-
Medical journals
- Career
Seroprevalence of Hantavirus Antibodies among Chronic Hemodialysis Patients in the Czech Republic
Authors: M. Pejčoch 1,2; P. Pazdiora 3,4; J. Eiselt 5; V. Hájek 6; E. Veselá 7; J. Vlasák 8; J. Benešová 9; A. Kubátová 10; B. Kříž 1,11
Authors‘ workplace: National Institute of Public Health, Prague 1; Regional Istitute of Public Health, Brno 2; Charles University, Faculty of Medicine, Plzeň 3; Regional Institute of Public Health, Plzeň 4; Teaching Hospital, Plzeň 5; Hospital & Health Centre, Klatovy 6; Military Hospital, Plzeň 7; FMC - DSW Ltd., Sokolov 8; Fresenius Medical Care – DS, Ltd., Sokolov 9; District Public Health Office, Klatovy 10; Charles University, 3rd Medical Faculty, Praha 11
Published in: Epidemiol. Mikrobiol. Imunol. 59, 2010, č. 1, s. 48-51
Overview
A total of 348 serum samples were collected from 301 hemodialysis patients with chronic renal failure of four healthcare settings in Western Bohemia. The sera were screened for the presence of hantavirus antibodies using ELISA kits (PROGEN Biotechnik GmbH) with Hantaan and Puumala antigens. Specific anti-Puumala antibodies were detected in five patients (1.7 %). Although hantaviruses are known to cause primarily acute renal damage (interstitial nephritis) in Eurasia, chronic effects of hantavirus infection and the detection of specific antibodies in hemodialysis patients have also been reported. Nonetheless, the detection of seropositivity is not proof of an etiological link between hantaviruses and chronic renal failure. The hantavirus seropositivity rate in hemodialysis patients was not significantly higher than that in the general population. Our findings are consistent with the literature data and do not contradict the contribution of hantaviruses to the pathogenesis of chronic renal damage in the Czech Republic.
Key words:
hantaviruses – hemodialysis patients – nephritis – antibodies – Czech Republic.Introduction
Diseases caused by hantaviruses are not yet well known in Central Europe and thus may escape detection or be misdiagnosed. Despite this fact several attempts has been made to asses and clarify epidemiological, microbiological and social features of this disease [14, 15, 17]. They are classified in the group of emerging infections. Although the diseases caused by hantaviruses have been known for a long time, the causative agent was not identified until 1976. In 1978, the Korean scientist Ho Wang Lee described the causative agent of Korean hemorrhagic fever and named it after the Hantaan river where he first isolated the virus [3]. The genus Hantavirus was newly recognized in the family Bunyaviridae. Hantaviruses are negative-sense single-stranded RNA viruses. Over 30 hantavirus species are currently known.
In nature, hantaviruses circulate among their specific rodent hosts. As a result of co-evolution, each hantavirus genotype has a specific host. Hantaviruses cause persistent asymptomatic infections in rodents, but are also implicated in severe diseases in humans. Rodents excrete hantaviruses in urine, faeces and saliva into the environment. The respiratory tract is the most common route of entry for the hantavirus that is transmitted to humans through the inhalation of contaminated aerosols. In Eurasia, hantaviruses primarily affect the kidney, causing hemorrhagic fever with renal syndrome (HFRS), or its milder form, nephropathia epidemica (NE). The disease results in renal failure that may progress to chronicity. HFRS is a systemic infection. The retroperitoneum is the major site of vascular leak and the kidneys suffer tubular necrosis. Both syndromes are accompanied by myocardial depression and hypotension or shock [9].
The following hantavirus genotypes are circulating in nature in Central Europe: Puumala (PUU) whose host is the bank vole (Myodes glareolus), Dobrava-Belgrade (DOB) harboured by the yellow-necked mouse (Apodemus flavicollis) and Tula (TUL) circulating among common voles (Microtus arvalis). The Hantaan (HTN) genospecies is typically found in Asia but not in Europe. Three genospecies, i.e. PUU, DOB and TUL, have been detected in the Czech Republic [7], with DOB virus posing the highest risk and even causing death in humans (unpublished data). TUL virus can also be responsible for infection in humans, but only one mild case has been reported to date [16].
Hantavirsues are generally considered to cause acute renal damage in Eurasia. Nonetheless, chronic hantavirus infection has also been reported, even in hemodialysis patients. We decided to screen a group of dialysis patients from Western Bohemia for the presence of specific hantavirus antibodies.
Patients & Methods
Serum samples for laboratory testing of specific hantavirus antibodies were obtained from three healthcare settings in Western Bohemia. A total of 107 serum samples from 1997 and 107 serum samples from 2000 were provided by the serum bank of the Hemodialysis Centre of the Teaching Hospital in Plzeň. The sera were collected from 167 patients, with some of them being sampled repeatedly. Thirty-five sera samples from 2000 were obtained from the EuroCare Centre, Military Hospital Brno. Seventy sera collected from 70 patients in June 2000 were from the NMC Hemodialysis Centre Ltd. in Sokolov. Other 29 sera from July and August 2000 were provided by the Hemodialysis Centre of the Department of Internal Medicine in Klatovy. Thus a total of 301 patients, 150 males and 151 females, male to female ratio 1 : 1.01, age range 20-87 years, mean age 61.0 years, were screened. For the patients sampled twice (in 1997 and 2000), the age at the first blood sample collection was taken into account.
The blood samples were stored frozen at a temperature of - 20 °C until the analysis. The antibodies were detected by commercial ELISA kits with the Hantaan and Puumala antigens (PROGEN BIOTECHNIK GmbH, Heidelberg, Catalog Nos. PR59065 and PR59056). The Hantaan antigen was used because no commercial kits with the Dobrava antigen have been available on the market, and these two genotypes are closely similar. Therefore, anti-Dobrava antibodies were detected by the kits with the Haantan antigen. The results were evaluated according to the manufacturer’s instructions. To demonstrate the reproducibility of the serology results, some of the sera were examined up to seven times, before the final result was determined. The sera were screened for the presence of specific IgG and IgM antibodies.
Results
The aforementioned kits were used to examine 348 sera from 301 chronic hemodialysis patients. In five (1.7 %) patients, four females and one male, the levels of specific antibodies suggested hantavirus infection. Six patients with borderline levels of antibodies were retested several times to be excluded after failing to give positive results.
Case histories of patients positive for hantavirus antibodies:
Patient No. 1.: A 78-year-old woman who presented with pneumonia and renal failure in 1997. Since October 1997, she had been on dialysis. Diagnosis: nephropathy of unclear etiology with cortical cysts as a cause of renal failure. In 2001, she died of heart failure.
Patient No. 2: A 77-year-old woman, a forest worker, who had been on dialysis since May 1995. Diagnosis: suspected diabetic nephropathy. She died in 2000.
Patient No. 3: A 44-year-old man who was diagnosed with renal failure of unclear etiology. He has been on dialysis since 1991 after presenting with uremia and signs of chronic renal failure progression. He has chronic viral hepatitis B.
Patient No. 4: A 72-year-old woman who lived in a small house with a garden and worked in a vegetable warehouse with the presence of rodents developed a flu-like illness with fever in 1992. She was diagnosed with impaired renal function and had been on dialysis after worsening of renal function in 1994. The patient died of heart failure in 2002. Signs of glomerulonephritis were found at autopsy.
Patient No. 5: A 73-year-old woman who had been on dialysis since June 1997 for chronic progressive pyelonephritis died in 1999.
1. Serology results of five positive patients. 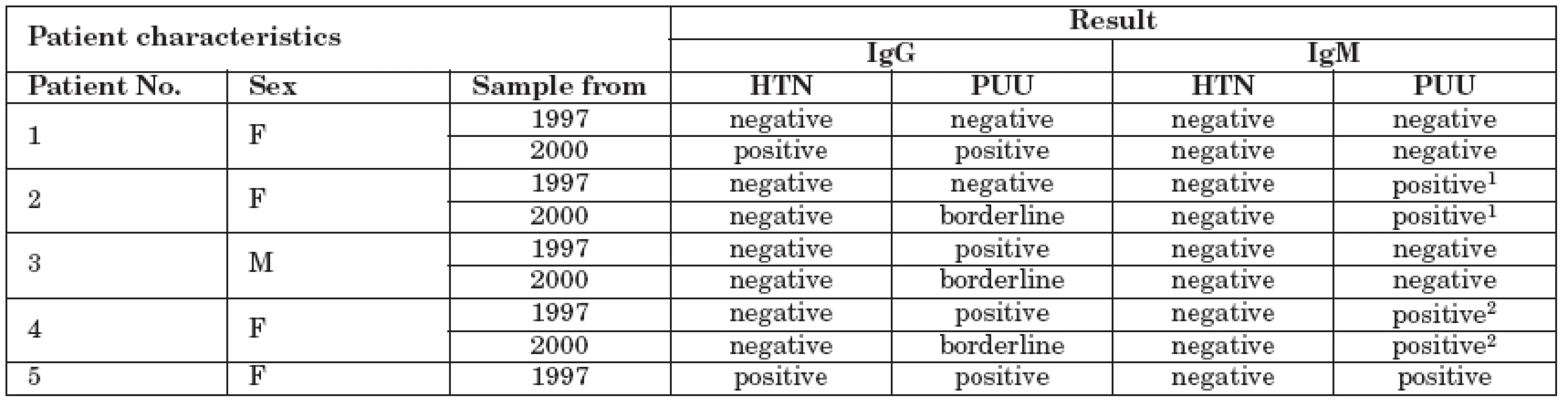
Notes: 1 Anti-PUU IgM highly positive in 1997 and lower positivity detected in 2000; 2 Anti-PUU IgM positive in both 1997 and 2000 at the same level Discussion
In Europe, hantavirus primarily affects the kidney and causes a form of HFRS called nephropathia epidemica. The disease usually has an acute course with the following five stages: (i) febrile, (ii) hypotensive, (iii) oliguric, (iv) polyuric, and (v) convalescent. The mortality rate in Europe is low. The patients usually recover completely. Delayed effects or a chronic form of the disease are relatively rare to be reported.
Elisaf et al. have described 32 Greek patients admitted to hospital for hemorrhagic fever with renal syndrome, but gave no details on the causative agents. Two of these patients developed renal dysfunction persistent for more than one year. Three recovered patients were diagnosed with type I distal renal tubular acidosis at 1 to 5 year follow-up and two of them had reduced renal concentration capacity. The development of chronic renal dysfunction was independent of severity of the initial acute disease [1]. In a Mayo Clinic study, 165 serum samples from patients with acute or chronic renal disease, 34 serum samples from patients with congenital renal disease, and 100 serum samples from healthy adults were tested by EIA for the presence of IgG, IgM and IgA antibodies against Hantaan and Puumala viruses. Specific antibodies were detected in 26 % of patients from the first group, in 3 % of patients with congenital renal disease, and in none of the third group. The positive patients from the first group had the following diagnoses: acute tubulointerstitial nephritis (9 patients), necrotizing glomerulonephritis (12) or IgA nephropathy (9), and other renal disorders (15). IgA, IgG and IgM positivity was found in 37 %, 33 % and in 17 % of cases, respectively. The remaining 13 % of cases were positive for all three types of antibodies [6]. Shutov et al. have reported that some patients with chronic renal failure from the middle Volga river valley had a history of hemorrhagic fever with renal syndrome. They concluded that hemorrhagic fever with renal syndrome contributed to the development of chronic renal failure in endemic areas [11]. Novo et al. have described Puumala virus infection in a 15-year-old boy with severe acute renal failure whose renal function subsequently remained mildly altered for more than two years [5]. Settergren et al. have also reported a persistently reduced glomerular filtration during the convalescence period [10].
There are only scarce literature data available on the prevalence of hantavirus antibodies in hemodialysis patients. Tsianos et al. screened 114 chronic hemodialysis patients (age range 14 to 73 years) for the presence of antibodies against hepatitis C virus and hantaviruses. Hantavirus antibodies were detected in 15 patients (13.2 %). Their presence correlated neither with transfusion nor with time on hemodialysis. It has been recommended to screen hemodialysis patients for the presence of hantavirus antibodies, in particular those with unclear cause of chronic renal failure [13]. In Israel, the presence of antibodies against Hantaan virus in chronic hemodialysis patients was investigated by Smetana et al. using indirect immunofluorescence. They found IgG antibodies against Hantaan virus in 11.6% of 43 screened patients and in 12.5% of 40 healthy control volunteers. Most of these study subjects originated from Asia and no difference was found in the level of infection between chronic hemodialysis patients and healthy people [12]. In 1990, Grcevska et al. from Skopje, Macedonia, have reported two cases of acute renal damage caused by hantavirus infection, one of which developed chronic renal failure with polyuria [2].
In our group of hemodialysis patients, we detected antibodies against hantaviruses in five of 301 (1.7%) study subjects. Most positives were females (80%). The PUU genotype was the causative agent in all five cases. Data from patient No. 2 showed the dynamics during the examination of serial serum samples. Her levels of anti-PUU IgM antibodies decreased over three years, while IgG antibodies switched from negativity to borderline values. The other patients exhibited a less typical course of infection. It must be noted, however, that hantavirus serology results are often inconclusive [4].
Like others, we detected both IgG and IgM hantavirus antibodies, anti-PUU antibodies in particular, in a proportion of hemodialysis patients. The average antibody prevalence rate in the Czech population is reported to be 0.8% (our unpublished data), and 1.97 [8]. Seroprevalence of hemodialysis patients differs not statistically significantly from the seroprevalence of the population. However involvement of hantaviruses in renal failure could not be excluded. It is obvious that hantaviruses may also contribute to chronic renal damage in the Czech Republic.
Acknowledgement
This work was supported by grant No. NR/ 9420-3/07 of the Grant Agency of the Ministry of Health, Czech Republic. We thank E. Pekna and S. Plosova for expert technical assistance.
Do redakce došlo 20. 8. 2009
Doc. MUDr. B. Kříž, PhD.
Státní zdravotní ústav
Šrobárova 48
100 42 Praha 10
e-mail: bohukriz@szu.cz
Sources
1. Elisaf, M., Korakis, H., Siamopoulos, K.C. Chronic renal dysfunction in hemorrhagic fever with renal syndrome patients. Renal Failure, 1993, 15, 623–627.
2. Grcevska, L., Polenakovic, M., Oncevski, A., Zografski, D. et al. Different pathohistological presentations of acute renal involvement in Hantaan virus infection: report of two cases. Clin Nephrol, 1990, 34, 197–201.
3. Lee, H.W., Lee, P.W., Johnson, K.M. Isolation of the etiologic agent of Korean hemorrhagic fever. J Inf Dis, 1978, 137, 298–308.
4. Lundkvist, A., Apekina, N., Myasnikov, Y., Vapalahti, O. et al, Dobrava hantavirus outbreak in Russia. Lancet, 1997, 350, 781–782.
5. Novo, R., Gagnadoux, M. F., LeGuenno, Y., Gubler, M. C., Niaudet, P. et al. Chronic renal failure after Puumala virus infection. Pediatr Nephrol, 1999, 13, 934–935.
6. Patnaik, M., Velosa, J.A., Peter, J.B. Hantavirus-specific IgG, IgM, and IgA in acute and chronic renal disease versus congenital renal disease in the United States. Amer J Kidney Dis, 1999, 33, 734–737.
7. Pejčoch, M., Heroldová, M., Zejda, J., Treml, F. et al. Detection of hantavirus antigen in rodents in the Czech Republic. (in Czech) Epidemiol Mikrobiol Immunol, 2003, 52, 18–24.
8. Pejčoch, M., Kříž, B. Prevalence of antibodies against hantaviruses among the adult population of the Czech Republic. Centr Eur J Publ Health, 2003, 11, 169–172.
9. Peters, C.J., Simpson, G.L., Levy, H. Spectrum of hantavirus infection: Hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Ann Rew Med, 1999, 50, 531–545.
10. Settergren, B., Trollfors, B., Fasth, A. Hultberg, B. et al. Glomerular filtration rate and tubular involvement during acute disease and convalescence in patients with nephropathia epidemica. J Inf Dis, 1990, 161, 716–720.
11. Shutov, A., Maximova, E., Potrashkova, K., Bruzgacheva, O. Haemorrhagic fever with renal syndrome and chronic renal failure. Lancet, 1996, 348, 1594–1595.
12. Smetana, Z., Mendelson, E., Soupaev, Z., Smetana, S. S. Serological survey of hantavirus antibodies in chronic hemodialysis patients. Clinical Nephrology, 1994, 42, 203–204.
13. Tsianos, E.V., Dalekos, G.N., Elisaf, M., Zervou, E. et al. High freguency of antibodies to Hantaan virus and hepatitis C virus in chronic haemodialysis patients - coincidence or cross - reaction. J Intern Med, 1993, 234, 607–610.
14. Vacková, M., Beran, J., Douda, O., Soukup, J. Epidemiologic problems in hantavirus infections. (in Czech) Epidemiol Mikrobiol Imunol, 2000, 49, 11–15.
15. Vacková, M., Douda, P., Beran, J., Gál, P. et al. Serologic detection of hantavirus antibodies. (in Czech) Epidemiol Mikrobiol Imunol, 2002, 51, 74–77.
16. Vapalahti, O., Lundkvist, A., Kukkonen, S. K., Cheng, Y. et al. Isolation and characterization of Tula virus, a distinct serotype in the genus Hantavirus, family Bunyaviridae. J Gen Virol, 1996, 77, 3063–3067.
17. Zelená, H., Januška, J. Serological characteristics of hantaviruses from clinical specimens analyzed in 1998-2008 in the Department of Virology, Public Healh Institute, Ostrava. (in Czech) Epidemiol Mikrobiol Imunol, 2009, 58, 115–120.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2010 Issue 1-
All articles in this issue
- Geography of the HIV/AIDS Pandemic: Analysis of Selected Available Papers and Studies
- Exotic Pets as a Potential Source of Salmonella
- Nocardia farcinica as the Causative Agent of a Brain Abscess in a Patient with Interstitial Lung Disease
- Changes in the Bacterial Spectrum in Severe Burn Wounds
- The Incidence of Tularemia in Slovakia in 1997-2008
- Molecular Epidemiology of Varicella Zoster Virus
- A Steady Rise in Incidence of Pertussis Since Nineties in the Czech Republic
- Multilocus sequence types in Czech neonatal GBS strains from 2004 to 2008
- Seroprevalence of Hantavirus Antibodies among Chronic Hemodialysis Patients in the Czech Republic
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Nocardia farcinica as the Causative Agent of a Brain Abscess in a Patient with Interstitial Lung Disease
- The Incidence of Tularemia in Slovakia in 1997-2008
- Exotic Pets as a Potential Source of Salmonella
- A Steady Rise in Incidence of Pertussis Since Nineties in the Czech Republic
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

