-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHighly active antiretroviral therapy during gestation: effects on a rat model of pregnancy
Vysoce účinná antiretrovirová terapie během těhotenství: vliv na těhotenství na krysím modelu
Cíl:
Posoudit nepříznivé účinky chronického užívání zidovudinu/lopinaviru/ritonaviru v těhotenství na krysím modelu.Typ studie:
Prospektivní experimentální studie.Pracoviště:
Gynekologicko-porodnická klinika, Federální Univerzita, São Paulo (UNIFESP).Metody:
40 březích EPM-1 potkanů albínů bylo náhodně rozděleno do čtyř skupin po 10 zvířatech: kontrolní (Ctrl) skupina (neošetřené) a tři experimentální skupiny (Exp1, Exp2 a Exp3), které obdržely zidovudin/lopinavir/ritonavir v odpovídajících dávkách 10/13,3/3,3; 30/39,9/9,9 a 90/119,7/29,7 mg/kg/den, od prvního do 20. dne březosti. Krysám byla aplikována dávka denně. Tělesná hmotnost byla zaznamenána ve dnech 0, 7, 14 a 20. V okamžiku, kdy byly krysy usmrceny, byl zaznamenán počet živých a mrtvých plodů, placenta a hmotnost placent a plodů. Plody byly vyšetřeny stereoskopickým mikroskopem, kdy byly hodnoceny vnější abnormality. K porovnání úmrtnosti mezi skupinami byl použit χ2 test.Výsledky:
Mezi skupinami nebyl v průběhu těhotenství zaznamenán statisticky významný rozdíl v nárůstu tělesné hmotnosti. Průměrné přírůstky mezi 7. a 20. den byly 45,70 ± 5,27 g pro Ctrl, 48,49 ± 3,64 g pro Exp1; 45,39 ± 6,22 g pro Exp2 a 44,19 ± 6,78 g pro Exp3. Nicméně, 7. percentil přírůstku hmotnosti byl nižší ve skupinách Exp2 a Exp3 a 14. percentil ve skupině Exp2. Další posuzované parametry se mezi skupinami Exp2 a Exp3 významně nelišily.Závěr:
Chronická expozice březích krys vysokým dávkám zidovudinu/lopinavirem/ritonavirem měla za následek významné snížení tělesné hmotnosti matky, ale nebyla spojena s významnými nepříznivými důsledky pro plod.Klíčová slova:
biologické stanovení, antiretroviral, těhotenství, krysa, teratologie
Authors: L. P. Carvalho; R. S. Simões; Edward Araujo Júnior
; Oliveira Filho R. M.; L. Kulay Júnior; M. U. Nakamura
Authors place of work: Department of Obstetrics, São Paulo Federal University (UNIFESP), São Paulo, SP, Brazil 1; Department of Infectology, University José do Rosário Velano, Alfenas, MG, Brazil 2; Department of Obstetrics and Gynecology, São Paulo University (USP), São Paulo, SP, Brazil 3; Department of Pharmacology, Institute of Biomedical Sciences, São Paulo University (USP), São Paulo, SP, Brazil 4
Published in the journal: Ceska Gynekol 2014; 79(2): 128-133
Summary
Objective:
To assess the adverse effects of the chronic use of zidovudine/lopinavir/ritonavir in a rat pregnancy model.Type of article:
Original paper.Design:
A prospective experimental study.Setting:
Department of Obstetrics, São Paulo Federal University (UNIFESP).Methods:
40 pregnant EPM-1 albino rats were randomly allocated into four groups of 10 animals each: control (Ctrl) group (untreated) and three experimental groups (Exp1, Exp2 and Exp3), which received zidovudine/lopinavir/ritonavir in the corresponding doses of 10/13.3/3.3; 30/39.9/9.9 and 90/119.7/29.7 mg/Kg/day from the first up to the 20th day of pregnancy, respectively. The rats were treated by gavage daily. Body weights were recorded on days 0, 7, 14 and 20. At term, the rats were sacrificed and the implantation sites, number of live and dead fetuses and placentas, resorptions and fetal and placental weights were recorded. The fetuses were evaluated for external abnormalities under a stereomicroscope. The chi-square test was used to compare death rates between groups.Results:
Weight gain during pregnancy no showed significant differences between groups. Average weight gains between the 7th and 20th day were 45.70 ± 5.27 g for Ctrl; 48.49 ± 3.64 g for Exp1; 45.39 ± 6.22 g for Exp2 and 44.19 ± 6.78 g for Exp3. However, the percentage weight gain in the 7th was lower in groups Exp2 and Exp3 and in the 14th in the Group Exp2. All other parameters assessed did not differ significantly between groups. Exp2 and Exp3 in relation of the others.Conclusions:
The chronic exposure of pregnant rats to high doses of zidovudine/lopinavir/ritonavir in association resulted in a significant reduction in maternal body weight gain but was not associated with significant adverse fetal parameters.Keywords:
biological assay, antiretroviral, pregnancy, rat, teratologyINTRODUCTION
The human immunodeficiency virus (HIV) over time has shown a tendency to stabilize the number of infected persons. However, in women, the rate of the epidemic is growing. In the 80s, beginning of the pandemic period, the ratio of men / women infected was 20 : 1. Currently, for every 1.6 infected men, there is 1 infected woman [4].
Disturbing fact has been the predominant age in females, between 13 to 19 years. This fact is associated with unprotected sex, early onset of sexual activity, cultural, socioeconomic and biological conditions of women themselves, which makes it vulnerable to HIV. The prevalence of infection in the reproductive period favors vertical transmission [5]. Most studies suggest that vertical transmission occurs in 70% of cases the intrapartum, and 30% of cases occurring in utero. Breastfeeding has 15 % additional risk of transmission [9, 10, 23].
In 90s began the study of antiretroviral in HIV positive pregnant women as Protocol 076, verifying the reduction of transmission of 25.5% among newborns of women who used placebo to 8.3% for children of mothers who used AZT [11]. Since then, several studies have demonstrated the effectiveness of protective measures for vertical transmission. In Brazil, in 2011, 745 cases of acquired immunodeficiency syndrome (AIDS) in children under 5 years were reported with an incidence rate of 5.4 / 100,000. The use of antiretroviral drugs for pregnant women and newborn, the indication for cesarean delivery and breast feeding with artificial milk, decreased by 25% AIDS cases among children under 5 years in the last ten years in our country [4].
Among the several treatment protocols, highly active antiretroviral therapy (HAART), usually includes one nucleoside analog (DNA chain terminator), one protease inhibitor and either a second nucleoside analog (“nuke”) or a non-nucleoside reverse transcription inhibitor [14]. Though the association of antiretroviral drugs has been shown to be largely safe for the concept [31], it is supposed that association of multiple drugs may introduce changes in their pharmacokinetics [19] and carry on unpredictable results on maternal and/or fetal compartments. The association resulted in a significant reduction of viral load in plasma in such an extent that sometimes it leads to undetectable levels of the virus [13].
Despite the reduction of vertical transmission, some antiretroviral drugs may be associated with teratogenicity, preterm delivery, intrauterine growth restriction, and may be developing resistance to antiretroviral use [22, 23]. Carvalho et al. [7] founded deleterious effects on fertility, high maternal mortality with the administration of ritonavir to pregnant rats, suggesting an important compartmentation of the drug. Cunha et al. [12] showed that the administration of a combination of lopinavir plus ritonavir to pregnant rats can causes morphological as well as functional changes in maternal and fetal liver and kidneys and, in higher than therapeutic doses, might be toxic to those animals. Though one cannot extrapolate drug effects from animals to human beings, the study of the effect of drugs on animal pregnancy is the best model to understand the effect of such drugs on humans. Carvalho et al. [8] showed that high dosing of zidovudine/lopinavir/ritonavir association during the entire rat pregnancy period can cause morphological changes in maternal liver, kidneys and pancreas. On the other hand, the corresponding fetal organs were not affected.
This experimental way of studying the effect of drugs on pregnancy has been adopted by the Experimental Obstetrics Discipline, Department of Obstetrics, São Paulo Federal University (UNIFESP), Brazil. Drugs have been studied alone or in association with others. The aim of such studies is to verify any pharmacokinetic change in the antiretroviral drugs that can provoke adverse effects. It is well known that there are many trials of drug effects on rat pregnancy. We decided to test lower doses of zidovudine/lopinavir/ritonavir association, corresponding to 1, 3 and 9 times the human therapeutic doses. These same doses have previously been investigated in an experimental model study that appraised the effects on maternal and fetal prole.
MATERIALS AND METHODS
Female Wistar rats (Rattus norvegicus albinus) of the EPM-1 variant, weighing approximately 200g each, were provided by the Center for Development of Experimental Models of UNIFESP. The study was approved by the local Animal Care Committee (Report 0402/09) and followed the guidelines proposed by the Canadian Council on Animal Care [24].
The animals were kept in plastic cages under controlled room temperature (22°C) and artificial light by fluorescent lamps with a constant day/night cycle (lights on 07 : 00–19 : 00), with free access to pelleted Purina rat food (Agribrands Purina do Brasil Ltda, Vilhena, RO, Brazil) and tap water. After a 7-day period of adaptation, the animals were mated in the proportion of one healthy male to three females during two hours. The immediate 24-hour period after mating was taken as day 0 (zero) of pregnancy if spermatozoids were detected in vaginal smears [16]. Forty pregnant rats were randomly divided into four groups. The control group (Ctrl) received distilled water and the experimental groups (Exp1, Exp2 and Exp3) received zidovudine/lopinavir/ritonavir in the corresponding doses of 10/13.3/3.3; 30/39.9/9.9 and 90/119,7/29.7 mg/Kg/day by gavage from the first up to the 20th day of pregnancy. Body weights were recorded for all animals at days 0, 7, 14 and 20 of pregnancy and expressed as percentage of body weight gain [26, 27].
At term, the animals were weighed; anesthetized using a mixture of xylazine (20 mg/kg) and ketamine (100 mg/kg) administered by the intraperitoneal route and submitted to laparotomy and hysterotomy. The following parameters were recorded: ovular implantations, ovular reabsorptions, number of live and dead fetuses and fetal and placental weights. The fetuses were inspected in detail under a stereoscopic microscope for gross external malformations: limb shortening, spina bifida, cleft lip, cleft palate, polydactyly and rachisquisis [27].
Data were expressed as mean ± standard deviation (SD). ANOVA and the Kruskal-Wallis multiple comparisons test were used for statistical analyses. The Chi-square test was used to compare death rates between groups. Significance was set at 5%.
RESULTS
Mean weight gain in the control group ranged from 12.77 g (± 2.93) to 58.47 g (± 8.73). In the experimental groups, mean weight gain from day 1 to 20 of gestation was 11.73 g (± 2.54) to 60.22 g (±5 .52), 8.78 g (± 0.67) to 54.17 g (± 12.71) and 9.01 g (± 0.88) to 53.20 g (± 14.85) in groups Exp1, Exp2 and Exp3, respectively. As shown on Table 1, these differences were significant in the Exp2 and Exp3 groups on the 7th day and Exp2 on the 14th day in relation to the others. None of the other parameters analyzed (number of fetuses, number of placentas, fetal weight and placental weight) differed significantly between the groups (Table 2).
Tab. 1. Body weight gain (%) of pregnant rats treated with zidovudine/lopinavir/ritonavir throughout gestation† 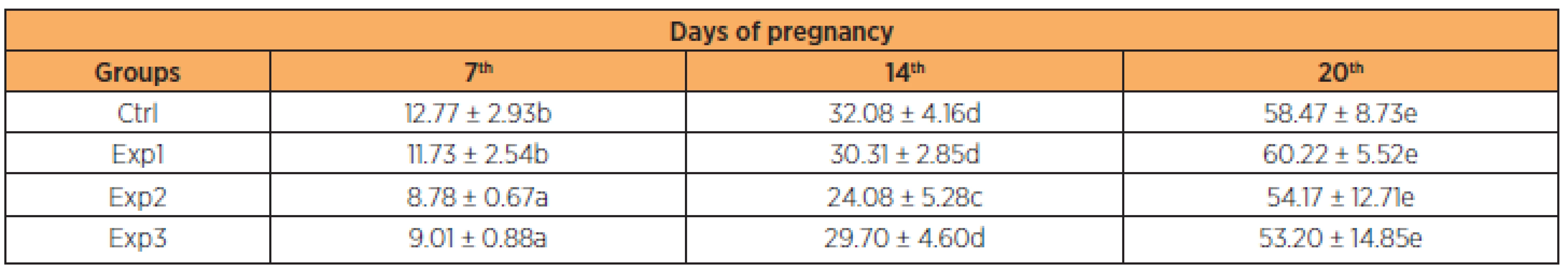
†Data presented in % (mean ± standard deviation). Groups of pregnant animals (n = 10 for each group) were treated once a day by gavage with distilled water (Ctrl) or zidovudine/lopinavir/ritonavir (Exp1, 10/13.3/3.3 mg/kg; Exp2, 30/39.3/9.9 mg/kg; Exp3, 90/119.7/29.9 mg/kg) throughout gestation. Significant differences were detected on the 7th, 14th and 20th days of the pregnancy periods (e > d > c > b > a; p < 0.05). Tab. 2. Effects of zidovudine/lopinavir/ritonavir on pregnancy parameters in rats † groups 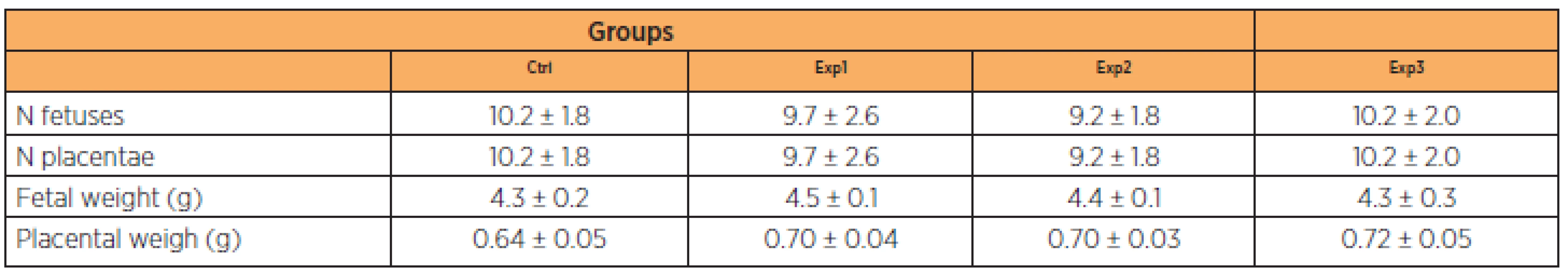
†Data are mean ± standard deviation (n = 10 animals in each group). There were no significant differences in any of the parameters between the groups. Ctrl, animals treated with vehicle; Exp, animals treated with oral zidovudine/lopinavir/ritonavir throughout pregnancy; Exp1, 10.0/13.3/3.3 mg/kg; Exp2, 30.0/39.3/9.9 mg/kg; Exp3, 90/119.7/29.9 mg/kg. DISCUSSION
Although funding for high-power anti-HIV therapy has increased in recent years, public and private health resources are still limited, especially in developing countries. The costs of AIDS treatment are especially high. Thus, the agents of first choice most used in developing countries are the most common and low-cost drugs, such as AZT and ritonavir, usually produced by pharmaceutical companies located in China, where the production costs is much cheaper. In view of this, we decided to study the action of the combination of zidovudine/lopinavir/ritonavir on rat pregnancy in order to ascertain the possible side effects of this association.
Studies using zidovudine in monotherapy have failed to find changes in maternal weight gain, maternal mortality or fetal malformation, even at high doses (60 and 100 mg/kg, by day) [26, 32]. However, a study with isolated use of ritonavir, using nine times the therapeutic dose, found high maternal mortality (40%) with the probable cause of death being multiple organ failure [7]. The use of lopinavir/ritonavir in the same doses used in this study showed no maternal mortality or fetal malformation [32]. This may be due to the use of ritonavir associated with another antiretroviral, because the association with lopinavir acts only as an enhancer, through the inhibition of cytochrome P450 3A [19].
Since maternal weight gain depends on the initial and final weight of the rats, we considered the most reliable weight percentage difference in order to analyze such data, because there is a weight variation in the beginning of the experiment between the different groups investigated [32]. As shown in Table 1, the pregnant rats treated with zidovudine/lopinavir/ritonavir in the beginning of the pregnancy (to the 7th day) showed less body weight gain in the Exp2 and Exp3 groups and in the 14th less gain in the Exp2 group compared to the others. These changes were statistically significant, though at the end of the experiment there was compensation and the final gain was similar in the various groups of the experiment.
A fixed dose combination of the protease inhibitors lopinavir and ritonavir is currently available for clinical use, with a recommended daily dose of 800mg of lopinavir plus 200 mg of ritonavir, given once or in two divided doses. However, in our experiment we gave them only once a day [3, 12].
This weight reduction is presumably due to dose-dependent gastrointestinal side effects as a function of ritonavir exposure through the 7th–14th day period of gestation, and can be related to the fact that rats lack some of the multiple factors involved in the pathogenetic mechanisms proposed for humans, which include the interference of several regulatory proteins such as sterol regulatory enhancer binding protein-1, the proteasome, mitochondrial DNA polymerase gamma and GLUT-4 [6]. Also, the short time of exposition to the drugs (3 weeks) could have played some role in this result [21]. Other authors have suggested the association of the drugs used in this study cab cause neuritis for a short time. Therefore, the animals can slowly move in their cages during the first and second part if pregnancy. This fact may explain our data on the weight of pregnant rats [1].
In rats, ritonavir markedly enhanced the pharmacokinetics of lopinavir, probably due to inhibition of presystemic and systemic metabolism, leading to increased exposure to this potent HIV protease inhibitor [30]. Few pharmacokinetic data during pregnancy are available for the dual protease inhibitor combinations that include low doses of ritonavir to boost concentrations of the coadministered protease inhibitor [2]. This finding applies to two relevant issues. First, no clear-cut dose-dependent effect can be inferred from this result. However, it can be related to the individual sensitivity of the animals to the drugs. Such individual variation could be linked to the functional levels of P glycoprotein (P-gp) [18], a membrane efflux pump pertaining to the superfamily of ATP binding cassette proteins [17]. As known, this protein is encoded by the ‘multidrug resistence’ genes Mdr 1a and Mdr 1b [15] and is expressed in tissues involved with drug absorption, metabolism and excretion [28]. P-gps limit penetration of potentially harmful or therapeutic hydrophobic compounds, thus providing protection of an organism against potentially toxic compounds in the environment. Accordingly, a placental P-gp may play an important role in the protection of the developing fetus [20]. Other barriers in which P-gps are importantly involved are the blood-brain, the blood-nerve; the blood-testis and the intestinal barrier (see Smit et al. [29]). Predictably, the absence of P-gp can lead to adverse effects when an organism is exposed to drugs such as antineoplastic agents, cardiac glycosides, beta-blockers, calcium channel blockers and HIV-protease inhibitors [25].
CONCLUSION
Taking into consideration the modern antiretroviral triple therapy for HIV infection, it is frequently difficult to predict the sort of interactions among the drugs used and which drug might be responsible for a particular effect. This challenge can be overcome by future biological assays using animal models to shed more light on the effect of associated antiretroviral drugs on the mother and fetus. However, our observations that zidovudine/lopinavir/ritonavir at doses of just one order of magnitude higher than those used in humans can cause effects on rat pregnancy outcome should not be underestimated. Finally, the chronic exposure of pregnant rats to high doses of associated zidovudine/lopinavir/ritonavir resulted in a significant reduction in maternal body weight gain, an effect that was not associated with significant adverse affects on the fetal parameters.
Prof. Edward Araujo Júnior, PhD (Corresponding author)
Department of Obstetrics, Federal University of São Paulo (UNIFESP)
Rua Carlos Weber, 956 apt. 113 Visage
Vila Leopoldina
São Paulo – SP, Brazil
CEP 05303-000
e-mail: araujojred@terra.com.br
Zdroje
1. Barreto, RL., de Jesus Simões, M., Amed, AM., et al. Stavudine effects on rat pregnancy outcome. J Obstet Gynaecol Res, 2004, 30, p. 242–245.
2. Best, BM., Stek, AM., Mirochnick, M., et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy.J Acquir Immune Defic Syndr, 2010, 54, p. 381–388.
3. Bierman, WF., van Vonderen, MG., Veldkamp, AI., et al. The lopinavir/ritonavir-associated rise in lipids is not related to lopinavir or ritonavir plasma concentration. Antivir Ther, 2011, 16, p. 647–655.
4. Brazil. Ministry of Health. Epidemiological Bulletin Year 1 – No 01, December, 2012. Available in website: http://aids.gov.br. Accessed November 25, 2013.
5. Brazil. Ministry of Health. Epidemiological Bulletin Year VII – No 01, December, 2010. Available in website: http://aids.gov.br. Accessed November 25, 2013.
6. Carr, A., Samaras, K., Burton, S., et al. A syndrome of peripheral lipodytrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhititors. AIDS, 2003, 17, F51–F58.
7. Carvalho, AM., Oliveira-Filho, RM., Simões, MJ., et al. Effect of chronic ritonavir administration on pregnant rats and their fetuses. Clin Exp Obst Gyn, 2004, 31, p. 229–231.
8. Carvalho, LP., Wagner, A., Simões, RS., et al. Effects of combined zidovudine-lopinavir-ritonavir therapy during rat pregnancy. Clin Exp Obstet Gynecol, 2013, 40, p. 345–349.
9. Chouquet, C., Burgard, M., Richardson, S., et al. Timing of mother-to-child HIV-1 transmission and diagnosis of infection based on polymerase chain reaction in the neonatal period by a non-parametric method. AIDS, 1997, 11, p. 1183–1184.
10. Cohan, D. Perinatal HIV: special considerations. Top HIV Med, 2003, 11, p. 200–213.
11. Connor, EM., Sperling, RS., Gelber, R., et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med, 1994, 331, p. 1173–1180.
12. Cunha, AM., Hagemann, CC., Simões, RS., et al. Effects of lopinavir-ritonavir combined therapy during the rat pregnancy. Morphological and biochemical aspects. Eur J Obstet Gynecol Reprod Biol, 2007, 133, p. 60–63.
13. De Clercq, E. Antiretroviral drugs. Curr Opin Pharmacol 2010, 10, p. 507–515.
14. Germinario, RJ. Anti-retroviral protease inhibitors – ‘a two edge sword?’ IUBMB Life, 2003, 55, p. 67–70.
15. Gros, P., Ben Niriah, YB., Croop, JM., Housman, DE. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature, 1986, 323, p. 728–731.
16. Hamilton, JB., Wolfe, JM. The effect of male hormone substance upon birth and prenatal development in the rat. Anat Rec, 1938, 70, p. 433.
17. Higgins, CF. ABC transporters: from microorganisms to man. Ann Rev Cell Biol, 1992, 8, p. 67–113.
18. Juliano, RL., Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta, 1976, 455, p. 152–162.
19. Kageyama, M., Namiki, H., Fukushima, H., et al. Effect of chronic administration of ritonavir on function of cytochrome P450 3A and glycoprotein in rats. Biol Pharms Bull, 2005, 28, p. 130–137.
20. Lankas, GR., Wise, LD., Cartwright, ME., et al. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod Toxicol 1998, 12, p. 457–463.
21. Leonard, E., McConisey, G. Metabolic complications of antiretroviral therapy in children. Ped Infect Dis J, 2003, 22, p. 77–84.
22. Loutfy, MR., Walmsley, SL. Treatment of HIV infection in pregnant women: antiretroviral management options. Drugs, 2004 ,64, p. 471–488.
23. Minkoff, HL. Human immunodeficiency virus infection in pregnancy. Obstet Gynecol, 2003, 101, p. 797–810.
24. Olfert, ED., Cross, BM., McWilliam, AA. Canadian Council on Animal Care/Guide to the Care and Use of Experimental Animals. 2nd ed. Bradda Printing Services: Ottawa, Ontario, Canada 1993.
25. Pavek, P., Staud, F., Fendrich, Z., et al. Examination of functional activity of P-glycoprotein in the rat placental barrier using rhodamine 123. J Pharmacol Exp Ther, 2003, 305, p. 1239–1250.
26. Pereira Fontes, TM., Simões, RS., Martins Oliveira, FH.,et al. Extended administration of the association of zidovudine plus ritonavir during rat pregnancy: maternal and fetal effects. Clin Exp Obstet Gynecol, 2007, 34, p. 175–178.
27. Quintino, MP., Nakamura, MU., Simões, MJ., et al. Chronic use of indinavir in albino rat pregnancy (Rattus norvegicus albinus, Rodentia, Mammalia): Biological assay. J Obstet Gynaecol Res, 2011, 37, p. 1212–1215.
28. Schinkel, AH. Disruption of the mouse mdr 1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell, 1994, 77, p. 491–502.
29. Smit, JW., Huisman, MT., van Tellingen, O., et al. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal exposure. J Clin Invest, 1999, 104, p. 1441–1447.
30. Ter Heine, R., Van Waterschoot, RA., Keizer, RJ., et al. An integrated pharmacokinetic model for the influence of CYP3A4 expression on the in vivo disposition of lopinavir and its modulation by ritonavir. J Pharm Sci, 2011, 100, p. 2508–2515.
31. Tuomala, RE., Shapiro, DE., Mofenson, LM., et al. Anti-retroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med, 2002, 346, p. 1863–1870.
32. Wagner, A., Nakamura, MU., Simões, RS., et al. Chronic action of association of zidovudine, lamivudine and ritonavir on pregnant rats. A biologic assay. Clin Exp Obstet Gynecol, 2011, 38, p. 28–32.
33. Walmsley, S,, Bernstein, B., King, M., et al. Lopinavir-Ritonavir versus Nelfinavir for the initial treatment of HIV Infection. N Engl J Med, 2002, 346, p. 2039–2046.
Štítky
Dětská gynekologie Gynekologie a porodnictví Reprodukční medicína
Článek vyšel v časopiseČeská gynekologie
Nejčtenější tento týden
2014 Číslo 2- Alergie na antibiotika u žen s infekcemi močových cest − poznatky z průřezové studie z USA
- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Isoprinosin je bezpečný a účinný v léčbě pacientů s akutní respirační virovou infekcí
-
Všechny články tohoto čísla
- Role of fatty acids in sperm membrane
- Breech presentation – an analysis of resultsin one perinatal center
- Effect of resilience in women on the outcome of her first IVF/ICSI
- Intrauterine fetal death syndrom: analysis of cases from 2008 to 2012 in Institute for the care of mother and child
- Highly active antiretroviral therapy during gestation: effects on a rat model of pregnancy
- Gynekologicko-porodnické oddělení Nemocnice Znojmo klinické centrum pro léčbu enometriózy
- Folic acid and prevention of the neural tube defects
- The use of synthetic mesh implants in surgical treatment of pelvic organ prolapse
- New views on the functional morphology of human clitoris
- Guidlines for the good laboratory practice in assisted reproduction – clean environment
- Tuky z mořských ryb a jejich význam od těhotenství až do konce kojení
- Podrobné hodnocení morfologie plodu při ultrazvukovém vyšetření ve 20.-22. týdnu těhotenství– doporučený postup
- Vyjádření výboru České gynekologické a porodnické společnosti ČLS JEP k pohotovostním službám zajištěným jediným lékařem
- Role of prebioptic and bioptic methodsin the screening and diagnosis of cervical cancer
- Ovarian epithelial malignant tumors in adolescence
- Česká gynekologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Role of prebioptic and bioptic methodsin the screening and diagnosis of cervical cancer
- Intrauterine fetal death syndrom: analysis of cases from 2008 to 2012 in Institute for the care of mother and child
- New views on the functional morphology of human clitoris
- Breech presentation – an analysis of resultsin one perinatal center
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



