-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pracovní den sekce technologie léků
„Pokroky v lékových formách”
Vyšlo v časopise: Čes. slov. Farm., 2016; 65, 192-203
Kategorie: Souhrny přednášek
Brno, 7. září 2016
Dne 7. září 2016 se v Brně na půdě Veterinární a farmaceutické univerzity konal druhým rokem pracovní den Sekce technologie léků s názvem Pokroky v lékových formách. Pořádajícími organizacemi byly Sekce technologie léků České farmaceutické společnosti ČLS JEP a Vzdělávací institute Farmaceutické fakulty VFU Brno. Akci již druhým rokem finančně podpořila společnost Česká lékárna Holding, a.s. – provozovatel sítě lékáren Dr. Max, které patří jménem organizačního výboru poděkování. Cílem akce bylo představení současných trendů v oblasti Farmaceutické technologie.
Konferenci navštívilo 74 účastníků z různých oblastí farmacie – z akademické oblasti (Farmaceutická fakulta VFU Brno, Farmaceutická fakulta UK Hradec Králové, Farmaceutická fakulta UK v Bratislavě, Vysoká škola chemicko-technologická v Praze, Fakulta chemicko-technologická Univerzita Pardubice, Vysoká škola báňská – Technická univerzita Ostrava, Technická univerzita, Liberec), výzkumných ústavů (Výzkumný ústav veterinárního lékařství Brno), farmaceutických firem (Sotax, Zentiva, k.s., Teva) i nemocničních a veřejných lékáren.
V úvodu pracovního dne účastníky přivítala předsedkyně Sekce technologie léků ČFS ČLS JEP doc. PharmDr. Kateřina Kubová, Ph.D. a následně promluvil k účastníkům děkan Farmaceutické fakulty VFU v Brně MUDr. Tomáš Parák, Ph.D. Následoval odborný program, který byl rozdělen do tří sekcí.
V dopolední sekci vystoupil PharmDr. Josef Mašek, Ph.D. z Výzkumného ústavu veterinárního lékařství Brno, který představil účastníkům zajímavou přednášku Nanomateriály pro konstrukci cílených terapeutik. Velmi současná byla také přednáška na téma Antiseptické mukoadhezivní filmy s nanostrukturním jílovým materiálem od doc. PharmDr. Jana Gajdzioka, Ph.D. (Ústav technologie léků FaF VFU, Brno). Dopolední sekci uzavřel doc. Ing. Petr Zámostný, Ph.D. (Vysoká škola chemicko-technologická Praha) s přednáškou Vliv kluzných látek na dezintegrační stabilitu tablet paracetamol-kofein-fenylefrin aneb „Formulační detektivka“, ve které představil postup technologů při odhalování faktorů způsobujících problémy při formulaci lékových forem.
Po pauze na oběd následovala druhá sekce zaměřená na obecnější témata, ovšem se vztahem k farmaceutické technologii. Sekci zahájila doc. PharmDr. Kateřina Kubová, Ph.D. (Ústav technologie léků FaF VFU, Brno) s přednáškou Mikrobicidy v boji proti HIV – současný stav klinických studií, ve které se věnovala dosaženým výsledků v dané oblasti. Na své si přišli také účastníci a zejména účastnice, které zajímá oblast kosmetiky. Přednáškou Současné trendy v péči o zuby a dutinu ústní je velmi zaujala doc. PharmDr. Ruta Masteiková, CSc. (Ústav technologie léků FaF VFU, Brno). Druhá sekce byla uzavřena PharmDr. Alešem Francem, Ph.D. (Ústav technologie léků FaF VFU, Brno). Jeho přednáška s názvem Příprava tvrdých tobolek s vysoce uniformním obsahem léčiva k detoxifikaci při lékových závislostech představila přínos farmaceutické technologie při odvykání závislých na léčivech typu benzodiazepinů.
V závěrečném bloku se objevila témata: Studium lisovatelnosti a vlastností tablet ze směsného suchého pojiva pro tablety dispergovatelné v ústech (PharmDr. Jitka Mužíková, Ph.D., Mgr. Šárka Tumová, Katedra farmaceutické technologie FaF UK, Hradec Králové), Matricové orální filmy jako moderní nosiče pro podání léčiv (PharmDr. Veronika Pechová, doc. PharmDr. Jan Gajdziok, Ph.D., Ústav technologie léků, FaF VFU, Brno), Flow through dissolution technique for microspheres (Michel Magnier, MSc., SOTAX AG, Basel, Switzerland, Ing. Iva Martincová, SOTAX Pharmaceutical Testing, s.r.o.) a Depotní suspenze účinné látky řízené velikostí částic těžce rozpustného léčiva (Mgr. Robert Lehotský, Mgr. Daniel Pěček, Zentiva).
Na pracovním dni byly též prezentovány výsledky farmaceutických technologů brněnské i hradecké Farmaceutické fakulty formou posterových prezentací.
Za organizační výbor (doc. PharmDr. Kateřina Kubová, Ph.D., doc. PharmDr. et Mgr. David Vetchý, Ph.D., doc. PharmDr. Jan Gajdziok, Ph.D.) doufáme, že se účastníkům Pracovní den sekce technologie léků líbil a že je po odborné stránce obohatil. Současně věříme v navázání osobních i pracovních vazeb, které přispějí k dalšímu rozvoji oboru, a doufáme, že se daný cíl podařilo splnit. Poděkování patří také všem kolegům, kteří při organizaci akce pomáhali. Pevné doufáme, že budeme moci účastníky tzv. Technologického dne přivítat na půdě naší univerzity zase příští rok.
za organizační výbor
doc. PharmDr. Kateřina Kubová, Ph.D.
Nanomaterials for targeted drug delivery systems
JOSEF MAŠEK
Department of Pharmacology and Immunotherapy, Veterinary Research Institute, Brno, Czech Republic
e-mail: masek@vri.czTargeted drug delivery is a method of delivering an active pharmaceutical ingredient (API) to a target organ, tissue or cell. Increased local concentration of a drug in a targeted region is achieved, whereas unwanted action in other parts of the body is suppressed. Formulation of API into nanoparticulate delivery systems exhibits one of the basic premises to achieve targeted drug delivery. Specific properties of nanomaterials are given by their size and shape as well as by their surface characteristics. The surface of nanoparticles can be functionalised or modified using different biopolymers, specific antibodies or targeting ligands to achieve active targeting of API into specific cells or to achieve prolonged circulation time in the blood vessels. Long-circulating nanoparticulate drug formulations (e.g. stealth liposomes) are predominantly delivered via enhanced permeation and retention effect (EPR effect) into tumour tissue. Controlled release, triggered release and release of the drug based on stimuli-responsiveness (e.g. temperature, pH) of nanocarriers are other crucial characteristics of many clinically tested delivery systems.
Although most of the approved nanoparticulate drug delivery systems are based on liposomes, a number of other delivery systems composed of biocompatible polymers, drug conjugates as well as inorganic materials are being developed and tested. A special group of therapeutic formulations are theranostics combining therapeutic and imaging agents.
Targeted drug delivery and nanoparticle-based formulations exhibit enormous potential for increasing drug potency and lowering the toxicity of currently used drugs as well as for the formulation of new drugs based on nucleic acids, proteins and peptides. Administration of API by alternative routes and drug targeting to the brain through the blood-brain barrier are important milestones that might be achieved using nanotechnology in the future.
Acknowledgements: This work was supported by project CZ AZV-ČR 16-30299A of the Ministry of Health of the Czech Republic and project GAP503/12/G147 of the Czech Science Foundation.
Antiseptic mucoadeshive films containing clay nanocompositive
JAN GAJDZIOK, HANA LANDOVÁ, DAVID VETCHÝ, PETR DOLEŽEL
University of Veterinary and Pharmaceutical Sciences Brno, Pharmaceutical Faculty, Department of Pharmaceutics, Brno, Czech Republic
e-mail: gajdziokj@vfu.czIntroduction: The present-day local therapy of infectious stomatitis is insufficiently effective due to a short residence time of the liquid or semisolid medical formulations used. An innovative dosage form based on mucoadhesive polymers that feature a prolonged residence time on the oral mucosa may be a solution of this problem. This formulation consists of a mucoadhesive oral film with incorporated nanocomposite biomaterial that is able to release the carried drug directly at the target area.
The study was aimed to prepare, test, and statistically evaluate mucoadhesive oral films (MOFs) based on the perspective mucoadhesive polymer carmellose in the form of its sodium salt and acid non-woven textile. Films were formulated using two promising techniques: solvent casting and impregnation. An innovative nanotechnologically modified clay mineral (vermiculite) with intercalated antiseptic drugs – chlorhexidine diacetate and digluconate was incorporated into their structure. Multivariate data analysis was used to evaluate the effects of the non-woven textile and incorporation of the active substance on the physico-mechanical and mucoadhesive properties of formulated MOFs. Prepared films demonstrated properties suitable for clinical use.
Experimental methods
Materials
A fraction < 45 µm of clay mineral vermiculite (Ver – Letovice, CZ) was used. Chlorhexidine diacetate (CA) and chlorhexidine digluconate (CG) (20% in H2O) were obtained from Sigma Aldrich, CZ and employed as APIs to prepare organovermiculite nanocomposites. Carmellose sodium (NaCMC – Blanose 7LF-PH, Ashland Aqualon, USA) served as the basic mucoadhesive and film-forming polymer. In some samples (Table 1), an acid form of carmellose (textile – Hcel HT, Holzbecher Medical, CZ) was incorporated into the structure of the film as a non-woven textile. In all cases, glycerol (Gly) (Kulich, CZ) acted as a plasticizer. Mucin from porcine stomach (Type II, Sigma-Aldrich, Co., USA) as a 5% dispersion was used to prepare artificial mucus. All other chemicals used in this experiment were of analytical grade.
Preparation of organovermiculites
The ethanolic solutions of CA and CG were prepared in concentrations according to the 0.5x CEC of Ver, and then stirred and heated with Ver suspended in water. After centrifugation, solid products were dried, and samples for the experiment were named Ver_CA and Ver_CG.
Preparation of mucoadhesive films
A 4% dispersion of NaCMC and Gly (3%) was prepared. Ver_CA and Ver_CG were added to the dispersion at two different concentrations to ensure the amount of chlorhexidine 10 or 20 mg in final MOFs. Subsequently, ten different batches of MOFs were prepared, five using the solvent casting method (samples without textile), and five using the innovative method of impregnation of the textile from the acid form of carmellose (Table 1)1).
Tab. 1. Table 1. Composition of prepared films 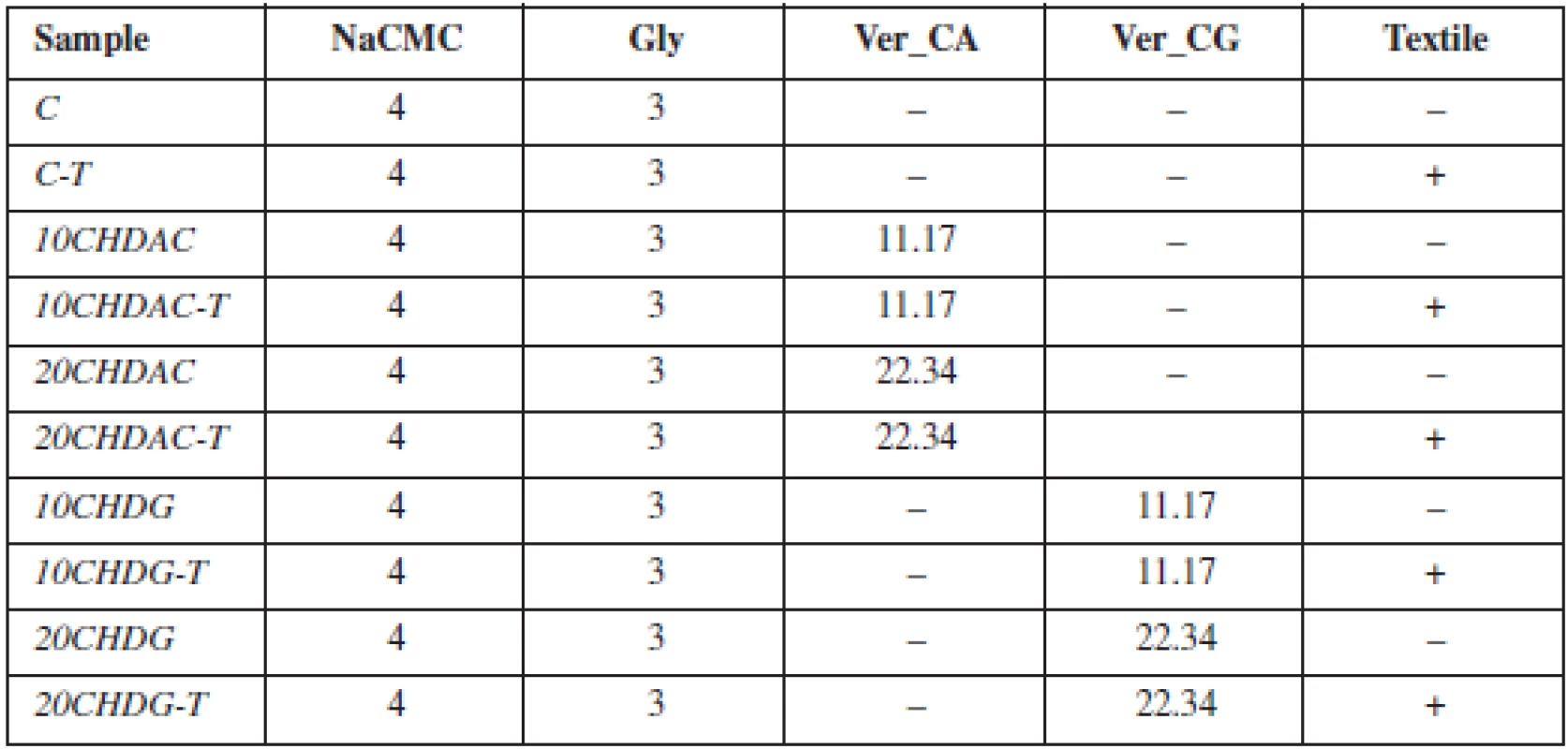
aFor all preparations, water was added to the final weight of 100 g The weight of MOFs was measured in ten circular (15 mm) samples selected at random from each batch (Table 2).
Film thickness was evaluated by optical analysis using a microscope (STM-902 ZOOM, Opting, CZ). Sample thickness was measured at fifteen different places on 3 films (Table 2).
Surface pH was measured using a contact pH meter (Flatrode, Hamilton, CH). An electrode was dipped into the MOF sample, and the value was recorded after stabilization.
A modified disintegration apparatus was used to determine residence time (RT)1). A basket for tablet insertion was replaced with a plastic slab. Oral mucosa was simulated using a cellophane membrane covered with mucin dispersion. The time necessary for complete detachment or erosion of the film was recorded (Table 2).
A CT3 Texture Analyzer (Brookfield, USA) was used for tensile testing of the prepared MOFs1). The strength and work done during this process and the deformation of the film at the moment of tearing were measured. The texture analyzer with a cylindrical probe was used for a puncture test. The strength needed to puncture fixed samples, the work done during this process, and the deformation of the film at the moment of penetration were measured. Values measured by the texture analyzer were recalculated for the film thickness 100 µm.
Multivariate data analysis
Methods of principal component analysis (PCA) and cluster analysis (ClustA) were used for descriptive evaluation of the experimental data. Prior to modelling, the variables were adjusted by autoscaling (i.e., mean centering), and scaling by standard deviation. The influence of formulation variables (Table 1) on the parameters of mechanical resistance, in vitro residence time, and surface pH were subsequently evaluated using multiple linear regression (MLR) analysis with analysis of variance. Suitability of the MLR models was assessed on the basis of characteristics such as R-square regression (which describes each model’s explained variability), R-square of prediction (which expresses the model’s predictive ability), and coefficient of variation (CV%; the average modelling error expressed as a percentage of the mean). Statistical evaluations were conducted using the program Unscrambler X (v. 10.3, Camo Software, NOR).
Results and discussion: The results are summarized in Table 2 and Figure 1.
Fig. 1 Principal component analysis: a – scores plot, b – correlation loadings plot 
This study describes the unique approach of preparing mucoadhesive oral films from the well-established mucoadhesive polymer carmellose (the sodium salt and/or acid form of the non-woven textile) with an incorporated nanotechnologically modified clay mineral (vermiculite) intercalated with the antiseptic drug chlorhexidine. The multivariate data analysis was employed to evaluate the influence of the formulation and process variables on the properties of the final medical preparation. This evaluation was subsequently complemented by testing the antimicrobial and antimycotic activity of prepared films (results not presented) with the aim of finding the most suitable composition for clinical application.
Conclusion: Generally, the best results were obtained with the sample containing 20 mg of chlorhexidine diacetate carried by vermiculite, with carmellose in the form of non-woven textile in its structure.
Acknowledgement: This work was supported by the Ministry of Health of CZ (projects: 1 RVO-FNOs/2012 and NT14477).
References
1. Vetchý D., Landová H., Gajdziok J., Doležel P., Daněk Z., Štembírek J. Determination of dependences among in vitro and in vivo properties of prepared mucoadhesive buccal films using multivariate data analysis. Eur. J. Pharm. Biopharm. 2014; 86, 498–506.
Investigating lubricant effects on disintegration stability of paracetamol-caffeine-phenylephrine tablets
PETR ZÁMOSTNÝ1, DOMINIKA THIMOVÁ1, BARBORA KREIBICHOVÁ1,2
1University of Chemistry and Technology Prague, Faculty of Chemical Technology, Department of Organic Technology
2Zentiva k.s., Praha
e-mail: petr.zamostny@vscht.czCombined drugs continue to increase their popularity because they are known to improve patient compliance as well as they represent an opportunity for pharmaceutical companies to deliver a higher added value product. The combined drug formulation is quite often based on a mixture of the so-called “mono-components”. They represent the core formulations of individual APIs used for manufacturing separate drugs thereof. While the drug-drug and drug-excipient cross-interactions are generally known and can be avoided in the combined drug formulation procedure, the excipient-excipient interaction or physical property effects may cause unexpected problems during pilot-batch manufacturing and stability tests of the combined product.
The aim of this work is to report a case study of successful investigation of stability problems caused by excipient-excipient interaction in a combined drug product. The work involves multicomponent mixtures containing the active pharmaceutical ingredients paracetamol, caffeine, and phenylephrine hydrochloride for tablet compression, the physical properties of which exhibited substantial change in physical properties during stability tests combined with significant batch-to-batch variability of the properties. Most notably, the changing physical properties developed into the prolonged disintegration of tablets, which increased from 1–3 minutes to 15–20 minutes within 2 days of aging. Our goal was therefore to discover the possible cause of prolonged disintegration and suggest a modification to alleviate the problem.
The first part of this work explored the parametric sensitivity of tablets compressed from different batches of tablet mixtures. The tests involved a study of how the disintegration time is affected by the pressure during compression, the pressure of pre-compression, the compression time, the tablet moisture before and after compaction, thermal stress, and other conditions. The experiments confirmed the problem of the increasing tablet disintegration time and the effect was quantified for each batch. Moisture and thermal stress proved to play a crucial role in the process. Tablets compressed from a mixture with the highest moisture content had the most favorable disintegration profile. On the other hand, tablets with the lowest moisture content showed an extreme increase in their disintegration time as soon as a few minutes after compaction. Even a moderate thermal stress of the prepared tablets (40 °C) lead to a significant deterioration of their disintegrating properties independent of the moisture content. On the other hand, experiments with another, laboratory prepared batch dried down to 4% moisture and then again down to 1.5% moisture, showed the moisture content effect is more complex and it plays a different role in aging of uncompressed mixture and the compressed tablets. Figure 1 shows the high-moisture mixture produces extreme disintegration time sensitivity on the tablet age. The sensitivity is reduced when the mixture is dried to a less moisture content. Nevertheless, it can be hardly decided, whether the moisture content or the time passed between the two drying steps, is the most important factor in the prolonged disintegration.
Fig. 1. Tablet disintegration time for fresh (■) and dried mixture (▲) compressed at 14 kN, and dried mixture (●) compressed at 12 kN 
Next, the properties of the mixture components that could be responsible for affecting the tablet disintegration were investigated. The physical and chemical properties of the individual mixture components, their eutectic phases, crystalline and amorphous forms and stability were studied. No changes in phase composition of APIs were detected using both the XRD and DSC measurements. Therefore, the effects of excipients were investigated looking for their cross-interactions. The main object of investigation was stearic acid, a substance with a very low melting point and interesting crystalline variability.
Some reports of interactions between stearic acid and polyvinyl pyrrolidone were already reported by Desai et al.1). We examined the pseudo-binary mixtures of Stearic acid 50 (SA) and Collidone 25 (polyvinyl pyrrolidone, PVP) using DSC. The SA content was 30, 50, and 70% wt. %. All mixtures exhibited onset in 38–41 °C range corresponding to the melting of the eutectic pseudo-binary mixture. However, only the mixture having 70% SA showed a peak corresponding to the solidification of the eutectic liquid involving the re-crystallizing SA. In other samples the solidification produced a glass phase. This conclusion is also supported by the fact that those samples did not show the melting peak on second heating pass.
Further disintegration and dissolution tests showed that the unstable disintegrating properties of tablets were caused by the interactions between Stearic acid 50 and Collidone 25. The glass phase forming both during the tablet compression and the subsequent tablet aging probably hinders the access of liquid to soluble particles of the formulation, affecting the water intake and hence the disintegration of the tablet.
In conclusion, the interaction of stearic acid and polyvinyl pyrrolidone was identified as a cause of tablet disintegration time variability. The interaction was found to develop during the aging of the mixture, the tablet compression, and the aging of the tablets, the rates of the processes being affected by moisture and temperature. The combination results in extreme variability of the product properties. Several solutions were proposed to avoid the problem of unstable disintegration. The most radical but probably also the most robust solution would be to use other lubricants during tablets production. For example, replacement of SA50 by magnesium stearate removed all disintegration-related problems. Alternatively, changing the SA/PVP proportion could prevent the glass phase formation and suppress the problems. Theoretically, the problem may by also alleviated by using pure SA instead of SA50, but it seems impractical from the commercial point of view. Magnesium stearate seems to be suitable for this purpose. Yet another possibility of eliminating the disintegration problems is optimizing the process of tablet pre-compression and compression in terms of pressures, dwell time and humidity. Adjusted compression conditions could guarantee the right level of tablet disintegration that would comply the stability programs, but they would put additional demands on controlling the process thoroughly.
Abbreviations
SA – stearic acid
SA50 – stearic acid of 50% purity (the rest is mostly palmitic acid)
PVP – polyvinyl pyrrolidoneFinancial support from specific university research (MSMT No 20/2016) is gratefully acknowledged.
References
1. Desai D., Kothari S., Huang M. Solid-state interaction of stearic acid with povidone and its effect on dissolution stability of capsules. Int. J. Pharm. 2008; 354(1–2), 77–81.
Microbicides in fighting against HIV: the current status of clinical trials
KATEŘINA KUBOVÁ, VERONIKA TKADLEČKOVÁ
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic
e-mail: kubovak@vfu.czThe original purpose for research of microbicides is to slow down the HIV epidemic among the world’s population until an effective vaccination will be developed. Generally, microbicide is defined as a chemical substance that kills, neutralizes or blocks the HIV virus and/or other sexually transmitted pathogens used in a variety of topical application regimes. Microbicides are applied on the vaginal and rectal mucosa. They can have also the contraceptive function1).
Currently, clinical trials of microbicides are performed by the organization The Microbicide Trials Network (MTN), which was established in 2006 in the USA. The MTN brings together international investigators and community and industry partners whose work is focused on the development and rigorous evaluation of promising microbicides2).
Microbicides include the active substances of different pharmacological groups. In the past, most of them (surfactants, buffering systems, and anionic polymers) were shown completely ineffective in clinical trials. Nowadays, a partial efficacy of antiretroviral agents (tenofovir, dapivirin) was proved in the prevention of sexual transmission of HIV in women. The favourite dosage forms for incorporation of microbicides are carbomers/polycarbophil vaginal gels or vaginal rings.
In the clinical trial CAPRISA 004 (phase IIb, 2007–2010, 900 women, placebo-controlled), 1% tenofovir gel was tested. The results showed that tenofovir gel, applied before and after sex, reduced HIV incidence by 39% overall and by 54% in women who used the gel consistently3).
The clinical trial ASPIRE (phase III, 2012–2015, 2,629 women, placebo-controlled) was designed to evaluate the safety and efficacy of the dapivirine vaginal ring (25 mg) for the prevention of HIV-1 infection in healthy, sexually active, HIV-negative women. The results showed that dapivirine vaginal ring reduced HIV incidence by 61% in women older than 25 years, whereas only by 10% in women younger than 25 years, probably due to low adherence4).
Nowadays in a clinical trial (phase I), a highly anticipated multifunctional vaginal ring – (tenofovir/ levonorgestrel) is being investigated.
Financial support IGA VFU Brno Czech Republic, project 306/2016/FaF is gratefully acknowledged.
References
1. Alexander N. J., Baker E., Kaptein M., Karck U., Miller L. Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004; 82(1), 1–12.
2. The microbicide Trials Network, http://www.mtnstopshiv.org/ (5. 9. 2016).
3. Abdool Karim Q., Abdool Karim S. S., Frohlich J. A., Grobler A. C., Baxter C., Mansoor L.E. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of hiv infection in women. Science 2010; 329, 1168–1174.
4. Palanee-Phillips T., Schwartz K., Brown E. R., Govender V., Mgodi N., Kiweewa F. M., Nair G., Mhlanga F., Siva S., Bekker L. G., Jeenarain N., Gaffoor Z., Martinson F., Makanani B., Naidoo S., Pather A., Phillip J., Husnik M. J., van der Straten A., Soto-Torres L., Baeten J. Characteristics of women enrolled into a randomized clinical trial of dapivirine vaginal ring for HIV-1 prevention. PLoS One 2015; 10(6), doi: 10.1371/journal. pone. 0128857.A study of compressibility and properties of tablets from a coprocessed dry binder for orally disintegrating tablets
JITKA MUŽÍKOVÁ, ŠÁRKA TUMOVÁ
Department of Pharmaceutical Technology, Charles University, Faculty of Pharmacy in Hradec Králové, Czech Republic
e-mail: muzikova@faf.cuni.czOrally disintegrating tablets (ODTs) combine the advantages of solid and liquid dosage forms. They improve patient compliance, convenience, bioavailability and rapid onset of the action of a drug. They are suitable for pediatric and geriatric patients, for patients who cannot swallow and for people who are travelling because water is not needed for administration1). One of the methods of ODTs production is direct compression. This process is simple but makes high demands on flowability and compressibility of tableting materials. Dry binders are basic excipients for direct compression2). In ODTs, sugars and sugar alcohols (very often mannitol) are used as dry binders. Due to the requirement of fast tablet disintegration, tableting materials must contain superdisintegrants or effervescent additives1, 3). Excipients can be connected using coprocessing to coprocessed dry binder4). Examples of coprocessed dry binders for ODTs are Pharmaburst®, Ludiflash®, Pearlitol Flash®, Disintequik™ ODT, Prosolv® ODT G2.
This work deals with the study of the compressibility and properties of tablets from Prosolv® ODT G2, which contains mannitol (67.3%), fructose (4.9%), crosspovidon (4.3%), microcrystalline cellulose (21.6%) and colloidal silicon dioxide (2%). Prosolv ODT G2 was tested in combination with two lubricants: magnesium stearate and sodium stearyl fumarate. For this purpose, two concentrations (0.5% and 1%) of lubricants were used. Combinations with the model drugs acetylsalicylic acid and ascorbic acid were also included. Prosolv® ODT G2 was also compared with Prosolv® SMCC 50 in the properties studied. Compressibility was evaluated using the energy profile of the compression process5). The tensile strength and disintegration time of the tablets were tested.
The total energy values of tableting materials with Prosolv® ODT G2 were not significantly affected by lubricants, acetylsalicylic acid increased the total energy. In the case of Prosolv® SMCC 50, the values of total energy were higher. Plasticity decreased in the case of Prosolv® ODT G2 under the influence of lubricants and drugs. Plasticity values for Prosolv® SMCC 50 were higher and more balanced. Lubricants in the mixtures with Prosolv® ODT G2 increased; however, ascorbic acid decreased the tensile strength of the tablets. Tablets from the Prosolv® SMCC 50 mixtures were significantly stronger, but lubricants decreased the tensile strength of the tablets. The disintegration time of tablets with Prosolv® ODT G2 was very short. It was proved that lubricants and acetylsalicylic acid prolonged the disintegration time in contrast to ascorbic acid which decreased the disintegration time. Tablets from Prosolv® SMCC 50 mixtures had a longer disintegration time.
References
1. Hahm H. A., Augsburger L. L. Orally disintegrating tablets and related tablet formulations. In: AugsburgerL. L., Hoag S. W. (eds.) Pharmaceutical dosage forms: Tablets, Vol. 2, 3th ed. New York: Informa Healthcare USA, Inc. 2008.
2. BolhuisG. K., de Waard H. Compaction properties of directly compressible materials. In: Çelik, M. (ed.) Pharmaceutical powder compaction technology, 2nd ed. New York: Informa Healthcare USA, Inc. 2011.
3. Velmurugan S., Sundar V. Oral disintegrating tablets. IJCPS 2010; 1, 1–12.
4. Gupta P., Nachaegari S. K., Bansal A. K. Improved excipient functionality coprocessing. In: Katdare, K.A., Chaubal, M. V., eds. Excipient developement for pharmaceutical biotechnology, and drug delivery systems. New York: Informa Healthcare USA 2006.
5. Ragnarsson G. Force-Displacement and Network Measurements. In: Alderborn G., Nyström, Ch. (eds.) Pharmaceutical powder compaction technology. New York: Marcel Dekker Inc. 1996.Oral matrix films as innovative drug carriers
VERONIKA PECHOVÁ, JAN GAJDZIOK
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic
e-mail: gajdziokj@vfu.czOral films, namely buccal mucoadhesive films and orodispersible films, are classified among oromucosal preparations. Oromucosal preparations are defined as solid, semi-solid or liquid preparations containing one or more active substances intended for administration to the oral cavity and/or the upper part of the throat to obtain a local or systemic effect. Mucoadhesive preparations are intended to be retained in the oral cavity by adhesion to the mucosal epithelium and usually contain hydrophilic polymers, which during wetting by saliva produce a hydrogel that adheres to the buccal mucosa. Buccal mucoadhesive films are advanced mucoadhesive preparations in the form of single - or multilayer patches of suitable polymeric materials1). They provide better patient compliance compared to buccal tablets owing to their flexibility that causes only minor discomfort to the patient. Buccal mucoadhesive films are suitable dosage form for drugs with low oral bioavailability, because buccal application circumvents possible degradation processes in the gastrointestinal tract as well as hepatic first-pass metabolism. Furthermore, buccal films are preferably used for the local treatment of oral mucosa2). Orodispersible films are intended for rapid dissolution and subsequent swallowing without the need of liquid intake. They are mainly used for administration of medicines requiring rapid onset of action (antimigraine drugs, antiemetic agents, antipsychotics, antihistamines etc.)3). The two main techniques used to prepare oral films are solvent casting and hot melt extrusion. Innovative manufacturing processes represent ink-jet or flexographic printing, characterized as application of the active substance on a placebo oral film with specific techniques4). Although manufacturers and developers of oral films perform a variety of tests to control critical quality attributes, the European Pharmacopoeia recommends only to ensure suitable mechanical strength to resist handling without crumbling or breaking and to carry out a dissolution testing to demonstrate the appropriate release of the active substance1). Oral films are nowadays available on the world pharmaceutical market, but only two products are available in the Czech Republic5).
References
1. European Directorate for the Quality of Medicines & Health Care. The European Pharmacopoeia 8th edition 2014 (8.1). http://online6.edqm.eu/ep801/ (5. 9. 2015).
2. Gilhotra R. M., Ikram M., Srivastava S., Gilhotra N.A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014; 28, 81–97.
3. Dixit R. P., Puthli S. P.Oral strip technology: overview and future potential. J. Control. Release. 2009; 139, 94–107.
4. Borges A. F., Silva C., Coelho J. F., Simões S. Oral films: current status and future perspectives: I – Galenical development and quality attributes. J. Control Release. 2015; 206, 1–19.
5. SÚKL. Databáze léků. http://www.sukl.cz/modules/medication/search.php (5. 9. 2016).Flow through dissolution techniqure for microspheres
MICHEL MAGNIER
SOTAX AG, Nordring 1, Aesch, 4147, Switzerland
e-mail: michel.magnier@sotax.comInitially described in CIBA by the Dr Langenbucher as the “column type method”, the Flow-Through Cell has been designed in Basel, in partnership with the SOTAX Company to overcome limitations faced with paddle and baskets, as maintaining sink conditions for poorly soluble compounds. The Flow-Through Cell technique has been later described in Pharmacopeias, based on standardized results obtained during the first industry and academic researches. After 40 years of development, the Flow-Through Cell is now the only dissolution technique which can be used for APIs, intermediates and final products. The FTC can therefore give information all along the dosage form lifecycle. The FTC dissolution has become a method of choice for several dosage forms (lipidic forms, powders, suspensions, microspheres, liposomes, medical devices). For every kind of dosage form, university professors have initiated scientific research that has been scaled up in the industry with manufacturers and have been described in parallel in regulation. As an example, the use of the Flow-Through Cell for microspheres has started around 2005 in the University of Connecticut. The first system used was a closed loop system with UV Fiber optic. In 2006, FTC was used to define accelerated conditions based on high temperature conditions. In 2007, the pH impact was studied. In 2010, the University of Connecticut worked in parallel on the use of FTC for liposomes, and in 2011 the FTC started to be described as a possible Quality Control Tool for microspheres. A second paper issued in 2011 involved regulation authorities and described the method validation on Risperidone. In parallel, general articles on microspheres were calling for a reference method. In 2015, the first level A in vivo in vitro correlation was described. In 2015, a pro-pharmacopeia document was issued: stimuli to revision process: performance test for parenteral dosage forms, including microspheres and liposomes. A future step will now be the inclusion of the FTC test for parenteral dosage forms in the Pharmacopeia.
Stručné shrnutí přednášky v českém jazyce
Ing. Iva Martincová – zástupce SOTAX AG pro Českou a Slovenskou republiku
SOTAX Pharmaceutical Testing s.r.o., Průmyslová 1306/7, 102 00 Praha 10, tel.: +420 774 771 277
e-mail: Iva.Martincova@sotax.comThe utilization of surface tension measurement for the evaluation of the critical micelle concentration of cetyl trimethylammonium bromide
BARBORA VRANÍKOVÁ, TEREZA KRCHOVOVÁ, KRISTÝNA OLÁHOVÁ, PETRA SVAČINOVÁ, PAVEL ONDREJČEK, ZDEŇKA ŠKLUBALOVÁ
Department of Pharmaceutical Technology, Faculty of Pharmacy in Hradec Králové, Charles University, Hradec Králové, Czech Republic
e-mail: vranikovab@faf.cuni.czIntroduction: Surfactants are a group of compounds containing both a hydrophobic long-chain part (tail) and a hydrophilic polar part (head) in their structure. According to the character of the polar head, they may be divided into four categories – anionic, cationic, non-ionic and zwitterionic surfactants1). It is well known that under the critical concentration, the surfactant is dispersed mostly in monomers. Above that concentration, however, the surfactant will aggregate and form micelles in the bulk solution. This concentration is defined as the critical micelle concentration (CMC). CMC values can be determined by a number of techniques including surface tension, electrical conductivity, light scattering, electron paramagnetic resonance and analytical ultracentrifugation2, 3). Conductivity meters and tensiometers are the two most popular methods for determining CMC3).
The surface tension method uses a surface tensiometer and measures the point at which the solution surface is saturated with a surfactant. The proportion of molecules presented at the surface of a liquid or as micelles in the bulk of liquid depends on their concentration. At low concentrations, surfactants occupy the surface of the liquid. As the surface becomes crowded with surfactant, additional molecules arrange into micelles, and surface tension becomes independent of the surfactant concentration2, 4).
Experimental methods: Cetyl trimethylammonium bromide (CTAB) (Sigma-Aldrich spol. s.r.o., Czech Republic) was dissolved in ultrapure water and acetate buffer of pH 5.5 to obtain a solution with a concentration of 600 mg/L and 500 mg/L, respectively. These stock solutions were subsequently diluted to concentrations of 500, 450, 400, 350, 300, 250, 200, 100 and 50 mg/L for ultrapure water and 400, 300, 250, 200, 175, 150, 125, 100, 75, 50, 25 and 10 mg/L for acetate buffer.
The surface tension measurement was performed using a processor tensiometer Krüss type K 100 (Krüss GmbH, Germany) equipped with a thermostat. The equilibrium surface tension of all prepared solutions was evaluated using the ring method at 25 °C three times for each concentration.
Results and discussion: The results from the measurements of CTAB in ultrapure water are presented in Figure 1. The resulting curve was interposed with two linear line segments. The CMC value was calculated using the equations describing line segments (–0.07x + 58.2 = 0.003x + 34.701). The obtained CMC value of 321.9 mg/L (0.88 mM) corresponds with the results given by the manufacturer (0.92–1.0 mM)5) and scientific literature (0.8 mM)6).
Fig. 1. Relation between concentration and surface tension of CTAB in ultrapure water 
Figure 2 shows the results of the evaluation of the surface tension of CTAB solutions in acetate buffer of pH 5.5. The resulting curve was interposed with two linear line segments. The CMC value was calculated using the equations describing line segments (–0.991x + 48.888 = –0.0012x + 40.565). The CMC value of CTAB in acetate buffer (85 mg/mL or 0.23 mM) is lower in comparison with CMC in ultrapure water. The lower value of CMC in acetate buffer can be explained by the electrolyte effect on micelle formation. The electrolyte neutralizes the charge at the micelle surface, reduces the thickness of the ionic layer around the surfactant ionic heads and, therefore, reduces the electrostatic repulsions between them, helping in this way the micellization process6). A similar effect can be observed in the presence of ionogenic drugs. The decreased value of CMC can cause a reduction in emulsifying efficiency of the surfactant.
Fig. 2. Relation between concentration and surface tension of CTAB in acetate buffer 
Conclusion: The critical micelle concentration can be determined by a number of techniques including the surface tension method. The CMC value of CTAB in acetate buffer was lower (0.23 mM) in comparison with CMC in ultrapure water (0.88 mM). This lower value of CMC for acetate buffer can be explained by the electrolyte effect. Therefore, the presence of ionogenic drugs can cause a reduction in emulsifying efficiency of the surfactant.
References
1. MalmstenM. Surfactants and Polymers in Drug Delivery. Vol. 122. New York: Marcel Dekker 2002.
2. Kerwin B. A.Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008; 97, 2924–2935.
3. Song Y., Sun R., Zhao K., Pan X., Zhou H., Li D. An induction current method for determining the critical micelle concentration and the polarity of surfactants. Colloid Polym. Sci. 2015; 293, 1525–1534.
4. http://www.biolinscientific.com/attension/applications/ (8. 8. 2016)
5. http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Shete/2/h6269pis.pdf (8. 8. 2016)
6. Bahri M. A., HoebekeM., Grammenos A., Delanaye L., Vandewalle N., Seret A. Investigation of SDS, DTAB and CTAB micelle microviscosities by electron spin resonance. Colloid Surface A. 2006; 290, 206–212.
Štítky
Farmacie Farmakologie
Článek Nové knihy
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2016 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Optická metoda měření velikosti a refrakčního indexu nanočástic Ag@Fe3O4
- Výhrada svědomí při výkonu profese lékárníka ve Slovenské republice
- Židé v evropské farmacii od 7. do počátku 20. století
-
Pracovní den sekce technologie léků
„Pokroky v lékových formách” - Výstava Léčivé rostliny a jejich dvojníci
- Prof. RNDr. Viliam Foltán, CSc. jubiluje
- Prof. RNDr. Luděk Beneš, DrSc. – in memoriam
- Nové knihy
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Výhrada svědomí při výkonu profese lékárníka ve Slovenské republice
- Židé v evropské farmacii od 7. do počátku 20. století
- Prof. RNDr. Luděk Beneš, DrSc. – in memoriam
- Optická metoda měření velikosti a refrakčního indexu nanočástic Ag@Fe3O4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání