-
Medical journals
- Career
Laparoscopic liver resection for alveolar echinococcosis
Authors: Farkasova M. 1; Kunovsky L. 1,2; Prochazka V. 1; Jr Husa P. 3; Blazek J. 4; Poredská K. 2; Mazenec J. 5; Vojvodova A. 6; Andrasina T. 6; Kala Z. 1
Authors‘ workplace: Department of Surgery, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Department of Gastroenterology and Internal Medicine, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Department of Infectious Diseases, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Infectious Diseases, Hospital Kyjov, Czech Republic 4; Department of Pathology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 5; Department of Radiology and Nuclear Medicine, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 6
Published in: Gastroent Hepatol 2019; 73(2): 126-131
Category:
doi: https://doi.org/10.14735/amgh2019126Overview
Echinococcosis is a rare parasitic infection in Central Europe. The most commonly affected organ is the liver. There are two types, cystic and alveolar echinococcosis with different types of treatment management. A radical liver resection remains the method of choice in the management of alveolar echinococcosis. We report a case of a 40-year-old woman with an echinococcal alveolar liver lesion successfully treated with a laparoscopic resection of the 5th liver segment. The laparoscopic technique, with all its benefits over open surgery, provides a safe and efficient treatment of selected types of echinococcal cysts located in the liver. It is a mini-invasive surgical approach which enables lower postoperative discomfort, quicker recovery and better cosmetic outcome.
Keywords:
echinococcosis – liver resection – laparoscopy – surgery
Introduction
Human echinococcosis is a rare zoonotic infection caused by parasites of the genus Echinococcus. The most frequent clinical forms of the disease are cystic echinococcosis (CE) caused by Echinococcus granulosus (EG) and alveolar echinococcosis (AE) caused by Echinococcus multilocularis (EM) [1]. The reported incidence rate of echinococcal infections in the Czech Republic between 2008 and 2017 was 0.023 cases per 100 000 person-year [2]. In endemic areas, the annual incidence of AE ranges from 0.03 to 1.2 per 100 000 persons [3].
EM is a small tapeworm (1.2–3.7 mm) that infects mainly foxes as a definitive host, and to a lesser extent dogs, cats, coyotes and wolves [4]. Humans are usually infected by food or water contaminated by carnivores’ faeces containing echinococcus eggs [5]. The egg hatches in the small bowel and releases an oncosphere that penetrates the intestinal wall and migrates through the circulatory system into the liver [4]. Primary extra-hepatic locations of EM are rare (1–3%), but EM can spread to other organs by infiltration or metastasis formation [3,6].
In the liver, the oncosphere develops into a larval stage creating multilocular cyst [4]. EM cysts are more poorly limited than EG cysts because there is no sharp separation between the parasitic tissue and the liver parenchyma. The initial phase of infection is always asymptomatic, usually for several years due to slow growth rate. Common symptoms of hepatic cysts are right epigastric pain, nausea, vomiting and jaundice [7].
Case report
We present a 40-year-old female patient, who was examined for decompensated hypertension. Concerning her medical history, she had diabetes mellitus type 2 treated by oral antidiabetics and underwent a laparoscopic resection of an ovarian cyst. During the series of tests and examinations, an abdominal ultrasound showed several non-specific lesions of the liver. The magnetic resonance imaging (MRI) of the liver was performed and revealed two small hemangiomas in segments 4 and 8 and one lesion 43×65×80 mm in size located in the segment 5 (Fig. 1A,B). In differential diagnosis, metastatic process or echinococcal cyst were considered. The tumour markers were negative. A serology test confirmed echinococcosis (cystic form). The patient had no relevant epidemiological anamnesis apart from a dog in her household. Regarding the echinococcosis therapy, she was treated with albendazole for 1 year at the department of infectious diseases. During the treatment she started to have intermittent abdominal pain in right upper quadrant. Therefore and in order to exclude dissemination to other organs, she had a positron emission tomography – computed tomography (PET/CT) scan that showed neither a progression of the liver lesion nor a dissemination to other organs (Fig. 2A,B). However, the liver lesion had a more alveolar appearance according to the PET/CT. For that reason, her case was presented at a multidisciplinary committee and a surgical resection was indicated.
1. Echinococcal liver lesion on MRI – A. T1 weighted image in portal phase of contrast enhancement – axial scan; B. T1 weighted image – axial scan. In the right liver lobe in the 5th segment there is a post-contrast T1 hypointense hypovascular focal lesion with hyperintense margin of hyperperfusion, the centre of the lesion remains unenhanced (A). On the T1 weighted image the lesion is hypointense, on the margin we can see small hyperintense areas – small cystic formations (B). Source: Department of Radiology, Hospital Kyjov.
Obr. 1. Echinokokové postižení jater na MR – A. T1 vážená sekvence po podání kontrastní látky v portální fázi v axiální rovině; B. T1 vážená sekvence v axiální rovině. V pravém laloku jater v 5. segmentu je postkontrastně v portální fázi T1 hypointenzní hypovaskularizované ložisko s hypervaskularizovaným hyperintenzním lemem (A). Na T1 je léze hypointenzní, po obvodu patrny drobné hyperintenzní okrsky v rámci drobných cystických kolekcí (B). Zdroj: Radiologické oddělení, nemocnice Kyjov.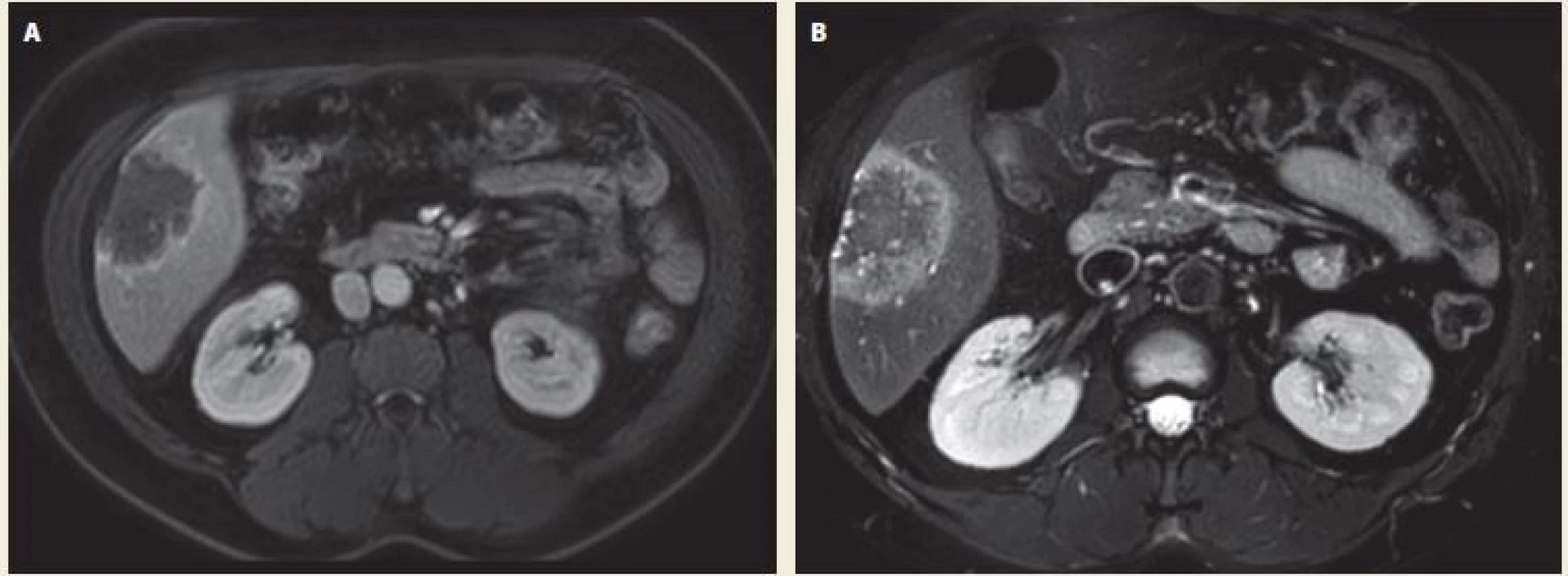
2. Echinococcal liver lesion on PET/CT after 1-year treatment with albendazole – A. contrast enhanced CT – axial scan; B. fusion – axial scan. After intravenous application of a contrast dye, there is a discrete saturation hem around a hypodense lesion in the 5th liver segment. In the fusion images of the 5th liver segment, there is a metabolically inactive hypodense lesion with a discrete post-contrast hem opacity and a complete disappearance of a 5 uorodeoxyglucose (FDG) accumulation over the surrounding hepatic parenchyma. Source: Department of imaging methods, T. Bata Regional Hospital, Zlín.
Obr. 2. Echinokokové postižení jater na PET/CT po roční terapii albendazolem – A. CT po aplikaci kontrastní látky intravenózně – axiální řez; B. fúze – axiální řez. Po aplikaci kontrastní látky intravenózně je patrný diskrétní lem sycení kolem hypodenzního ložiska v 5. segmentu jater. Na fúzních snímcích v 5. segmentu jater je patrno metabolicky neaktivní hypodenzní ložisko s diskrétní postkontrastní opacifikací lemu a úplným vymizením akumulace 5 uorodeoxyglukózy (FDG) oproti okolnímu jaternímu parenchymu. Zdroj: Oddělení zobrazovacích metod, Krajská nemocnice T. Bati, Zlín.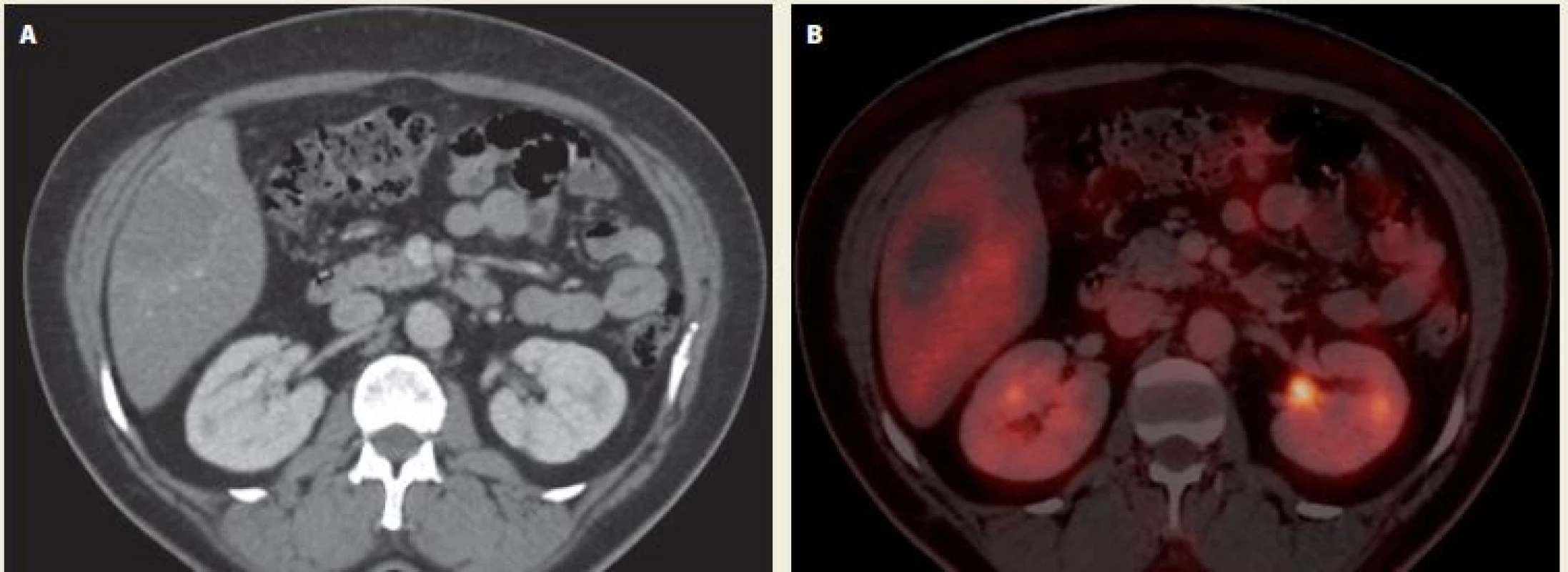
Surgery procedure
We have decided to perform a laparoscopic resection due to a favourable lesion location. We started by diagnostic laparoscopy, lesion was 8 cm in diameter located in the segment 5 (Fig. 3A), there was no evidence of peritoneal dissemination. Five trocars were inserted in standard positions. The hepatoduodenal ligament was secured by Pringle’s maneuver during the resection. An atraumatic haemostat was used to clamp the hepatoduodenal ligament in order to interrupt a blood flow in the hepatic artery and the portal vein and to control a bleeding from the liver parenchyma during the dissection. A distance between the lesion and the principal plane (Rex-Cantlie line) was too close, therefore a cholecystectomy had to be performed. The liver resection was performed with 2 cm safety margins (Fig. 3B,C,D). An argon plasma coagulation was applied to improve a haemostasis in the liver parenchyma (Fig. 3E). The liver specimen was extracted in a protective bag from suprapubic Pfannenstiel incision (Fig. 3F). An abdominal drain was inserted to control a postoperative bleeding or a bile leak. The blood loss was 700 ml, the operation time 220 minutes.
3. A. An echinococcal lesion in liver segment S5 (view of visceral surface of the liver); B. demarcation of the resection line by an electrocoagulation tool (view of diaphragmatic surface of the liver); C. interruption of liver parenchyma by an electrocoagulation tool; D. clipping of the vessel during the interruption of the liver parenchyma tissue; E. the use of argon plasma coagulation in secondary haemostasis of the liver resection surface; F. placing the specimen in a protective bag before extraction.
Obr. 3 A. Echinokokové ložisko v segmentu S5 (pohled na viscerální plochu jater); B. vytyčení resekční linie elektrokoagulačním nástrojem (pohled na diafragmatickou plochu jater); C. pčerušování jaterního parenchymu elektrokoagulačním nástrojem; D. ošetčení cév klipováním při přerušování jaterního parenchymu; E. ošetření resekční plochy jater pomocí argon plazma koagulace; F. vložení resekátu do ochranného vaku před jeho následnou extrakcí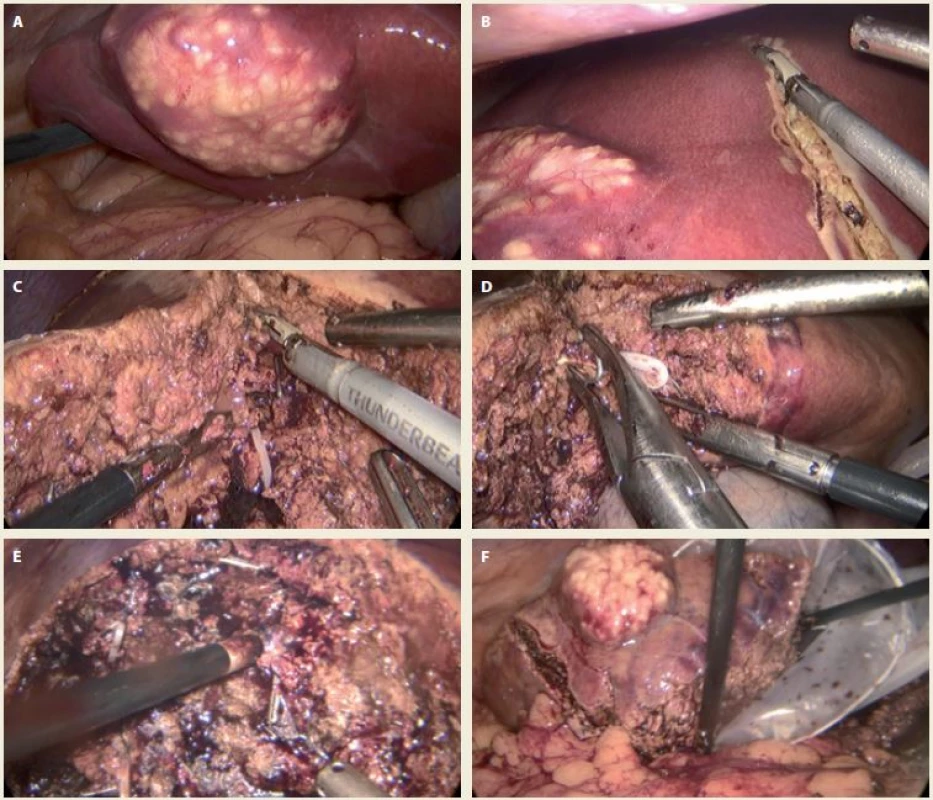
Postoperatively, the patient could walk and eat 1 day after surgery. There was no bile leakage from the drain. There were no postoperative complications. The patient was discharged 7 days after the surgical procedure. The final histopathology results reported a necrotic liver tissue with an eosinophilic inflammatory reaction (corresponding to parasitic disease) without any evidence of a malignant tumour (Fig. 4A,B). The patient is still on albendazole therapy as a secure postoperative therapy to avoid a recurrence of the disease.
4. Histological section – A. necrosis with acellular membrane material on the right. Hepatic parenchyma with centrolobular steatosis on the left. Haematoxylin- eosin staining 10×; B. necrosis with acellular membrane material at the bottom of the image. The necrotic tissue is bounded by a giant cellular resorptive reparative reaction with fibrosis and chronic inflammatory infitrate involving eosinophilic granulocytes at the upper margin. Haematoxylin-eosin staining 50×.
Obr. 4. A. Nekróza s materiálem acelulárních membrán napravo. Parenchym jater s centrolobulární steatózou nalevo. Barvení hematoxylin-eozinem 10×; B. nekróza s materiálem acelulárních membrán ve spodní části obrázku. Nekróza je při horním okraji ohraničena obrovskobuněčnou resorptivně reparativní reakcí s fibrózou a chronickým zánětlivým infiltrátem s účastí eozinofilních granulocytů. Barvení hematoxylin- eozinem 50×.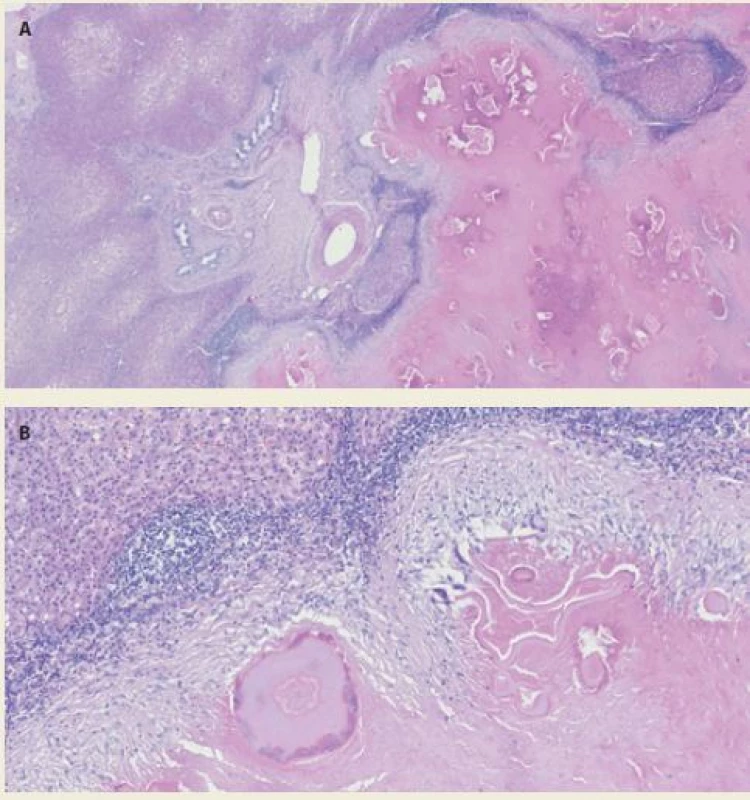
Discussion
AE is a potentially lethal disease. It is characterised by an initial asymptomatic period of 5–15 years. In the progressive phase, symptoms include abdominal pain, jaundice, anaemia or fever. A later stage is characterised by severe hepatic dysfunctions [8]. Distant metastases to lungs, brain or bones may be seen in the late stage of the disease [6,9]. In our case report, the patient was asymptomatic and diagnosed incidentally during examinations for decompensated hypertension.
The diagnosis of AE is mainly based on a clinical presentation, epidemiological evidence, serology tests and a radiological examination [10]. The MRI or CT scan of the liver are the standard imaging methods. It is also necessary to rule out metastases of the disease to other organs; we performed a whole-body PET/CT scan that ruled out distant metastasis.
Concerning the therapy of echinococcosis, in patients with cystic form, the preoperative therapy with albendazole is recommended, however with no exact treatment duration [3]. This recommendation was initially followed in our patient, too. As she was diagnosed with a CE according to the MRI scan and serology, she received one-year long therapy with albendazole.
On the other hand, in patients with alveolar form, a radical surgery (a liver resection) is the treatment of choice. However, it is possible only in 35% of patients due to a late diagnosis [11]. As the parasite’s growth resembles a malignant tumour, the procedures and techniques (including 2 cm safety resection margin) of oncological surgery are recommended. If a R0 resection is not possible, a palliative surgery is no longer recommended, and the patient should stay on a life-long anti-parasitic drug therapy [8]. A liver transplantation (LT) should be reserved for patients with advanced forms of the disease as a salvage therapy. A pericystectomy (removal of a cyst without a resection of healthy liver tissue) broadly used for CE treatment, is not possible in AE due to a solid nature of the lesion [3,11,12]. There are also percutaneous methods like ultrasound-guided PAIR (puncture, aspiration, injection and respiration) which is sometimes used as an alternative to surgery or if a surgery is contraindicated but only in a cystic form of the disease [13]. Our patient had a superficial lesion in the 5th segment, which was favourable for a laparoscopic, minor liver resection (segmentectomy).
After the first laparoscopic liver resection in 1991, dramatic progress has been made in the field of liver surgery. Nowadays even major liver resections are carried out laparoscopically. When compared to an open liver surgery, a laparoscopic liver resection is more feasible and safer in both benign and malignant liver lesions [14].
There are 20 meta-analyses comparing the results of laparoscopic and open approach. Most studies demonstrate a significantly quicker hospital discharge, an earlier return of bowel activity and lesser requirement of analgesics in patients after laparoscopic liver resections [15].
Postoperative administration of antiparasitic drugs for at least 2 years and a long-term follow-up are mandatory in all cases. The most preferred anti-parasitic drug is albendazole given orally at a dose of 10–15 mg/kg/day. Alternative drugs are mebendazole or amphotericin B as a salvage therapy. A long-term drug treatment is mandatory in all inoperable cases.
According to the current guidelines, a preoperative antiparasitic drug administration is not recommended (except in the case of LT) in AE, however can be considered in special cases (in order to downsize the lesion or to prevent from a parasitic dissemination).
Conclusion
Hepatic alveolar echinococcosis is a rare but a potentially life-threatening parasitic disease that should be considered in a differential diagnosis of liver lesions. If resectable, a surgical treatment should be a method of choice in patients with AE. Moreover, a laparoscopic approach is considered to be safe and effective as presented in our case report.
Submitted: 22. 1. 2019
Accepted: 12. 3. 2019
Lumír Kunovský M.D., Ph.D.
Department of Surgery
University Hospital Brno
Jihlavská 20
625 00 Brno
Czech Republic
Sources
1. Otero-Abad B, Torgerson PR. A Systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl Trop Dis 2013; 7 (6): e2249. doi: 10.1371/journal.pntd.0002249.
2. Státní zdravotní ústav. Infekce v ČR – ISIn a EPIDAT. [online]. Available from: http: //www.szu.cz/publikace/data/infekce-v-cr.
3. Brunetti E, Kern P, Vuitton DA et al. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114 (1): 1–16. doi: 10.1016/j.actatropica.2009.11.001.
4. Centers for Disease Control and Prevention. Parasites – Echinococcosis. Biology. [online]. Available from: https: //www.cdc.gov/parasites/echinococcosis/biology.html.
5. Sherlock S, Dooley J. Diseases of the liver and biliary system, 11th edition. Malden, MA Blackwell Science 2002 : 728.
6. Rosoľanka R, Antolová D, Nováková E et al. Severe life-threatening parasitic liver disease complicated by dissemination to the lungs. Gastroent Hepatol 2016; 70 (2): 145–149. doi: 10.14735/amgh2016csgh.info05.
7. Boyer TD, Manns MP, Sanyal AJ. Zakim & Boyer’s hepatology: a textbook of liver disease. 6th ed. Philadelphia: Elsevier Health Sciences 2012 : 1380.
8. Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis 2010; 23 (5): 505–512. doi: 10.1097/QCO.0b013 e32833d7516.
9. Madhusudhan KS, Srivastava DN, Dash NR et al. Alveolar echinococcosis of liver: a diagnostic problem in a nonendemic area. Curr Probl Diagn Radiol 2015; 44 (2): 221–226. doi: 10.1067/j.cpradiol.2014.08.006.
10. Du L, Zhang LQ, Hou LZ et al. Combined resection of the right liver lobe and retrohepatic inferior vena cava to treat hepatic alveolar echinococcosis: a case report. Medicine (Baltimore) 2017; 96 (38): e8097. doi: 10.1097/MD.0000000000008097.
11. Erhartová D, Froněk J, Stejskal F et al. Alveolární echinokokóza – vzácné onemocnění vyžadující multidisciplinární přístup. Gastroent Hepatol 2018; 72 (4): 287–292.
12. Stancu B, Andercou O, Pintea D et al. Laparoscopic simultaneous partial pericystectomy and total cystectomy for hydatid liver cysts – case report. Clujul Med 2015; 88 (3): 415–419. doi: 10.15386/cjmed-416.
13. Patkowski W, Krasnodębski M, Grąt M et al. Surgical treatment of hepatic Echinococcus granulosus. Prz Gastroenterol 2017; 12 (3): 199–202. doi: 10.5114/pg.2017.70473.
14. Cai X. Laparoscopic liver resection: the current status and the future. Hepatobiliary Surg Nutr 2018; 7 (2): 98–104. doi: 10.21037/hbsn. 2018.02.07.
15. Coelho FF, Kruger JA, Fonseca GM et al. Laparoscopic liver resection: experience based guidelines. World J Gastrointest Surg 2016; 8 (1): 5–26. doi: 10.4240/wjgs.v8.i1.5.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2019 Issue 2-
All articles in this issue
- Clinical practice guidelines for chronic hepatitis C virus infection
- Inflammatory bowel disease and male fertility
- Ectopic pancreas as a cause of upper gastrointestinal bleeding
- A gastric Dieulafoy’s lesion
- Involvement of the gastrointestinal tract in amyloidosis: when to think about it and how to diagnose
- Part 2 – Epidemiology of inflammatory bowel diseases in the Czech population: available data sources, prevalence of treated patients and overall mortality
- Laparoscopic liver resection for alveolar echinococcosis
- Validation of the Slovak Version of the SIBDQ Questionnaire in a Cohort of Inflammatory Bowel Disease Patients
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- A gastric Dieulafoy’s lesion
- Involvement of the gastrointestinal tract in amyloidosis: when to think about it and how to diagnose
- Ectopic pancreas as a cause of upper gastrointestinal bleeding
- Inflammatory bowel disease and male fertility
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

