT finding of a calcified lesion in the central section of the right parietal lobe. The lesion was 2 × 2 × 2.5 cm in size and showed perifocal oedema with a narrow hemosiderin rim on T2 scans (Fig. 3). On a post‑contrast MRI, the lesion rims were enhanced showing mild non‑homogenous central enhancement. Radiology findings were atypical and cavernoma or calcified meningioma were suspected. The patient was admitted to the Neurosurgery Department, Faculty of Medicine and University Hospital in Ostrava. Brain angiography was performed: the finding did not confirm pathologic vascularisation. In the preoperative period, one partial motor epileptic paroxysm occurred.
Fig. 1. Diffusely calcified lesion in the right parietal lobe in the brain (A) and bone window (B). 
Fig. 2. X-ray image of CT examination showing a calcified expansion in the parietal area. 
Fig. 3. Expansion of central area at the right side. 
Expansion of central area at the right side in T1 signal (A, B, D). After contrast medium administration, there is obvious contrast enhancement at the rim and mild non-homogenous internal enhancement in the sagittal (A) and axial plane (D). Perifocal oedema around the lesion in the axial plane in T2 (C). Surgery and postoperative course
Right ‑ sided parietal craniotomy was performed using electrophysiological monitoring. Durotomy exposed central sulcus widening with a convex, thickened arachnoid resembling an arachnoid cyst. After penetration into the cyst, we found a white, solid tumour at a depth of about 2 cm, with a vessel network on the surface. Because of the tough consistency of the tumour, slow de ‑ bulking using a CUSA (Cavitron Ultrasonic Surgical Aspirator) was carried out first, followed by careful separation from the surrounding brain tissue. In some areas, the tumour adhered strongly to the brain. The surrounding cortex was predominantly responsible for motor response of the left lower extremity. After surgery, the patient was woken up without complications. Objective neurology findings were limited to mild monoparesis of the left arm, with subjectively perceived left arm apraxiaand dysaesthesia. Post‑surgical MRI scan showed satisfactory findings without a re-sidual lesion or bleeding. The patient was discharged on the ninth day after the surgery. Upper left extremity apraxia subsided within six weeks. Follow‑up brain MRIs at 3, 9 and 17 month post‑surgery did not show any signs of tumour recurrence. Perifocal oedema also subsided (Fig. 4). The patient did not have any further epileptic seizures after removal of the lesion. She continues to take antiepileptic medication and is monitored by neurologists.
Fig. 4. Post-operative finding 1 year after CAPNON removal. 
Post-operative finding 1 year after CAPNON removal in the parietal lobe in axial (A), sagittal (B, C) and coronal plane (D). After contrast medium application (C, D). In T2 – disappearance of perifocal oedema (A). Histological examination
Histological examination that required repeated reading confirmed the diagnosis of calcifying pseudoneoplasm of the neuraxis. Histological preparations (Fig. 5) showed a lesion consisting of an acellular irregular chondroid matrix with some calcified areas. The matrix contained radially positioned spindle cells on the periphery, followed by a small amount of brain tissue with GFAP immunoreaction. We also found osseous metaplasia without giant cell reaction.
Fig. 5. The histological preparations. 
Acellular, chondoid matrix with focal calcification (A), fibrovascular stroma with myxoid changes and myofibroblasts proliferation (B), brain tissue border at the peripheral part of chondoid matrix (C), osseous metaplasia (D). Literature review
CAPNON is a rare lesion that may be found anywhere in the entire neural axis, may be intracranial or spinal. In general, it is considered to be a benign, non‑infiltrative lesion that may gradually increase in size. Symptoms occur due to mass effect and are determined by the lesion location [4,5].
Including our case, we found 72 cases of this lesion in the literature (Tab. 1). Analysis of these cases provided valuable data on the epidemiology and clinical and histopathological signs of this entity.
Tab. 1. Review of all reported cases. 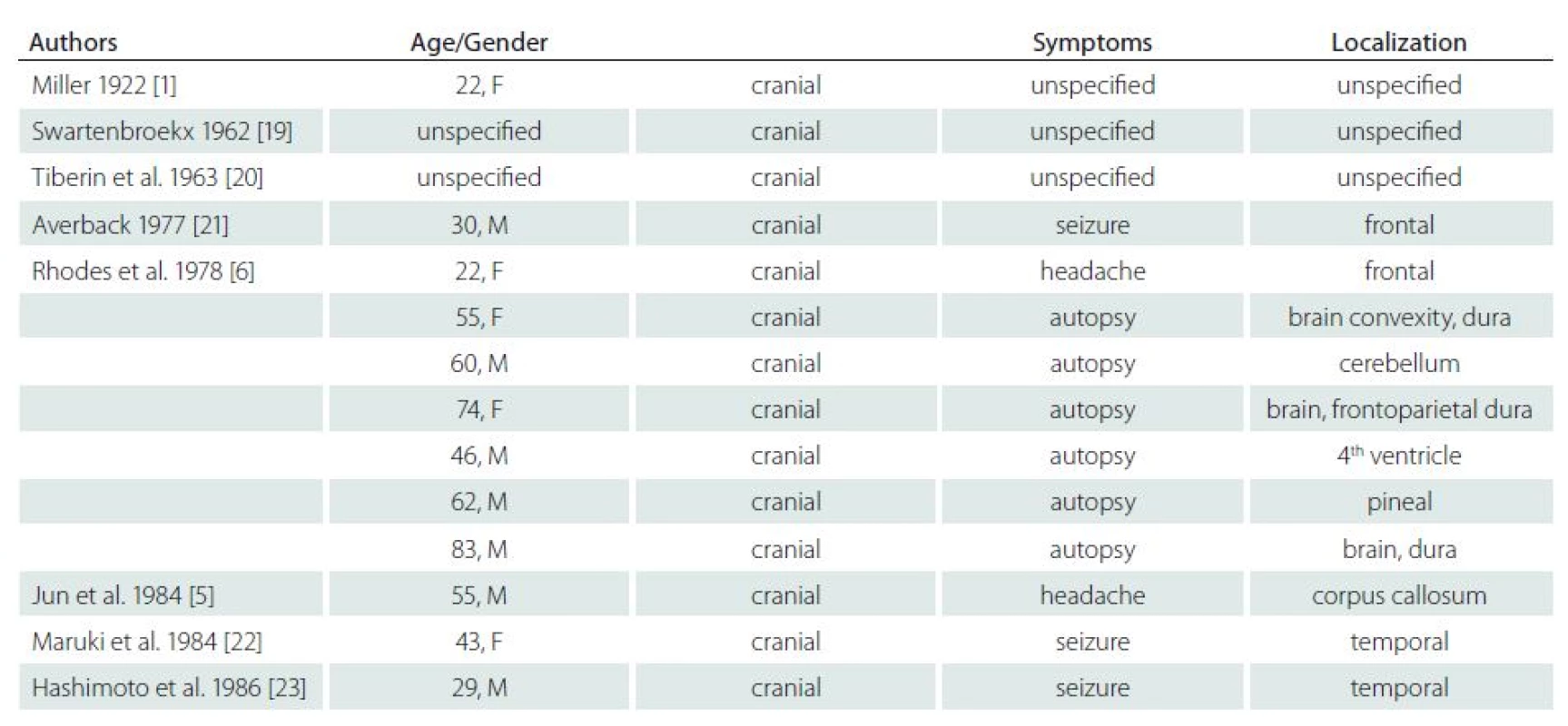
Ordered by year of publishing. Tab. 1. Review of all reported cases – continuing. 
Ordered by year of publishing. Tab. 1. Review of all reported cases – continuing. 
Ordered by year of publishing. It is notable that the number of reports on these rare lesions increases. We identified 16 published cases only until 1990, whilst we found 56 cases published after 1990: this is probably due to the increased availability of MRI examinations. In 45 cases (62%), CAPNON were intracranial, 22 (31%) were in the spinal canal and five (7%) were in the area of the cranio ‑ cervical junction (CCJ). Intracranial location was twice as frequent as the spinal location. The disease is more frequent in men – 1.33 : 1 (40 men : 20 women; gender was not stated in two cases). Older publications, reporting lower number of cases, stated ratio of 1.5 : 1 with a higher number of men [4].
With respect to tumour location, gender ratio was 1.15 : 1 (23 men : 20 women) for intracranial lesions and 1.45 : 1 (13 men : 9 women) for spinal lesions. Of the five CCJ cases, four were male patients. It seems that the proportion of men increases in the cranio ‑ caudal direction, and the incidence in men is more frequent with spinal location.
The mean age at CAPNON diagnosis was 45.6 years, median 49 years and range 2 to 83 years. The mean age of patients with intracranial, spinal and CCJ lesions was 44.3 (median 46), 46.5 (median 56.5) and 53.2 (median 53) years, respectively. Of the 45 cases of intracranial CAPNON, precise location was stated in 42 patients: 39 were intradural (93%) and 3 (7%) were extradural. The majority of cases were supratentorial (31/ 42; 74%); lesions were infratentorial in 11 patients (26%). It is of note that no lesions were found in the occipital lobe. There was no clear difference in location in other brain lobes.
Symptoms of intracranial CAPNON included: epilepsy (14/ 42, 33.3%); headaches (10/ 42, 24%) and posterior fossa symptoms (7/ 42, 16.7%; particularly cerebellar symptoms and cranial nerve palsies). The lesions were found accidentally during autopsy or as incidental lesions on the brain MRI/ CT in 16.7% (7/ 42) and 9.5% (4/ 42) of patients, respectively. Epileptic seizures were the initial symptom in 14 patients (including our patient): six women and eight men. All had supratentorial CAPNON, i.e. 14/ 31 patients with supratentorial lesions (45%) had epileptic seizures (Tab. 2). In half of these cases, CAPNON was located in the temporal lobe (7/ 14). In 21% of cases (3/ 14), both frontal and parietal lobes were affected; the lesion was located in the pineal region in one patient. Of the 10 CAPNON cases located in the temporal lobe, seven patients suffered from epileptic seizures (70%). Epileptic seizures occurred in 3/ 8 (37.5%) patients with a lesion located in the parietal lobe, and in 3/ 7 (43%) patients with a lesion located in the frontal lobe. The mean age of patients with seizures was 35.8 years, median 36.5 years – significantly lower than in other CAPNON cases (mean 48, median 54 years). Pain (local, radicular) was the dominant symptom in 82% of patients with spinal CAPNON, while 27% experienced paraparesis (gait disorder). Pain was the dominant manifestation (80%) in CCJ CAPNON, associated with a neurological deficit in one patient.
Tab. 2. Summary of patients with epileptic seizures. 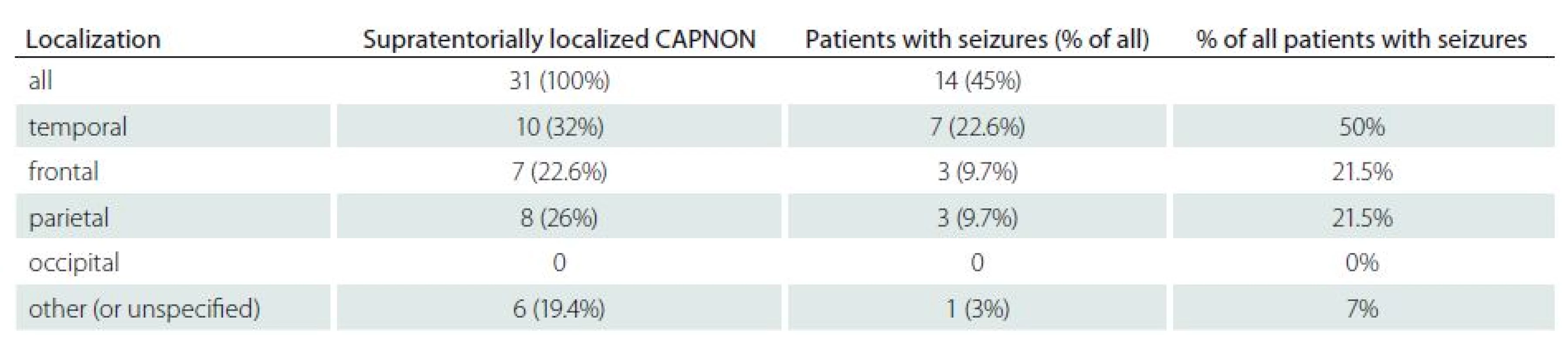
Discussion
The question on the origin of these lesions remains unanswered. It may be an atypical osseous metaplasia [6] or a reactive healing process to unknown provoking factors (e. g. trauma or inflammation) [7]. Rodriguez et al. described an association with ependymoma WHO grade II, possibly confirming the hypothesis of a reactive lesion [8]. We cannot exclude an association with an arachnoid cyst in our patient. Some authors suspect a developmental disorder (hamartoma) [9] or even a low grade neoplasm [10].
Histopathology of CAPNON typically exhibits a strange, acellular, basophilic, chondromyxoid matrix of lobular or focally nodular character [7,11]. This chondromyxoid matrix is usually surrounded by spindle‑like epitheloid cells in a palisade formation resembling a granuloma [7,11]. Furthermore, there are variable amounts of fibro‑vascular stroma, calcium deposits, osseous metaplasia and diffusely scattered psamomatous bodies [7,11]. The presence and amount of individual components is variable and individual [7]. Literature mentions that all CAPNON cases had positive vimentine presence tests. In the majority of cases, EMA antigen was present; S100 protein and GFAP antigen was usually negative [4].
In CAPNON diagnostics, we may use skull X‑ray imaging, CT and MRI scanning. On a plain X‑ray image, typical finding involves a solid calcified lesion or a radiolucent lesion with multiple calcifications [5,11]. A hyperdense lesion with clear borders and without contrast enhancement is typically found on CT scans of CAPNON patients [4,11]. Rarely, a soft tissue structure with peripheral calcification is detected [12,13]. MRI typically shows a homogenous lesion with low signal on T1 and T2 - weighted images. Rarely, an isodense signal on T1 and a hyperdense signal on T2 is seen [4,11]. Including our patient, perifocal oedema was found in a total of five patients (high signal on T2) [4,8,14]. Post‑contrast enhancement in the rim and non‑homogenous internal enhancement is typical for this lesion. Rarely, there is no contrast enhancement on MRI [11]. Muccio et al. reported a hypointensity on DWI and variable ADC level on an ADC map [15].
Differential diagnosis includes, particularly in case of intracranial lesions, a wide spectrum of lesions. As described above, low T1 and T2 signals – parameters essential for differentiation from other lesions – are typical for CAPNON. Calcified lesions with heterogenous signal intensity on T2 contrast or high T2 signal are more typical for a neoplasm than for the CAPNON [7]. Contrast enhancement – slight contrast enhancement in the rim areas or non‑homogenous internal contrast enhancement – is also typical for CAPNON [11]. Calcifications may be found in 9.3% of primary brain tumours, mainly oligodendrogliomas, gangliomas, meningiomas, ependymomas, papilomas of choroid plexus and astrocytomas [7,11]. Oligodendrogliomas bare signs of central or peripheral calcifications in 70 – 90% of cases, meningiomas in about 20% [16]. Calcifications in meningiomas are smaller, round and with lamellar layer structure. In CAPNON, calcification nodules are larger and have irregular shape. When compared to a primary brain tumour, only about 1% of calcifications are associated with metastases [11]. Since reactive gliosis with Rosenthal’s fibrils may develop around CAPNON, superficially located pilocytary astrocytomas with secondary calcification should be considered. Calcifications may also be found in the sellar region: in the majority of cases, this is a craniopharyngeoma or a pituitary stone for which this location is typical [5]; there is no report of a CAPNON in this area. Subsequent calcifications in an aneurysm sac or in arterio ‑ venous or cavernous malformations must also be considered. We often find calcifications in the epiphysis or the choroidal plexus of brain ventricles. These lesions are benign. When the lesion is spinal, calcified disc sequester, synovial cyst, neurinoma or meningioma should be considered. Calcifications may also be found after a spinal infection, or in an old calcified hematoma. MRI may help us to differentiate between these pathologies.
Radical surgical removal of the lesion is the treatment of choice whenever possible. No relapses have been reported after radical removal. However, recurrence has been seen after partial extirpation. Chang et al. described recurrence of a partially removed CAPNON in the C2 vertebrae after 24 months [17] and Bertoni et al. described recurrence three years after partial extirpation of CAPNON in the right side of the ponto ‑ cerebellar junction [3]. Incidental lesions may be monitored but since the CAPNON is diagnosed based on histological testing, other, more frequent calcified lesions with completely different prognoses have to be considered. As the literature demonstrates, asymptomatic CAPNON growth may occur, only later becoming symptomatic: follow‑up is, therefore, highly recommended [4,5,10]. Patients should be closely monitored using MRI, particularly during the early years, and especially in the case of histologically unverified lesions. Jun et al. described enlarging CAPNON in the corpus callosum during an 8‑year period [5] and Smith et al. reported progression of a spinal CAPNON in L2 – L3 segments after four years [10]. Kerr et al. reported posterior fossa lesion and growth during a two‑year period; the lesion continued to expand over further three years and signs of perifocal oedema and progressive headache occurred [4]. According to Kerr et al., deteriorating headaches may be a sign of CAPNON growth and an indication for lesion extirpation [4].
Conclusion
CAPNON is a rare, benign, slowly ‑ growing lesion of the central nervous system. In the majority of cases, the lesion is intracranial and CAPNON has slightly higher prevalence in men. Its origin remains unclear but most likely involves development of a reactive lesion of an unknown origin. If a CT scan shows a combination of a calcified lesion with hypointensity on the T1 and T2 MRI signal, CAPNON should be considered. Radical removal is the treatment of choice whenever possible. Incidental lesions must be monitored as they may grow and become symptomatic.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 23. 12. 2014
Accepted for print: 7. 7. 2015
Tomas Krejci, M.D.
Neurosurgery Department
Faculty of Medicine
University Hospital of Ostrava
17. listopadu 1790
708 52 Ostrava
Czech Republic
e-mail: tomk82@seznam.cz
Zdroje
1. Miller EA. Calculi within the brain. Report of a case of intracranial calcification with successful operation and recovery. Surg Gynecol Obstet 1922; 34 : 786 – 789.
2. Nonaka Y, Aliabadi HR, Friedman AH, Odere FG, Fukushima T. Calcifying pseudoneoplasms of the skull base presenting with cranial neuropathies: case report and literature review. J Neurol Surg Rep 2012; 73(1): 41 – 47. doi: 10.1055/ s ‑ 0032 ‑ 1321503.
3. Bertoni F, Unni KK, Dahlin DC, Beabout JW, Onofrio BM. Calcifying pseudoneoplasms of the neural axis. J Neurosurg 1990; 72(1): 42 – 48.
4. Kerr EE, Borys E, Bobinski M, Shahlaie K. Posterior fossa calcifying pseudoneoplasm of the central nervous system. J Neurosurg 2013; 118(4): 896 – 902. doi: 10.3171/ 2013.1.JNS121755.
5. Jun C, Burdick B. An unusual fibro‑osseous lesion of the brain. Case report. J Neurosurg 1984; 60(6): 1308 – 1311.
6. Rhodes RH, Davis RL. An unusual fibro‑osseous component in intracranial lesions. Hum Pathol 1978; 9(3): 309 – 319.
7. Aiken AH, Akgun H, Tihan T, Barbaro N, Glastonbury C.Calcifying pseudoneoplasms of the neuraxis: CT, MR imaging, and histologic features. Am J Neuroradiol 2009; 30(6): 1256 – 1260. doi: 10.3174/ ajnr.A1505.
8. Rodriguez FJ, Scheithauer BW, Fourney DR, Robinson CA. Ependymoma and intraparenchymal calcifying pseudoneoplasm of the neural axis: incidental collision or unique reactive phenomenon? Acta Neuropathol 2008; 115(3): 363 – 366.
9. Garen PD, Powers JM, King JS, Perot PL jr. Intracranial fibro‑osseous lesion. Case report. J Neurosurg 1989; 70(3): 475 – 477.
10. Smith DM, Berry AD 3rd. Unusual fibro‑osseous lesion of the spinal cord with positive staining for glial fibrillary acidic protein and radiological progression: a case report. Hum Pathol 1994; 25(8): 835 – 838.
11. Stienen MN, Abdulazim A, Gautschi OP, Schneiderhan TM, Hildebrandt G, Lücke S. Calcifying pseudoneoplasms of the neuraxis (CAPNON): clinical features and therapeutic options. Acta Neurochir (Wien) 2013; 155(1): 9 – 17. doi: 10.1007/ s00701 ‑ 012 ‑ 1502 ‑ 2.
12. Moser FG, Tourje EJ, Pressman BD, Blinderman EE. Calcifying pseudotumor of the cervical spine. Am J Neuroradiol 1994; 15(3): 580.
13. Mayr MT, Hunter S, Erwood SC, Haid RW jr. Calcifying pseudoneoplasms of the spine with myelopathy. Report of two cases. J Neurosurg 2000; 93 (Suppl 2): 291 – 293.
14. Shrier DA, Melville D, Millet D, Qian J, Millet D, Nelson Cet al. Fibro‑osseous lesions involving the brain: MRI. Neuroradiology 1999; 41(1): 18 – 21.
15. Muccio CF, Cerase A, Leone A, Dalena AM, Di Blasi A, De Simone M et al. Calcifying pseudoneoplasm of the neuraxis. Two case reports and review of CT and MR findings. Neuroradiol J 2012; 25(4): 453 – 459.
16. Grabowski M, Recinos P, Chen T, Prayson R, Vogelbaum M. Calcifying pseudoneoplasm of the neuraxis overlying the corpus callosum: a case report and review of the literature. Clin Neuropathol 2013; 32(6): 515 – 521. doi: 10.5414/ NP300640.
17. Chang H, Park JB, Kim KW. Intraosseous calcifying pseudotumor of the axis: a case report. Spine (Phila Pa 1976) 2000; 25(8): 1036 – 1039.
18. Fletcher AM, Greenlee JJ, Chang KE, Smoker WR, Kirby PA, O’Brien EK. Endoscopic resection of calcifying pseudoneoplasm of the neuraxis (CAPNON) of the anterior skull base with sinonasal extension. J Clin Neurosci 2012; 19(7): 1048 – 1049. doi: 10.1016/ j.jocn.2011.11.016.
19. Swartenbroekx A. A case of “brain stones”. J Belge Radiol 1962; 45 : 534 – 535.
20. Tiberin P, Beller AJ. Observations on so ‑ called brain stones or cerebral calculi. Neurology 1963; 13 : 464 – 476.
21. Averback P. Epileptogenic mineralization: pathological variants with good prognosis. Ann Neurol 1977; 2(4): 332 – 335.
22. Maruki C, Nakajima K, Shimoji T, Ito K, Matsumoto M, Ishii S. Brain stone. A case report. No Shinkei Geka 1984; 12(12): 1441 – 1445.
23. Hashimoto M, Tanaka T, Ohgami S, Yonemasu Y, Fujita M.A case of idiopathic brain stone presenting as psychomotor epilepsy. No Shinkei Geka 1986; 14(12): 1457 – 1461.
24. Nitta T, Ito M, Sato K, Ishii S. Brain stone in the cerebellum. Case report. Neurol Med Chir (Tokyo) 1987; 27(2): 150 – 153.
25. Garen PD, Powers JM, King JS, Perot PL jr. Intracranial fibro‑osseous lesion. Case report. J Neurosurg 1989; 70(3): 475 – 477.
26. Tokunaga H, Iwanaga H, Imanishi M, Koshimae N, Aoki H, Eishu B et al. A huge idiopathic brain stone in the posterior fossa. No Shinkei Geka 1995; 23(8): 711 – 716.
27. Qian J, Rubio A, Powers JM, Rosenblum MK, Pilcher WH, Shrier DA et al. Fibro‑osseous lesions of the central nervous system: report of four cases and literature review. Am J Surg Pathol 1999; 23(10): 1270 – 1275.
28. Tsugu H, Fukushima T, Takeno Y. Calcifying pseudotumor of the neural axi ‑ case report. Neurol Med Chir (Tokyo) 1999; 39(11): 762 – 765.
29. Albu G, Deák G, Mencser Z, Vajtai I. Fibro‑osseous lesion of the central nervous system. Orv Hetil 2001; 142(22): 1165 – 1167.
30. Tatke M, Singh AK, Gupta V. Calcifying pseudoneoplasm of the CNS. Br J Neurosurg 2001; 15(6): 521 – 523.
31. Liccardo G, Lunardi P, Menniti A, Floris R, Pastore FS,Fraioli B. Calcifying pseudo ‑ tumor of the spine: description of a case and review of the literature. Eur Spine J 2003; 12(5): 548 – 551.
32. Ghosal N, Thakre D, Murthy G, Hegde AS. Cerebral calculi in the temporal horn of the lateral ventricle: report of an unusual case. Histopathology 2007; 50(6): 817 – 818.
33. Park P, Schmidt LA, Shah GV, Tran NK, Gandhi D, La Marca F. Calcifying pseudoneoplasm of the spine. Clin Neurol Neurosurg 2008; 110(4): 392 – 395. doi: 10.1016/ j.clineuro.2007.12.006.
34. Apostolopoulos V, David KM, Malcolm A, King A. Intradural calcifying fibroblastic proliferation associated with a nerve root: a reactive process mimicking a nerve sheath tumor. Spine (Phila Pa 1976) 2009; 34(19): 712 – 715.
35. Montibeller GR, Stan AC, Krauss JK, Nakamura M. Calcifying pseudoneoplasm of the inferior colliculus: an unusual location for a rare tumor: case report. Neurosurgery 2009; 65(5): E1005 – E1006. doi: 10.1227/ 01.NEU.0000351770.69874.15.
36. Mohapatra I, Manish R, Mahadevan A, Prasad C, Sampath S, Shankar SK. Calcifying pseudoneoplasm (fibro osseous lesion) of neuraxis (CAPNON) – a case report. Clin Neuropathol 2010; 29(4): 223 – 226.
37. Tong D, Karunaratne N, Howe G, Spencer D, Manolios N. Clinical images: calcifying pseudoneoplasm of the neuraxis. Arthritis Rheum 2010; 62(3): 704. doi: 10.1002/ art.27293.
38. Hodges TR, Karikari IO, Nimjee SM, Tibaleka J, Friedman AH, Cummings TJ et al. Calcifying pseudoneoplasm of the cerebellopontine angle: case report. Neurosurgery 2011; 69 (Suppl 1): E117 – E120. doi: 10.1227/ NEU.0b013e3182155511.
39. Ghosal N, Furtado SV, Gupta K, Hegde AS. Fibro‑-osseous lesion of the pineal region resembling osteoblastoma: a case report. Neuropathology 2011; 31(2): 158 – 161. doi: 10.1111/ j.1440 ‑ 1789.2010.01140.x.
40. Ozdemir M, Bozkurt M, Ozgural O, Erden E, Tuna H, Caglar YS. Unusual localization of an unusual tumor: calcifying pseudoneoplasm of the foramen magnum. Clin Neuropathol 2011; 30(1): 25 – 27.
41. Jentoft ME, Scheithauer BW, Bertoni F, Abood C, Chang HT. Calcifying pseudoneoplasm of the neuraxis with single nerve rootlet involvement. Can J Neurol Sci 2012; 39(6): 840 – 842.
42. Nathoo N, Viloria A, Iwenofu OH, Mendel E. Calcifying fibrous tumor of the spine. World Neurosurg 2012; 77(3 – 4): 592. doi: 10.1016/ j.wneu.2011.04.022.
43. Bartanusz V, Ziu M, Jimenez DF, Henry JM. Calcifying pseudoneoplasm of the atlantoaxial joint in a child. J Neurosurg Spine 2013; 18(4): 367 – 371. doi: 10.3171/ 2013.1.SPINE12810.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek Webové okénkoČlánek Recenze knih a DVDČlánek Implementation ScienceČlánek Projekt ncRNAPain
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2015 Číslo 5- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Diagnostic in Patient with Acute Vertigo
- Implementation Science
- Contribution of Olfactory Tests to Diagnosis of Neurodegenerative Diseases
- Differential Diagnosis of Tauopathies – a Clinical Approach
-
Komentář k článku autorů Rusina et al.
Diferenciální diagnostika tauopatií – klinický pohled – komentář - Cognitive‑ communication Disorders in Patients with Dementia Due to Alzheimer’s Disease
- Normative Data for the Rey‑ Osterrieth Complex Figure Test in Older Czech Adults
- Projekt ncRNAPain
- Results of Early Endarterectomies after Transient Ischaemic Attack
- Methodology of Systematic Review Development I: the Effectiveness of Hyperbaric Oxygen Therapy on Mortality in Adults with Craniotrauma
- Non‑ organic Visual Loss in Children
- Calcifying Pseudoneoplasm of the Neural Axis – a Case Report
- Skull‑ base Osteomyelitis Misdiagnosed and Treated as Neuroborreliosis – a Case Report
- Recurrent Transient Global Amnesia – Four Case Reports
- Hypoglossofacial Anastomosis – Three Case Reports
- Acute Hyperkinetic Syndromes Treated with Stereotactic Neurosurgery Intervention – Three Case Reports
- Webové okénko
-
Analýza dat v neurologii
LIII. Atributivní riziko - Poznámky k nitrosvalové léčbě spasticity botulotoxinem
- Recenze knih a DVD
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Diagnostic in Patient with Acute Vertigo
- Recurrent Transient Global Amnesia – Four Case Reports
- Normative Data for the Rey‑ Osterrieth Complex Figure Test in Older Czech Adults
- Contribution of Olfactory Tests to Diagnosis of Neurodegenerative Diseases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



