-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The role of flavonoid osajin in renal ischemia-reperfusion model
Úloha flavonoidu osajinu na modelu ischemie-reperfuze ledviny
Byl sledován efekt flavonoidu osajinu extrahovaného z rostliny Maclura pomifera, Moraceae v podmínkách ischemie reperfuze ledviny. Ischémie v délce 60 minut s následnou reperfuzí způsobila vzestup markeru metabolismu volných kyslíkových radikálů malondialdehydu (MDA) v séru, vzestup hladin kreatininu a urey jako markerů ledvinného selhávání a pokles hodnot celkové antioxidační kapacity. Tyto změny doprovázela změna funkce ledvin, projevující se poklesem clearance creatininu. Patnáctidenní orální podávání osajinu v dávkách 5, 10 a 20 mg/kg/den se projevilo statisticky významným snížením hladin malondialdehydu, zvýšením hladiny superoxiddismutázy, zvýšením celkové antioxidační kapacity a zvýšením clearance. Tato data naznačují, že osajin má potenciální protektivní efekt proti renálním dysfunkcím, vyvolaným stavem ischémie s následnou reperfuzí. Závěry podporují i výsledky histopatologického vyšetření ledvinné tkáně.
Klíčová slova:
osajin – Maclura pomifera – antioxidant – renální ischemie-reperfuze – volné kyslíkové radikály
Authors: J. Nečas 1; L. Bartošíková 1; T. Bartošík 2; P. Fráňa 3; M. Pavlík 2
Authors place of work: Palacký University in Olomouc, Faculty of Medicine, Department of Physiology, Czech Republic 1; St Anne’s Faculty Hospital Brno, Clinic of Anesthesiology and Resuscitation, Czech Republic 2; St Anne’s Faculty Hospital Brno, IInd Clinic of Internal Medicine, Czech Republic 3
Published in the journal: Čes. slov. Farm., 2009; 58, 160-167
Category: Původní práce
Summary
The aim of the present study was to investigate the antioxidant effect of the flavonoid osajin isolated from the infructences of Maclura pomifera, Moraceae, against the damage inflicted by ROS in a rat model of renal ischemia-reperfusion injury. Renal ischemia-reperfusion was induced by clamping unilateral renal artery for 60 minutes. Renal function was assessed by estimating serum creatinine, blood urea nitrogen and creatinine clearance. Renal morphological alterations were assessed by histopathological examination of hematoxylin-eosin stained sections of the kidneys. Ischemia-reperfusion produced elevated levels of malondialdehyde (a metabolic marker of lipid peroxidation), decreased the total antioxidant activity and deteriorated the renal function as assessed by increased serum creatinine, blood urea nitrogen and decreased creatinine clearance as compared to control rats. The ischemic kidneys of rats showed severe hyaline casts, epithelial swelling, proteinaceous debris, tubular necrosis, medullary hemorrhage and an extensive inflammatory infiltrate. The oral administration of osajin at the doses of 5, 10, and 20 mg/kg for 15 days significantly decreased the malondialdehyde level, increased superoxide dismutase, the total antioxidant activity and creatinine clearance to nearly the normal level. These results together with those obtained from the histopathological examination clearly demonstrate the in vivo antioxidant effect of osajin in attenuating renal ischemia-reperfusion injury.
Key words:
osajin – Maclura pomifera – antioxidant – renal ischemia-reperfusion – free oxygen radicalsIntroduction
Many superior plant compounds have a protective effect on human health and they are used in the treatment of a wide range of diseases in popular medicine. Flavonoids belong to these classes of compounds. They normally occur in human diet, especially in fruit and vegetables, and their daily intake is about 1–2 g. Many of their effects on plant and animal cells are significant 1–4). In oncology the flavonoids are used as corroborative compounds, which reduce the side effects of cytostatics, and on the other hand, they enhance the therapeutical effects – this field is documented very well 5–8).
Flavonoids are often used in the treatment of inflammation because of their ability to inhibit the key enzyme in PG synthesis – COX 9–13). They were tested in post-transplantation conditions, too 14).
Flavonoids are effective antioxidants in the case of the oxidative injury 15). They prevent lipid peroxidation, scavenge free oxygen radicals, and inactivate pro-oxidative metal ions (iron, copper). The scavenging activity is one of the best known properties and it represents a significant therapeutic use 16). The antioxidant activity of the flavonoids depends on the number and position of hydroxyl groups in their molecules and on their glycosylation. The optimal properties were found in the flavonoids with the ortho-hydroxy structure on ring B; 2, 3 double bond, and the 4 oxo functional group on ring C, and 3 and 5 -OH groups on rings A and C 17, 18).
Among various reactive oxygen species (ROS) generated during the pathological processes, which cause or accompany I/R injury, superoxide plays the key role. In spite of being a free radical, it is not highly reactive, however the superoxide radical anion appears to play a central role, since other reactive intermediates are formed in reaction sequences starting with it. According to the fact that the physiological functions of the kidneys are conditioned by a remarkable consumption of oxygen, the elimination of superoxide radical during I/R is one of the possible therapeutic interventions. In this study we have examined whether it is possible to affect the origination of superoxide and its metabolic derivates experimentally through the oral administration of different doses of the flavonoid osajin.
EXPERIMENTAL PART
Methods
Plant material
Maclura pomifera (Raf.) Schneid. (Moraceae), fruits (2 kg) obtained from the Garden of Herbal Plants of the Department of Natural Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno. A voucher specimen (MPF 001030) is deposited in the herbarium of this Department.
Extract preparation
Osajin was extracted from the infructencence of Maclura pomifera, Moraceae, together with another flavonoid pomiferin. These two flavonoids were separated from each other by an addition of lead acetate which is able to react with the two hydroxyl groups at positions 3’ and 4’ on ring B of pomiferin to form a light yellow insoluble coagulate while osajin remains in the solution since it has only one hydroxyl group on ring B.
Procedure of the separation process
10 grams of a osajin-pomiferin mixture was heated with 15 grams of lead acetate trihydrate in 20 ml of methanol. A light yellow coagulate was produced and then filtered. The filtered coagulate was rinsed with hot acetone and dissolved in hot acetic acid (200 ml). Osajin (3.5 grams) was obtained by crystallization from the thickened methanol and acetone solution. The obtained substances were re-crystallized from methanol. The purity of osajin was HPLC - proved.
High-performance liquid chromatography (HPLC)
High-performance liquid chromatography was used to assess the quality of the separate fractions after flash chromatography, column chromatography, and for the identification and purity ascertainment of the isolated substances and prepared semi-synthetic derivates. Chromatography was performed with the apparatus Hewlett Packard 1100 with a DAD detector. The chromatograms at the wavelengths of 280 nm and 350 nm were registered. The columns LC-8 and Supelcosil ABZ+Plus were used for the measurements.
Maclura method: wavelengths of 280 nm and 350 nm. The mobile phase was a mixture of acetone nitrile and 40 mM formic acid (gradient elution – in the first minute 30 per cent 40 mM formic acid and 70 per cent acetone nitrile, up to 15 minute 100 per cent acetone nitrile). Flow 1 ml/min, column temperature 40 °C.
Muscar method: wavelengths of 280 nm and 350 nm. The mobile phase was a mixture of acetone nitrile and 40 mM formic acid (gradient elution – in the first minute 10 per cent acetone nitrile and 90 per cent 40 mM formic acid, up to 28th minute 100 per cent acetone nitrile). Flow 1 ml/min, column temperature 40 °C.
Fig. 1. High-performance liquid chromatogram of osajin from infructencence of Maclura pomifera, Moraceae (LC-8 – method Maclura) 
Fig. 2. High-performance liquid chromatogram of osajin from infructencence of Maclura pomifera, Moraceae (Supelcosil ABZ+Plus – method Muscar) 
Animals and therapy
The experiment was approved and monitored by the Ethical Committee of the University. The experiment was performed by the work team whose members are holders of the Certificate on Professional Competence issued by the Central Commission for Animal Protection pursuant to § 17 of the Act on Protection of Animals against Cruelty (No 246/1992 Coll.) of the Czech National Council.
This study was performed on 50 male Wistar SPF (AnLab, Germany) laboratory rats of identical age and comparable weight (250 ± 15 gr). The animals were housed at a standard controlled temperature, fed a standard diet for small laboratory animals, and given water ad libitum during their acclimation. After a recovery period, the animals were divided randomly into 5 groups and placed separately in glass metabolic cages.
The 40 animals from treated and placebo groups were subjected to renal ischemia (60 min) and subsequent reperfusion in general anaesthesia (2% Rometar-xylazinum – 0.5 ml + 1% Narkamon-ketaminum – 10 ml, a dose of 0.5 ml solution/100 g body weight).
The tested compound was applied to three groups of animals (n = 10) – treated groups – in three different doses of 5 mg.kg-1, 10 mg.kg-1, and 20 mg.kg-1 (for each animal group 1 selected dose) in a 0.5% Avicel solution orally once a day by a intragastric tube 19). To the fourth group (n = 10) – the placebo group – 0.5% Avicel solution only was applied in the same quantity (2 ml) and by the same application method as in the treated groups. The last group of the animals (n = 10) – the intact one – was left without I/R and treatment.
At the end of the experiment (the 15th day), all animals were exsanguinated in general anaesthesia (see above) and the selected biochemical parameters were analyzed. Samples of the kidney tissue were collected for histopathological examination.
Biochemical assay
In blood: superoxide dismutase (SOD), glutathion peroxidase (GSHPx), total antioxidative activity (TAA) using RANDOX testing kits (Dublin, Ireland); further in serum: malondialdehyde (MDA) – TBARs method, urea, creatinine, uric acid. In urine: creatinine, total protein, Diuresis.
Fig. 3. Osajin chemical structure C<sub>25</sub>H<sub>24</sub>O<sub>5</sub>, Mr = 404, M.p. = 188 to 189 °C, solubility – MeOH, Chloroform, EtoAc 
Statistical analysis
The obtained values of the biochemical parameters under study were processed by a Microsoft® Excel® table processor and statistically evaluated using a UNISTAT programme and ANOVA test. The value p ≤ 0.05 was considered to be significant.
Histopathological examination
The material was fixed in 10% formaldehyde and processed in the manual way. Two blocks were made of each sample, the sections being stained with hematoxylin-eosin. All evaluated samples were of outstanding quality, the evaluation being performed by a histopathologist without knowledge of the experimental protocol.
Evaluation principle: all samples in the material were evaluated and scored in 3 kidney topicalities separately, the result was added up, and in the end the average score of each medicated group was stated.
Scoring schedule:
1st topicality – kidney medulla – the grade of tissue destruction through bleeding (according to the extent) and presence of inflammatory infiltrate (max. +++) were evaluated here.
2nd topicality – cortex and glomerules – both extraglomerular (+) presence of hemorrhages and increased cellularity and extravasates in the glomerule (max. ++) were evaluated here.
3rd topicality – kidney channels – presence of regressive changes of the epithelia from edema to necrosis was evaluated here (+ in the case of necrosis and ± in the case of regression not reaching the grade of necrosis). In addition, the channel content was evaluated (protein and hyaline cylinders +). The maximum is ++.
The highest possible result (the worst) per one sample was 7 (7 times +).
Results
Biochemical results
The serum MDA values were statistically significantly increased in animals after I/R in the placebo group in comparison with the intact animals (p ≤ 0.01). After the oral administration of osajin at the doses of 5, 10 a 20 mg/kg/day for a period of 15 days, the MDA values statistically significantly decreased (p ≤ 0.01). The serum TAA values were statistically significantly reduced in the placebo animal group in comparison with the intact animal group and with the osajin-treated groups at the doses of 5, 10 a 20 mg/kg/day for the period of 15 days. The results of MDA and TAA assays are summarized in Table 1.
Tab. 1. Effect of osajin administration on MDA serum values and total antioxidative activity 
Values are means ± S.D.; ** p ≤ 0.01 treated vs. placebo group; ++ p ≤ 0.01 treated vs. intact group ; • • p ≤ 0.01 placebo vs. intact group The superoxide dismutase (SOD) values were statistically highly significantly increased (p ≤ 0.01) in the treated animals at the doses of 5, 10, and 20 mg/kg/day in comparison with the placebo group and the intact animal group. The SOD values were not statistically significantly different at mutual comparison of different osajin doses.
The GSHPx values were statistically highly significantly decreased (p ≤ 0.01) in comparison with the placebo group and the intact animal group. The GSHPx values were not statistically significantly different at the doses of 5, 10 and 20 mg/kg, see Table 2.
Tab. 2. Effect of osajin administration on the activity of enzymes participating in scavenging reactions 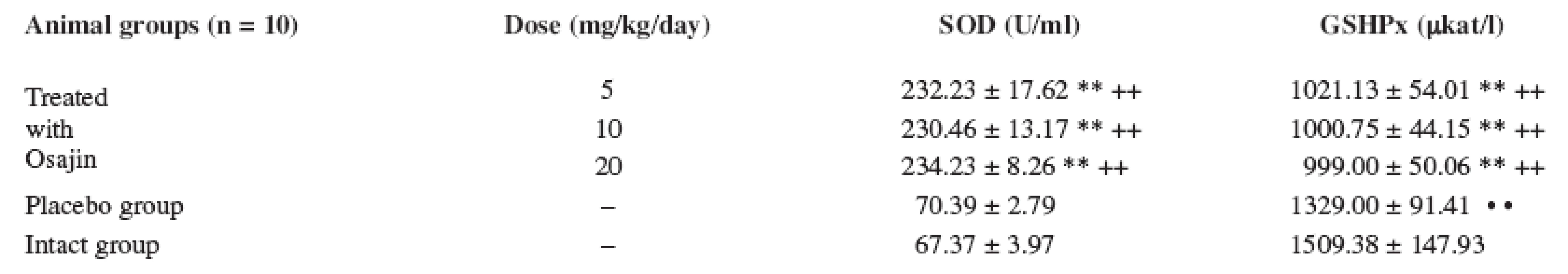
Values are means ± S.D.; ** p ≤ 0.01 treated vs. placebo group; ++ p ≤ 0.01 treated vs. intact group; • • p ≤ 0.01 placebo vs. intact group The serum creatinine values after I/R do not show significant changes in the placebo group and in the osajin-treated groups, either. Their values were higher in comparison with the intact animal group only (p ≤ 0.01; p ≤ 0.05). The applied osajin in the treated animals does not show statistically significant changes in the values of creatinine.
The serum urea values in the animals exposed to I/R are higher in comparison with the group of the intact animals (p ≤ 0.01; p ≤ 0.05), their values were not decreased by osajin administration. See Table 3.
Tab. 3. Effect of osajin administration on serum creatinine and urea value 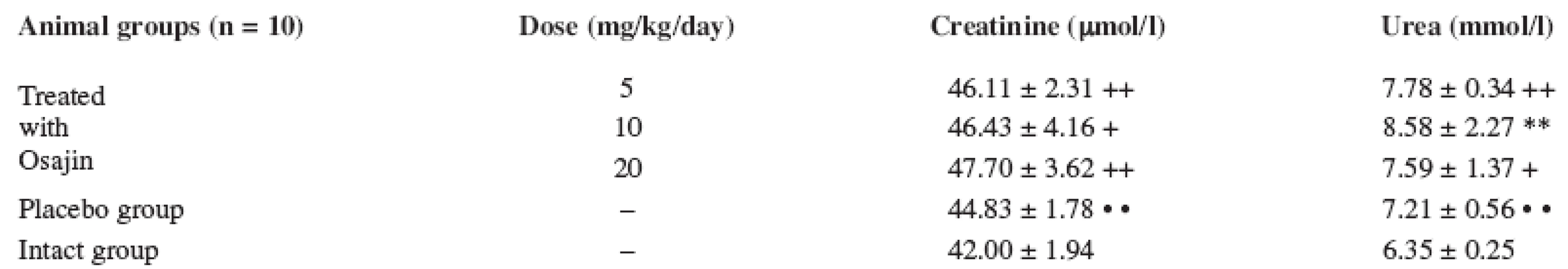
Values are means ± S.D.; ** p ≤ 0.01 treated vs. placebo group; ++ p ≤ 0.01 treated vs. intact group; • • p ≤ 0.01 placebo vs. intact group; + p ≤ 0.05 treated vs. intact group The urine creatinine values after the performed I/R were statistically significantly higher in the placebo group in comparison with the intact group (p ≤ 0.01). Osajin administration did not result in their decrease. An analogical effect was observed in the urea and total protein values.
The diuresis values in the animals after the performed I/R were statistically significantly decreased in the placebo group in comparison with the intact group (p ≤ 0.01). Osajin peroral administration did not result in a significant change. See Table 4.
Tab. 4. Effect of osajin administration on urine values of creatinine, urea, total protein and diuresis 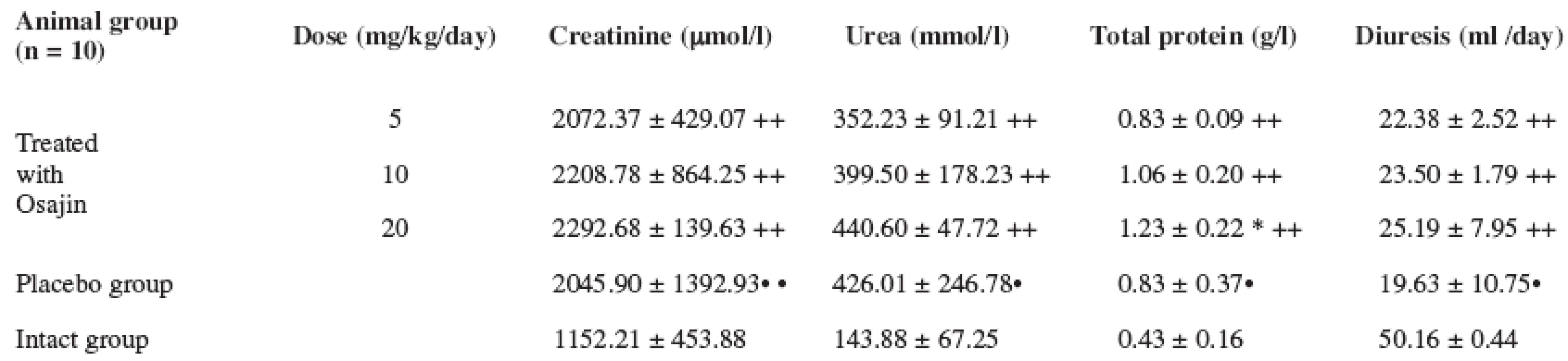
Values are means ± S.D.; * p ≤ 0.05 treated vs. placebo group; ** p ≤ 0.01 treated vs. placebo group; + p ≤ 0.05 treated vs. intact group; ++ p ≤ 0.01 treated vs. intact group; • • p ≤ 0.05 placebo vs. intact group; p ≤ 0.01 placebo vs. intact group For the kidney functional condition assessment, the 24 hour creatinine clearance was determined. The intact animal clearance corresponding to the value of 1376.07 ml/24 hours was used as the standard equal to 100%. At the clearance comparison in the placebo group, the clearance of 65.01% was ascertained. In the animals after osajin administration at the individual doses of 5, 10, and 20 mg/kg/day, the clearance was 73.09%, 81.24%, and 87.98%, respectively. See Table 5.
Tab. 5. Effect of osajin administration on creatinine clearance/24 hours 
Results of histopathological examination
Generally, the best result was achieved in the animal group after osajin administration at the dose of 5 mg/kg/day with a score of 3.3 (the best indexes in all 3 topicalities were here, too).
Placebo group: in the placebo group changes typical of ischemia with the score from 2 to 5.5 with the average 4.25 occurred. The presence of the inflammatory infiltrate with the admixture of polymorphonuclears (in 67 %) was also relatively frequent.
Intact group: hamorrhagia only incidentally, most probably caused by bruise, otherwise without pathological changes. See Figure 4.
Fig. 4. Light microscopy (hematoxylin and eosin stained sections) of rat kidneys: (A) Rat kidney treated with osajin (5 mg/kg/day) prior to renal ischemia/reperfusion (magn. 400×); (B) placebo rat kidney (cortex) subjected to ischemia/reperfusion (magn. 400×); (C) placebo rat kidney (medulla) subjected to ischemia/reperfusion (magn. 200×); (D) renal tissue of intact animals (magn. 400×) 
Discussion
Renal ischemia is well known as a frequent cause of renal failure during which a functional damage of the kidney occurs due to the combination of blood vessel vasoconstriction, tubular obstruction, and glomerular filtration malfunction 20–22).
In the conditions of I/R, highly reactive oxygen radicals are generated. The fact that free oxygen radicals play a significant role during the renal I/R is well known, being accompanied by superoxide dismutase, glutathione peroxidase depletion and reduction of the total antioxidant activity, which act as natural oxygen radical scavengers in the organism 23). SOD, GSHPx, and other antioxidants play an important role in the elimination of pathological changes caused by I/R through detoxication of oxygen radicals arising during this process 24, 25).
The aim of the performed study was the assessment of the nephroprotective effect of the flavonoid osajin separated from Maclura pomifera, Moraceae, in vivo using a rat model of renal I/R injury. Earlier in vitro studies confirmed the antioxidant properties of this flavonoid 2). The results of our study have confirmed that after I/R period the renal malfunction occurs in non-treated animals, which was demonstrated in the diuresis reduction (p ≤ 0.01; p ≤ 0.05) and a creatinine clearance decrease. The general model of the malfunction is characterized by the total antioxidant activity decrease, and the increase in MDA value (p ≤ 0.01).
The administration of osajin perorally at the doses of 5, 10, and 20 mg/kg/day secondary to renal ischemia, for the period of 15 days, showed distinct changes in the values of the biochemical parameters under study.
The antioxidant protection under the conditions of inducing oxidative injury is a complex system in which the separate antioxidant elements co-operate with one another. The function of one antioxidant often conditions the effects of another element in the system. Dismutation of superoxide by SOD is the first step of the enzymatic cascade leading to the complete detoxification of free radicals. However, the product of its activity, H2O2, is still a toxic agent. The second step corresponds to the transformation of H2O2 to H2O via hydroperoxidases such as catalase or glutathione peroxidase. In our experiment, the superoxide dismutase values were significantly lower in the animals after I/R, which is considered as the evidence for the consequence of this pathological process. In contrast to this, the SOD values were significantly increased (p ≤ 0.01) in the treated animals after osajin administration in comparison with the placebo and the intact animal groups. No relation between the effect and the different doses of 5, 10, and 20 mg/kg/day was observed. It is evident that the increase in the SOD activity can be noxious for the organism. Since it can produce H2O2 (a precursor of the hydroxyl radical) at a rate in excess of that at which hydroperoxides can remove it. In our experiment, statistically significantly decreased GSHPx values (p ≤ 0.01) were ascertained in comparison with the placebo and the intact animal groups without the statistical dependence on the dose. GSHPx is only able to act in the presence of a sufficient amount of reduced glutathione (GSH). GSH is oxidized to GSSG, and then GSSG oxidized glutathione is then reduced by the NADPH – dependent flavoenzyme glutathione reductase in order to maintain GSH-Px activity. Thus, the ratio of GSSG to GSH is believed to be an excellent antioxidant marker during intracellular oxidant stress.
Natural antioxidants can react with different radicals through different mechanisms; they can affect one another (synergy, inhibition) 26, 27). Therefore we have measured the total antioxidant activity which quantifies the capacity of radical elimination in a biological material sample. After 15 days of osajin peroral administration, the total antioxidant activity increased (p ≤ 0.01; p ≤ 0.05) in the treated groups in comparison with the placebo and the intact animal groups.
Osajin peroral administration for the period of 15 days after I/R induction reduced the MDA values statistically significantly independent of the applied doses. MDA, as a final product and a marker of free hydrogen radical metabolism generated during the pathological reactions following after I/R, was statistically significantly reduced (p ≤ 0.01) in comparison with the placebo group. Our results confirmed that the MDA serum values in I/R exposed animals were higher in the animals of the placebo group in comparison with the group of the intact animals, while osajin reduced the MDA values, by which it confirmed the capacity to prevent lipoperoxidation induced by I/R injury.
Animals that underwent renal I/R exhibited significant morphological and functional injuries which were characterized by a significant increase in the serum concentrations of creatinine and urea nitrogen as compared to the control, suggesting a significant degree of glomerular dysfunction caused by renal I/R.
Renal I/R also produced a significant reduction in creatinine clearance which was used as an indicator of glomerular filtration rate, and thus of the glomerular function.
The osajin peroral administration within the period of 15 days after renal ischemia in our experiment increased the clearance values in comparison with the animals exposed to I/R only. The animals exposed to I/R showed a statistically significant diuresis decrease in comparison with the intact animal group and the treated groups. The perorally administered flavonoid osajin improved the kidney functional parameters in diuresis, creatinine clearance, and urea excretion in urine; the other kidney parameters are probably misrepresented due to the functional compensation by the healthy kidney.
From the results of our experiment it can be deduced that the administration of osajin in the laboratory rats subsequently after I/R induction protects against I/R injury consequences. This was confirmed by the increase in both the antioxidant enzyme values and the total antioxidant activity which occur even at a dose of 5 mg/kg/day. This effect is also demonstrated in the functional parameter of clearance; as a result of this a higher metabolite excretion by urine occurs. The osajin protective effect was also confirmed by the histopathological examination of the kidney tissue. The protective effect depends on the applied dose in some parameters. Although the flavonoid osajin seems to be protective with a probable effect against the oxidative injury induced by the renal I/R, its effect and optimal method of application have to be explained.
Address for correspondence:
doc. MUDr. Jiří Nečas, CSc.
Palacký University in Olomouc,Faculty of Medicine, Department of Physiology, Czech Republic
Hněvotínská 3, 775 15 Olomouc
e-mail: necasjir@tunw.upol.cz
Received 9 Juny 2009
Accepted 8 July 2009
Zdroje
1. Havsteen, B. H.: Pharmacol. Ther., 2002; 96, 67–202.
2. Bartosikova, L., Necas, J., Suchy, V., Janostikova, E., Bartosik, T., Jurica, J., Florian, T., Klusakova, K., Frydrych, M.: Pharmazie, 2006; 61, 552–555.
3. Yao, L. H., Jiang, Y. M., Shi, J., Tomas-Barberan, F. A., Datta, N., Singanusong, R., Chen, S. S.: Plant. Foods Hum. Nutr., 2004; 59,113–122.
4. Valachovicova, T., Slivova, V., Sliva, D.: Mini Rev. Med. Chem., 2004; 4, 881–887.
5. Kandaswami, C., Perkins, E., Soloniuk, D. S., Drzewiecki, G., Middleton, E. Jr.: Cancer. Letters, 1991; 56,147–152.
6. Plowman, J., Narayanan, V. L., Dykes, D., Szarvasi, E., Briet, P., Yoder, O. C., Paull, K. D.: Cancer. Treat. Rep., 1986; 70, 631–635.
7. Scambia, G., Ranelletti, F.O., Benedetti-Panici, P., Piantelli, M., Bonanno, G., De Vincenzo, R., Ferrandina, G., Maggiano, N., Capelli, A., Mancuso, S.: Gynecol. Oncol., 1992; 45, 13–19.
8. Nishino, H., Tokuda, H., Satomi, Y., Masuda, M., Osaka, Y., Yogosawa, S., Wada, S., Mou, X. Y., Takayasu, J., Murakoshi, M., Jinnno, K., Yano, M.: Biofactors, 2004; 22,57–61.
9. Kuehl, F. A., Egan, R. W.: Science, 1980; 28, 978–984.
10. O‘Leary, K. A., de Pascual-Tereasa, S., Needs, P. W., Bao, Y. P., O‘Brien, N. M., Williamson, G.: Mutat. Res., 2004; 551, 245–254.
11. Seaver, B., Smith, J. R.: J. Herb. Pharmacother., 2004; 4, 11–18.
12. Selvam, C., Jachak, S. M., Bhutani, K. K.: Phytother. Res., 2004; 8, 582–584.
13. Bayer, J., Gomer, A., Demir, Y., Amano, H., Kish, D. D., Fairchild, R., Heeger, P.S.: Clin. Immunol., 2004; 110, 100–108.
14. Zhong, Z., Connor, H. D., Froh, M., Lind, H., Bunzendahl, H., Mason, R. P., Thurman, R. G., Lemasters, J. J.: Free Radic. Biol. Med., 2004; 36, 1248–1258.
15. Manach, C., Donovan, J. L.: Free Radic. Res., 2004; 38, 771–785.
16. Urquiaga, I., Leighton, F.: Biol. Res., 2000; 33, 55–64.
17. Mabry, T. J., Ulubelen, A.: J. Agric. Food Chem., 1980; 28(2), 188–195.
18. Aherne, S.A., O’Brien, N.M.: Nutrition, 2002; 18, 75–81.
19. Vetchy, D., Rabiskova, M.: Int. J. Pharm., 2002; 242, 353–356.
20. Greene, E. L., Paller, M. S.: Miner. Electrolyte Metab., 1991; 17, 124–132.
21. Paller, M.S.: Free Radic. Biol. Med., 1991; 10, 29–34.
22. Chatterjee P. K.: Naunyn-Schmiedebergęs Arch. Pharmacol., 2007; 376, 1–43.
23. De Vecchi, E., Lubatti, L., Beretta, C., Ferrero, S., Rinaldi, P., Galli Kienle, M., Trazzi, R., Paroni, R.: Kidney Int., 1998; 54, 857–863.
24. Subash, S., Subramnian, P.: Mol. Cell. Biochem., 2009; 327, 153–161.
25. Guz, G., Demirogullari, B., Ulusu, N. N., Dogu, C., Demirtola, A., Kavutcu, M., Omeroglu, S., Stefek, M., Karasu, C.: Clin. Exp. Pharmacol. Physiol., 2007; 34, 210–216.
26. Ratnam, D. V., Angola, D. D., Bhardway, V., Sahana, D. K., Kumar, M. N. V. R.: J. Control. Rel., 2006; 113, 189.
27. Bartosikova, L., Necas, J., Suchy, V., Jankovska, D., Janostikova, E., Bartosik, T., Klusakova, J., Jurica, J., Florian, T., Frydrych, M., Krcmar, J., Frana, P., Franova, J.: Czech and Slovak Pharmacy, 2006; 55, 78–83.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2009 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Cardioprotective effect of 2’,3,4’--trihydroxychalcone in preclinical experiment
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
- The role of flavonoid osajin in renal ischemia-reperfusion model
- Effects of combined hormonal deprivation and fungal elicitation on the production of coumarins in cell suspension cultures of Angelica archangelica L.
- Studies of the properties of tablets from directly compressible isomalt
- XLIX. sympozium z historie farmacie a veterinární medicíny
- Determination of the constituents of propolis of different geographical origin
- Z činnosti farmaceutických společností
- Na památku prof. RNDr. PhMr. Karla Paláta, CSc.
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Determination of the constituents of propolis of different geographical origin
- Studies of the properties of tablets from directly compressible isomalt
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání






