-
Medical journals
- Career
Micromodification of Virus-neutralisation Assay with Vital Staining in 96-well Plate and its Use in Diagnostics of Ťahyňa Virus Infections
Authors: H. Zelená; J. Januška; J. Raszka
Authors‘ workplace: Department of Virology, National Reference Laboratory for Arboviruses, Institute of Public Health in Ostrava
Published in: Epidemiol. Mikrobiol. Imunol. 79, 2008, č. 3, s. 106-110
Overview
Virus-neutralizační test (VNT) je v sérologii arbovirů považován za zlatý standard pro svou vysokou specificitu a senzitivitu. V Národní referenční laboratoři České republiky (NRL ČR) pro arboviry byla vyvinuta jeho mikromodifikace na mikrotitračních deskách s 96 jamkami s vitálním barvením buněčných kultur. Metoda je využívána pro detekci specifických protilátek proti různým virům, zejména arbovirům. Je popsán postup provedení VNT v mikromodifikaci pro virus Ťahyňa. Jsou prezentovány výsledky vyšetření protilátek antiŤahyňa u obyvatelstva v Moravskoslezském kraji dosažené pomocí VNT. Celková séroprevalence u 1001 vyšetřovaných osob byla 3,80 %. Nejvyšší podíl séropozitivních osob byl zjištěn u populace starší 59 let (17,53 %). Naopak u vyšetřovaných dětí do 5 let byla séroprevalence 0,00 % a ve věkové skupině 6–14 ůet 0,56 %.
Klíčová slova:
virus Ťahyňa – arboviry – neutralizace viru – vitální barvení – buněčná kulturaIntroduction
Ťahyňa virus
Ťahyňa virus (TAHV) is a member of the California serogroup of the genus Bunyavirus, family Bunyaviridae. Principal vectors of Ťahyňa virus are culicine mosquitoes, predominantly of the genus Aedes. Principal hosts of Ťahyňa virus are hares, rabbits, hedgehogs and small rodents.[2, 8]
Ťahyňa virus causes in humans so-called Valtice fever, a self-limiting febrile illness occurring in summer and early autumn. The rate of seropositivity depends on the location and age, highest rates (30-60 %) are found in elderly persons living close to frequently flooded areas with increased populations of mosquitoes [3, 4, 7]. At least 200 documented cases of Valtice fever have been published in Slovakia and Moravia since 1963 but many cases have remained probably unnoticed [1, 5, 7]. The disease also occurs in other European and non-European countries[2]. The closely related La Crosse virus is the main cause of arbovirus encephalitis in children in the USA [9].
Serologic examination in human population for specific anti-Ťahyňa antibodies is usually performed with the hemaglutination-inhibition (HIT) or plaque-reduction neutralisation tests (PRNT). No commercial assay for serologic diagnostics of Ťahyňa infections is available. Micromodification of virus-neutralisation assay in 96-well plates with vital staining of the cell cultures for detection of neutralizing anti-Ťahyňa antibodies was developed in the NRL for arboviruses of the Czech Republic.
Principle of virus-neutralisation test with vital staining of the cell cultures
Specific structures on the virus surface are blocked by virus-neutralisation antibodies, thereby the infectivity of the virus is neutralised. Manifestation of the virus infectivity may occure in different forms, depending on the selected host system, way of inoculation etc. Use of cytopathic effect on cell cultures is the best suited and most practical method for the routine diagnostics in virus-neutralisation test. It belongs to the most specific serologic reactions and in the case of arboviruses, presence of virus-neutralisation antibodies predicates directly the antiinfective immunity of the organism. The virus-neutralisation test is considered to be the golden standard for arbovirus serology.
There are three posibilities of quantitative arrangement of the virus-neutralisation assay: 1. Serially diluted virus and nondiluted serum (antibodies) – mostly used for identification of unknown virus isolate. 2. Nondiluted virus and serially diluted serum (antibodies) - the most convenient arrangement for the purpose of antibodies detection from clinical samples. 3. Serially diluted virus and serially diluted serum (antibodies) – also known as so-called box-titration – used only for special purposes.
Micromodification of VN assay in 96-well plate with vital staining was developed in the NRL for arboviruses for the purpose of serologic diagnostics of many arboviral infections, Ťahyňa virus including. Neutral red dye is used for the final visualisation of the reaction.
Vital staining differenciates the viability of the cell culture. Living cell culture is able to take in all the dye from diluent in the microplate well. The neutral red dye is absorbed in the cytoplasm and the whole cell culture gets conspicuously coloured in the form of clusters, islets or continuous red area on the bottom of the well. The cell cultures damaged by the virus are not able to absorb the dye which remains diffused in the diluent and the damaged cells don’t get coloured. The differences in the colour of the diluent and especially the morphology and colouring of the cell layer on the bottom of the wells are very distinct and observable macroscopically. Macroscopic differentiation of the cytopathic effect is possible even in the case of partially nonspecifically degenerated cells when the microscopical evaluating of cell cultures is no more feasible. [6]
The detailed test procedure and results of anti-Ťahyňa serosurvey in the area of Northern Moravia are presented.
Materials and methods
Description of the test procedure
Required number of tissue culture 96-well plates is prepared and marked (1 row for each sample, 8 serum samples can be tested in 1 plate). Two rows for negative controls and 2 rows for positive controls are used (negative and positive human sera). Universal diluent is MEM (minimal essential medium) buffered with 0.02 mol/l TRIS-HCl pH 8,1 (stock solution 2 mol/l TRIS-HCl pH 8,1 is diluted 1 : 100) + 7,5% bovine fetal serum. This mixture is called MEM-TRIS. Standardized volume of 0.025 ml per well of each component is used: 0.025 ml of serum in the respective dilution, (from 1 : 4 in serial double dilutions), 0.025 ml of diluent (MEM-TRIS), 0.025 ml of Ťahyňa virus (prototype strain TAH-92 cultivated on intracerebrally infected suckling mice) in working dilution (=100 TCID50, e.g. 5x10-4). After 10s vortexing the covered plates are incubated for 18 hours in +4 °C, then warmed up for 1 hour in 37 °C. 0.025 ml of cells suspension (pork kidney stable cell line = PS cells) in working dilution (e.g. 600 000 cells/ml i.e. 15 000 cells/well) is added. After 10s vortexing the covered plates are incubated for 3-4 days in humide incubator with 37 °C. 0.025 ml of neutral red dye in working dilution (1 : 10 000) is added (1 part of stock 0.2% aqueous solution of neutral red and 19 parts of MEM-TRIS) and vortexed for 10 sec. Covered plates are incubated for 1 day in humide incubator with 37 °C. It is possible to follow up the dynamics and continue incubation with neutral red. After disposal of the whole content of wells the results are read definitely.
First 3 wells of each row serve as serum controls, instead of the virus is added identical volume of pure diluent (MEM-TRIS) and only first 3 dilutions of serum are used: 1 : 4, 1 : 8 and 1 : 16.
Reading of the results
Only live cells are stained with neutral red dye and turn red in the end of the VNT. Dead cells damaged by the virus (cytopathic effect) are not able to be stained with the neutral red dye and remain colourless. First 3 wells of each row (serum control without added virus) must remain vital and macroscopically red coloured. If they are not vital (colourless), it means that the sample was toxic for the cells and the reaction is not specific up to the sample-dilution of more than 50 % damaged cells. Results of the VNT are expressed in the form of virus-neutralisation (VN) titer which is inverted value of the highest dilution of the sample which is able to neutralise the cytopathic effect of the virus for more than 50 % in the respective microwell.
Plate with completed virus-neutralisation test is showed in Fig. 1.
Image 1. Rows 1, 2, 3, 4. and 7 show positive results with VN titers 64, 32, 16, 64 and 16 respectively, whereas rows 5, 6 and 8 reveal negative results. 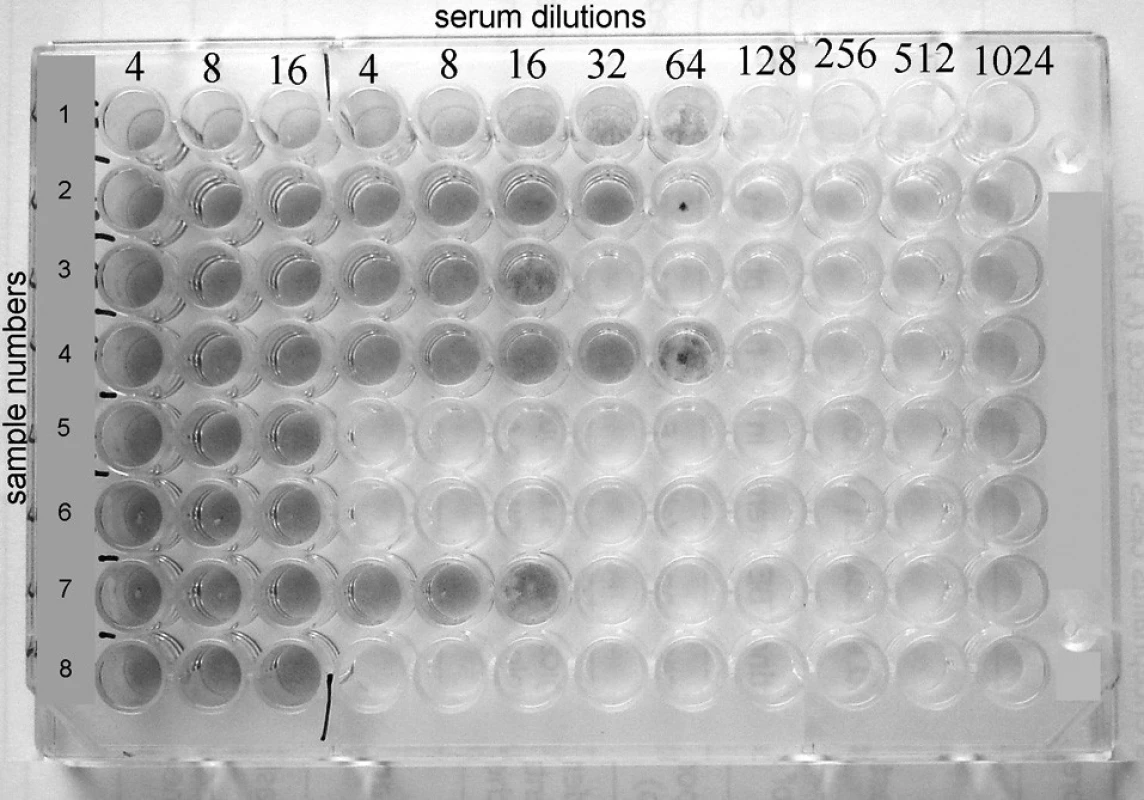
Rows 1, 2, 3, 4 and 7 show positive results with VN titers 64, 32, 16, 64 and 16 respectively, whereas rows 5, 6 and 8 reveal negative results.
Virus titration
Titration of the working virus dilution should be proceeded simultaneously with each run of virus-neutralisation test in a separate plate.Working dilution of virus is further diluted in 10-fold dilutions. Plate for virus titration is prepared and marked. Two whole columns of the plate are used for each dilution of the virus. Working dilution of the virus is marked as 10, further dilutions as 10-1, 10-2, 10-3 and 10-4. Last two columns serve as cell control (pure diluent instead of virus). Procedure of virus titration is identical with the proper virus-neutralisation test, only different dilutions of virus are used (pure diluent instead of virus in the cell controle columns) and instead of diluted serum is added only pure diluent.
For the VNT, 100 infectious doses of the virus (100 TCID50) should be used, it means 100x higher dilution of the virus which causes more than 50 % cytopathic effect.
To estimate the risk for Ťahyňa virus infections, human population in the region of Nortehrn Moravia (northeast part of the Czech Republic) was examinated for specific anti-Ťahyňa antibodies with virus-neutralisation assay in 96-well plate with vital staining of the cell cultures. From November 2006 to June 2007 1125 serum samples from 1001 persons tested for various reasons for other arboviruses in NRL were colected. For establishment of acute infection, in addition to positive case history, seroconversion or 4-fold increase of VN-titer in paired serum is required. For establishment of seropositivity without acute infection positivity in anti-Ťahyňa VNT is necessary.
Results
Results of anti-Ťahyňa survey
No case of seroconversion or significant increase of VN-titer was found in paired sera. 41 samples from 38 persons were positive for anti-Ťahyna antibodies. There were 25 males and 13 females positive in the age from 6 to 78 years. Age distribution of anti-Ťahyňa positive persons divided to 10-years age intervals is shown in Fig. 2.
Image 2. Age distribution of anti-Ťahyňa positive persons 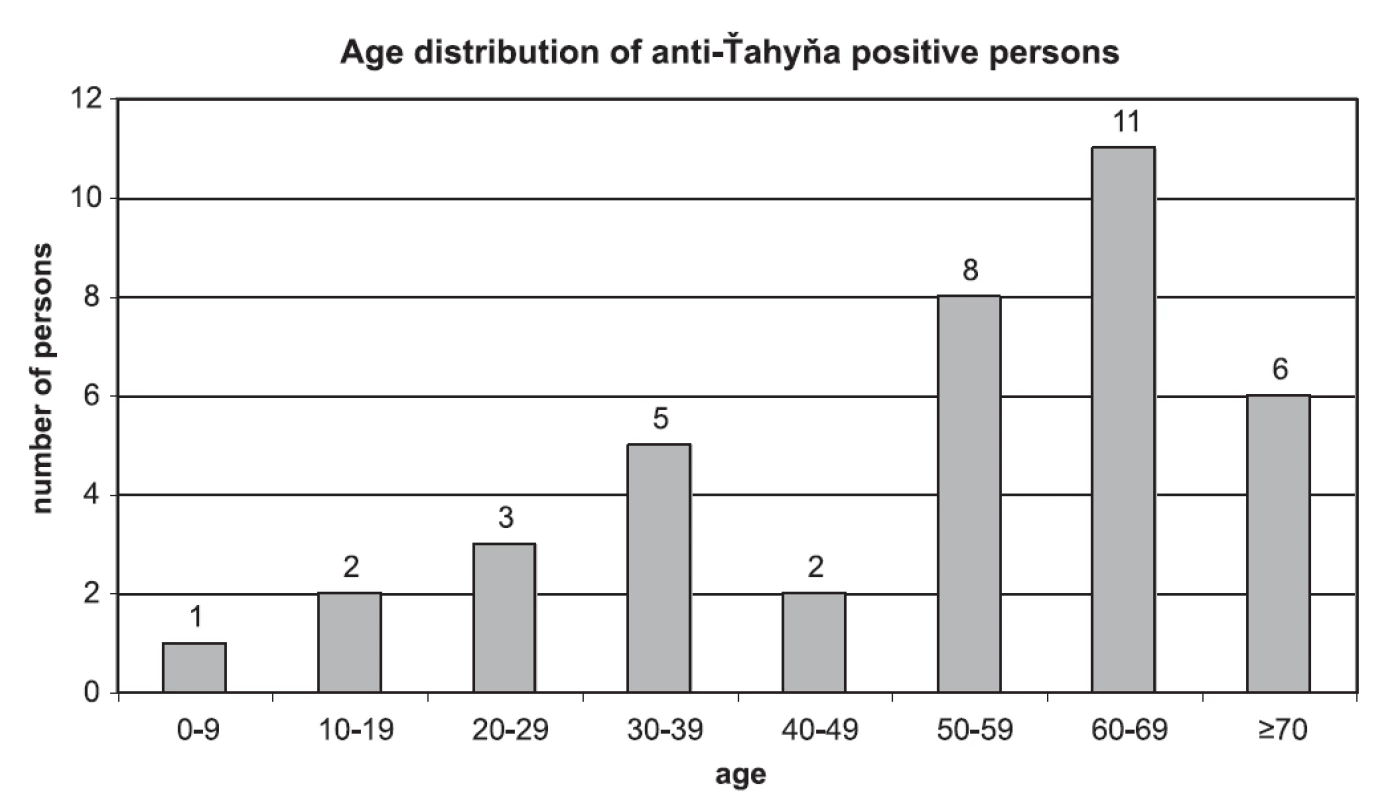
Virus-neutralisation titer of anti-Ťahyňa antibodies in positive samples ranged from 4 to 512, the most common titer was 16. Values of the titers and numbers of persons with respective titers: titer 4 (4 persons), titer 8 (7 persons), titer 16 (12 p.), titer 32 (4 p.), titer 64 (4 p.), titer 128 (2 p.), 256 (2 p.), 512 (3 p.).
Total seroprevalence among 1001 examinated persons was 3.80 % (38 persons). The rate of seropositivity was figured out and related to the commonly used basic epidemiologic age groups (0-5 years, 6-14 years, 15-59 years and ≥60 years). The highest positivity rate was found in persons older than 59 years – 17 of 97 persons were positive (17.53 %), in children, on the other hand, the seroprevalence was very low: no case of 31 examinated children in the age group 0-5 years and only 1 case of 180 children (0.56 %) in the age group 6-14 years. 20 seropositive persons of 693 (2.89 %) were found in the age group 15-59 years. Detailed data is demonstrated in table 1.
Table 1. Anti-Ťahyňa positivity in age groups 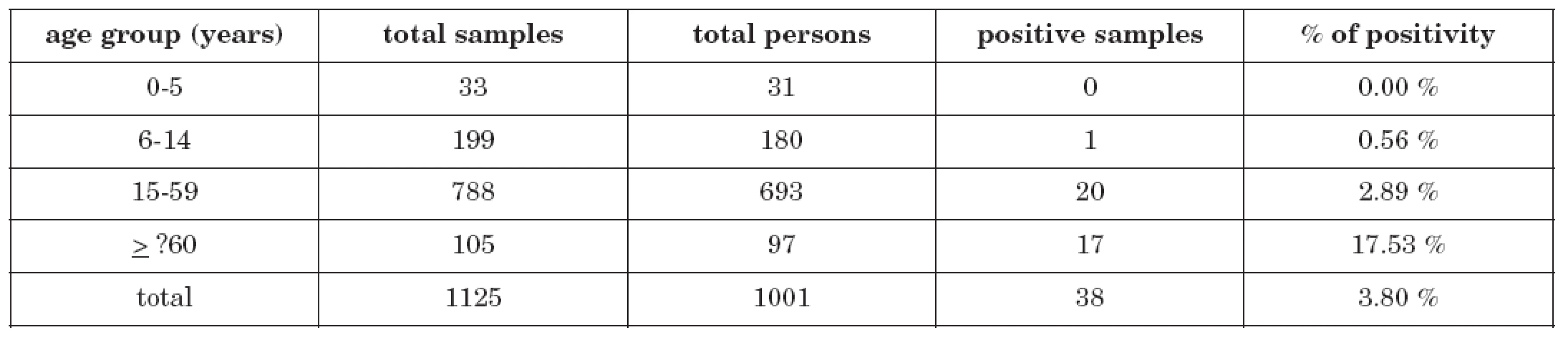
Conclusions and discussion
Virus-neutralisation assay for anti-Ťahyňa detection in 96-well plate with vital staining of the cell cultures developed in the NRL for arboviruses of the Czech Republic was shown as a simple, useful, high sensitive and specific tool for detection of anti-Ťahyňa antibodies in human blood. The same method is routinely used also for serologic diagnostics, serosurveys and confirmation of many other antiviral antibodies, arboviruses above all (tick-borne encephalitis virus, West Nile virus, yellow fever virus, chikungunya virus and others). In addition, this kind of assay may be used also for examination of all warm-blooded animals. Vital staining of cell cultures for detection of neutralisation antibodies is known in PRNT (plaque-reduction neutralisation test). VNT modification in 96-microplates with vital staining was firstly described by Januška et.al. [6] for tick-borne encephalitis virus diagnostics.
The micromodification with vital staining for the final visualisation of the reaction enables macroscopic reading of the results, hence tedious microscopic reading can be totally get rid of. This modification is more comfortable and precise and affords better reproducibility of the results comparing to PRNT or classical virus-neutralisation assay with only microscopic reading. Additionally, vital staining allows also observation of the dynamics of cytopathic effects.
Results of serosurvey focused on anti-Ťahyňa antibodies among popuation in the region of Northern Moravia showed the total seroprevalence of 3.80 % with the increasing positivity rate depending on the age but no case of acute Ťahyňa infection was found.
Northern Moravia was proved as endemic territory for Ťahyňa virus although the ascertained seroprevalence is lower comparing to earlier studies from the Breclav area during 1997 floods (53.8 % seropositivity) [3] and from the river basins in Central Bohemia during 2002 floods (16,5 %) [4, 5]. The disparity can be explained by the difference of environmental factors, the seropositivity is always higher in wetlands and in areas of frequently ocuring heavy rains and floods which support mosquito-vector populations. No of these circumstances occured in Northern Moravia during recent period with the exception of floods in 1997. Any significant increase of mosquito population wasn’t noticed in this territory. The dependence of seropositivity rate on the age is correspponding with the results of other surveys in the Czech Republic [3, 4, 5, 7].
The study was supported by the Grant Agency of the AS CR - Academy of Sciences of the Czech Republic [IAA 600930611 (Re)emerging mosquito-borne diseases].
Do redakce došlo 8. 4. 2008-06-19
MUDr. Hana Zelená
NRL pro arboviry - Zdravotní ústav
Partyzánské nám. 7
702 00 Ostrava
e-mail: hana.zelena@zuova.cz
Sources
1. Bárdoš, V., Medek, M., Kania, V., Hubálek, Z. Isolation of Tahyna virus from the blood of sick children. Acta Virol, 1975, 19, 447.
2. Hubálek, Z., Halouzka, J. Arthropod-borne viruses of vertebrates in Europe. Acta Scientiarum Naturalium Brno,1996, 10(4-5). ISSN 0032-8758.
3. Hubálek, Z., Halouzka, J., Juřicová, Z., Příkazský, Z., Žáková, J., Šebesta, O. Surveillance for mosquitoborne viruses in the Břeclav area after the 1997 flood. [in Czech] Epidem Mikrobiol Imunol, 1999, 48, 91-96.
4. Hubálek, Z., Zeman, P., Halouzka, J., Juřicová, Z., Šťovíčková, E., Bálková, H., Šikutová, S., Rudolf, I. Antibodies against mosquito-born viruses in human population of an area of Central Bohemia affected by the flood of 2002 [in Czech] Epidem Mikrobiol Imunol, 2004, 53, 112-120.
5. Hubálek, Z., Zeman, P. et al. Mosquitoborne viruses, Czech Republic, 2002. Emerg Infect Dis, 2005, 11(1), 116-118.
6. Januška, J., Daneš, L., Heinz, F. Laboratory diagnostic methods of arboviral and rodent-born viral infections. [in Czech] Praha: Avicenum, Praha, 1990. ISBN 80-201-0068-7.
7. Kolman, J.M., Kopecký, K., Minář, J., Hausenblasová, M. Prevalence of antibodies to Tahyna, Calovo and tick-borne encephalitis viruses in humans, Bohemia I. The Labe basin. [in Czech] Ceskosl Epidem Mikrobiol Imunol. 1972, 21, 79–85.
8. Kolman, J. M., Málková, D., Nemec, A., Smetana, A., Hájková, Z., Minář, J. The isolation of the Tahyna virus from the mosquito Aedes vexans in southern Moravia. J Hyg Epidem, 1964, 8, 380–386.
9. Szumlas, D.E., Apperson, C.S., Hartig, P.C., Francy, D.B., Karabatsos, N. Seroepidemiology of La Crosse virus infection in humans in western North Carolina. Am J Trop Med Hyg, 1996, 54, 332–337.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2008 Issue 3-
All articles in this issue
- Opomíjené virové infekce přenášené hematofágními členovci v České republice
- Od historických poznatkov a názorov až po súčasné úlohy na poli celiakie
- Reccurent Erythema Migrans as a Persistent Infection
- Aminoglykozidy a kolistín potláčajú tvorbu biofilmu u Klebsiella pneumoniae
- Micromodification of Virus-neutralisation Assay with Vital Staining in 96-well Plate and its Use in Diagnostics of Ťahyňa Virus Infections
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Opomíjené virové infekce přenášené hematofágními členovci v České republice
- Aminoglykozidy a kolistín potláčajú tvorbu biofilmu u Klebsiella pneumoniae
- Od historických poznatkov a názorov až po súčasné úlohy na poli celiakie
- Reccurent Erythema Migrans as a Persistent Infection
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

