-
Medical journals
- Career
Preeclampsia-associated homeostasis changes in pregnant woman after ART
Authors: V. Kaminskyi 1; O. Zhdanovych 1; T. Kolomiichenko 1; S. Kornienko 2; T. Anoshina 1; Obstetrics Of Department 1; Gynecology; Reproductology; Education National Medical Academy Of Postgraduate Shupyk; Kyiv; Ukraine; YV. Voronenko Rector; Md, Dr.Sc.; Professor Full; Ukraine Of The National Academy Of Medical Sciences Of Academician; Science State Prize Winner In Ukraine; Technology; Scientist Honoured; Ukraine Of Technologist
Authors‘ workplace: Odessa national medical university, Ukraine, rector V. M. Zaporozhan, Chairman of the Academic Council of the University, Academician of the NAMS of Ukraine, Doctor of Medical Sciences, Professor 2
Published in: Ceska Gynekol 2020; 85(6): 396-402
Category:
Overview
Objective: To determine the preeclampsia-associated features of homeostasis in pregnant woman after ART to clarify the possible mechanisms and factors of preeclampsia.
Design: A prospective study.
Setting: Department of Obstetrics, Gynecology and Reproductology, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine.
Methods: The 150 pregnant woman after ART were examined: 48 with preeclampsia (subgroup 1), 102 preeclampsia-free (subgroup 2). The liver enzymes in blood: alanine aminotransferase (ALT), aspartate aminotransferase (AST); the lipid metabolism indices: total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), aterogenity index (AI), triglycerides (TG), 25-hydroxy-vitamin D concentration, cytokines: interleukin-1, -2, -6, -8, -10 (IL-1, IL-2,, IL-6, IL-8, IL-10), tumor necrosis factor (TNF), number of platelets, platelet aggregate function, fibrinogen (F), activated partial thrombin time (aPTT), soluble fibrin monomer complexes (SFMC), D-dimer, von Willebrand factor (VWF) activity were measured. To compare the means Student’s t-test was used.
Results: In women with preeclampsia, the transaminase levels and lipidogram indices (TG, LDL, LDL, AI) are elevated. Blood vitamin D are decreased (25.92±4.76 vs. 38.42±5.12 ng/mL, p <0.05). Between subgroups 1 and 2 the differences in IL-1 (8.9 ±0.62 vs. 6.4±0.60 pg/mL, p <0.05), IL-8 (85.7±5.38 vs. 72.8±5.72 pg/mL, p <0,05) and IL-10 (2.3±0.81 vs. 4.7±0.27 pg/mL, p <0,05) were determined. Increased platelet aggregation was observed against relative thrombocytopenia background, higher F, SFMC, prolonged aPTT, increased D-dimer (328.1±23.6 vs. 186.2±15.4 ng/mL, p <0.05), and von Willebrand factor (1.48±0.21 vs. 0.76±0.27 IU/mL, p <0.05).
Conclusion: Preeclampsia in pregnancy after ART occurs if in first trimester the proatherogenic disorders of lipid metabolism, vitamin D deficiency, aggravated inflammatory response, prothrombotic changes in the hemostasis system with the endothelium damage exist. Such changes may be prognostic markers of preeclampsia.
Keywords:
assisted reproductive technologies – preeclampsia – liver lipid metabolism – vitamin D – interleukin – Homeostasis
INTRODUCTION
More than forty years ago, the first child after in vitro fertilization was born, and this gave hope of paternity for many couples [23]. Ever since assisted reproductive technologies (ART) have been increasingly used for infertility problem [9, 33]. The course of pregnancy in patients after IVF is characterized by a high frequency of obstetric and perinatal pathologies (threatened abortion and preterm delivery, placentation pathology, prematurity, intrauterine growth retardation, fetal hypoxia) [7, 16, 17, 31]. The risk of congenital malformations is discussed, although this may be result both ART per se and other factor effects (age of woman, concomitant gynecological and somatic pathologies, disorders of psycho-emotional adaptation, etc.) [12, 14, 15, 37].
Many researchers emphasize the high risk of preeclampsia during induced pregnancy, at least 2-fold, especially at pregnancy with twin and cryopreservation of embryos [4, 24, 30].
Along with the fact that preeclampsia is one of the leading cause of maternal and perinatal mortality, this disease and its complications cause a range of medical problems: induced preterm delivery, maternal and child diseases in the future. Newborns suffer from intragestation distress and intrauterine growth restriction, physical and neuro-mental development retardation [6].
The etiology of preeclampsia is not well understood. The definition of PE as an adaptation disease is in line with common concepts and allows us to explain some aspects of the disease [11]. However, there is no generally accepted theory that gives a unified picture of pathogenesis and satisfies the demands of practice [3, 25]. More than 30 hypotheses were tried to explain the causes and mechanisms of preeclampsia, but all of them light the isolated parts of the complex process.
Many researchers believe that preeclampsia is the result of pathological immune and hormonal reactions that progressing during gestation; incomplete remodeling of uterine vessels occurs, endothelial dysfunction develops. Endothelial dysfunction may involve the disintegration of the endothelial cell monolayer and loss of vascular integrity, which is clinically manifested by proteinuria [5]. At that, the trigger mechanisms are not fully defined and the search for new biomarkers of preeclampsia is in progress [18, 26, 35].
The objective of this study was to determine the preeclampsia-associated features of homeostasis in pregnant women after ART to clarify the possible mechanisms and factors of preeclampsia development.
MATERIALS AND METHODS
A total of 200 women were fully examined: 150 women with pregnancy after fertility treatment by ART (main group) and 40 healthy women with a non-induced pregnancy and without preeclampsia in history (control group). The supervision of pregnancy in dynamics showed that in 48 (32.0%) women from the main group the preeclampsia of varying severity was developed (p <0.05): 31 cases of early preeclampsia (including 8 severe preeclampsia) and 17 cases of late preeclampsia. In follow-up the main group was divided into 2 subgroups: 48 women with preeclampsia and 102 women with pregnancy not complicated by preeclampsia.
The examinations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) were performed on the automatic biochemistry analyzer «Cobas Integra 400» («Roche Diagnostics», Switzerland).
Low-density lipoprotein cholesterol (LDL-C) was calculated by the formula Friedewald W.T. et al. (1972):
LDL-С = TC – HDL-C – TG / 2.18 (mmol/L).
Very-low-density lipoprotein cholesterol (VLDL-C) was calculated from TG by the formula:
VLDL-C = TG / 2.18 mmol/L.
The aterogenity index (AI) was calculated by the formula of AM Klimov (1976).
AI = (TC – HDL-C) / HDL-C.
The examination of 25-hydroxy-vitamin D, 25-(OH)D was carried out by enzyme immunoassay. EUROIMMUN (Germany) analyzer and test system were used.
Using the test systems from Vector-Best Ukraine LLC by enzyme immunoassay method, the cytokines in peripheral blood were analyzed: interleukin-1 (IL-1), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor (TNF).
Common blood analysis was carried out on an Auto Hematology Analyzer Mindray ВC-3200 by the photometric and conductometric (electrical impedance) methods with Mindray reagents using. Laboratory examination of fibrinogen (F), activated partial thrombin time (aPTT), soluble fibrin monomer complexes (SFMC) and D-dimer was carried out by the semi-automated coagulometer Helena C-2 with Helena reagents using. Examination of von Willebrand factor (VWF) activity and ADP-induced (adenosine-2-phosphoric acid) platelet aggregation function was performed by the Chronolog 490-4D (USA) aggregometer.
Statistical analysis of the findings was performed using Biostat, Statistica 6.0 software from Install Shield Software Corporation (USA). Normal distribution variables are presented as mean and standard deviation (SD). Student’s t-test was used to compare the means. The significance level for all tests was p <0.05.
RESULTS
In women with preeclampsia, abnormal liver enzymatic function was detected, as evidenced by elevated transaminases (table 1) and lipid metabolism. Significant changes in lipidogram (TG, VLDL-C, LDL-C and AI increasing) were observed; this point out a predominance of proatherogenic fractions of the cholesterol, and corresponds to a high rate of obesity and metabolic syndrome.
1. The liver enzymes and lipidogram indices in pregnant woman after ART 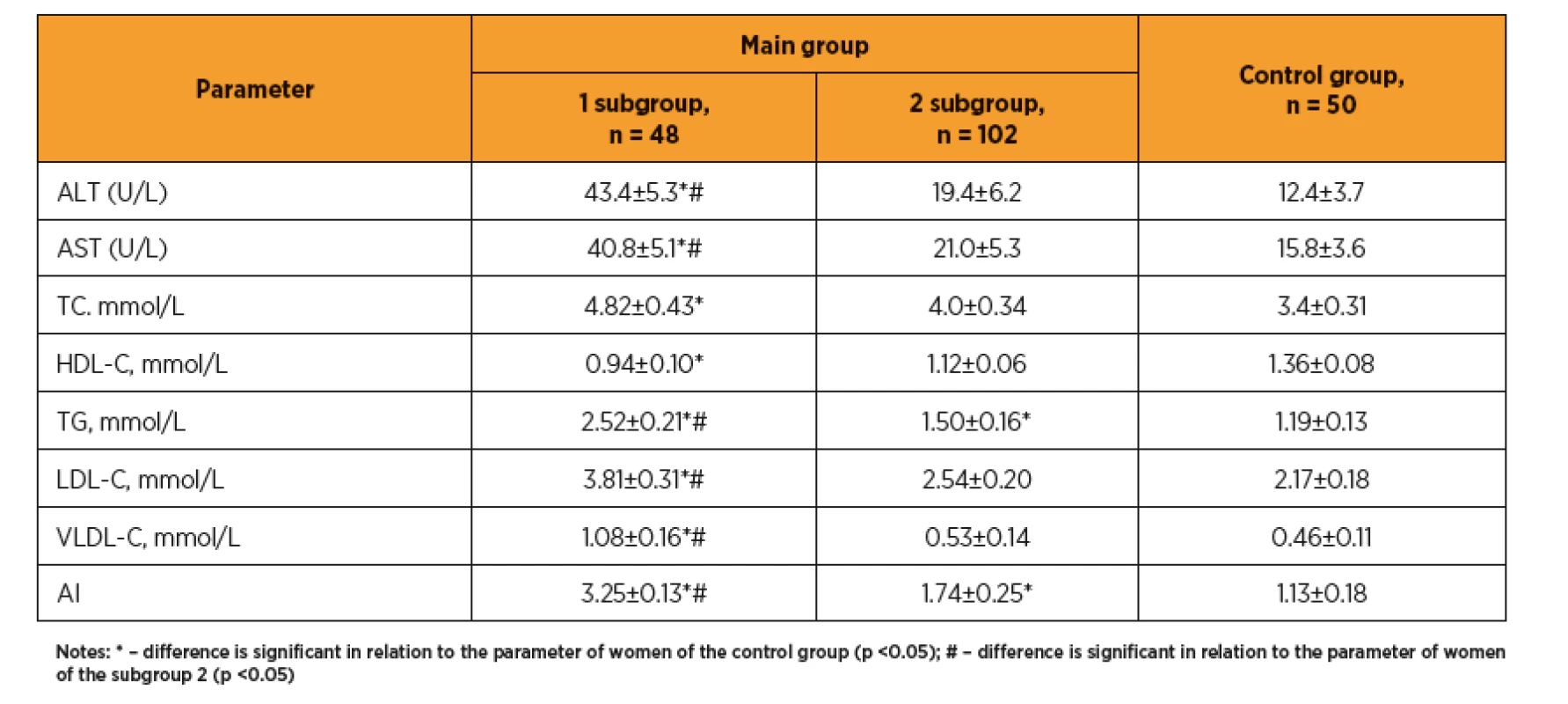
According to our data (figure 1), the average level of blood 25-hydroxycalciferol (vitamin D) in women with preeclampsia was significantly reduced as compared with control group (25.92±4.76 vs. 38.42±12 ng/mL, p <0.05). According to the classification for Central European countries (Warsaw, 2012), this level (under 30 ng/mL) is suboptimal, and in some patients this levels were even under 20 ng/mL, which is regarded as vitamin D deficiency.
Fig. 1 Vitamin D concentration in blood of pregnant woman after ART, ng/ml 
Preeclampsia is a severe complication of the pregnancy characterized by maternal aggravated systemic inflammatory response with immune system activation. Cytokines, chemokines and adhesion molecules are core for immune processes. Pro - and anti-inflammatory cytokines play a crucial role in the growth and function of the placenta. Any changes in these cytokines, misbalances in the pro - and anti-inflammatory cytokine ratios can be associated with many pregnancy disorders, including preeclampsia [2].
A study of the cytokine profile in the first trimester of ART pregnancy showed that in women with preeclampsia the levels of proinflammatory cytokines (IL-1, IL-2, IL-6, IL-8, TNF) were significantly increased as compared with control group and above the laboratory reference ranges, while the level of the proinflammatory cytokine with protective properties (IL-10) was significantly reduced (table 2). There is revealed a significant difference between the group of pregnant women after ART with preeclampsia and the group of pregnant women without this obstetric complication, in terms of IL-1 (8.9±0.62 vs. 6.4±0.60 pg/mL, p <0.05), IL-8 (85.7±5.38 vs. 72.8±5.72 pg/mL, p <0.05) and IL-10 (2.3±0.81 vs. 4.7±0.27 pg/mL, respectively, p <0.05). Thus, the preeclampsia develops in women whose first trimester of gestation is course at aggravated inflammatory response, as indicated by increasing of the proinflammatory cytokines and decreasing of the anti-inflammatory protective interleukins, which leads to damage of endothelial cells.
2. Cytokine profile in pregnant woman after ART, ng/ml 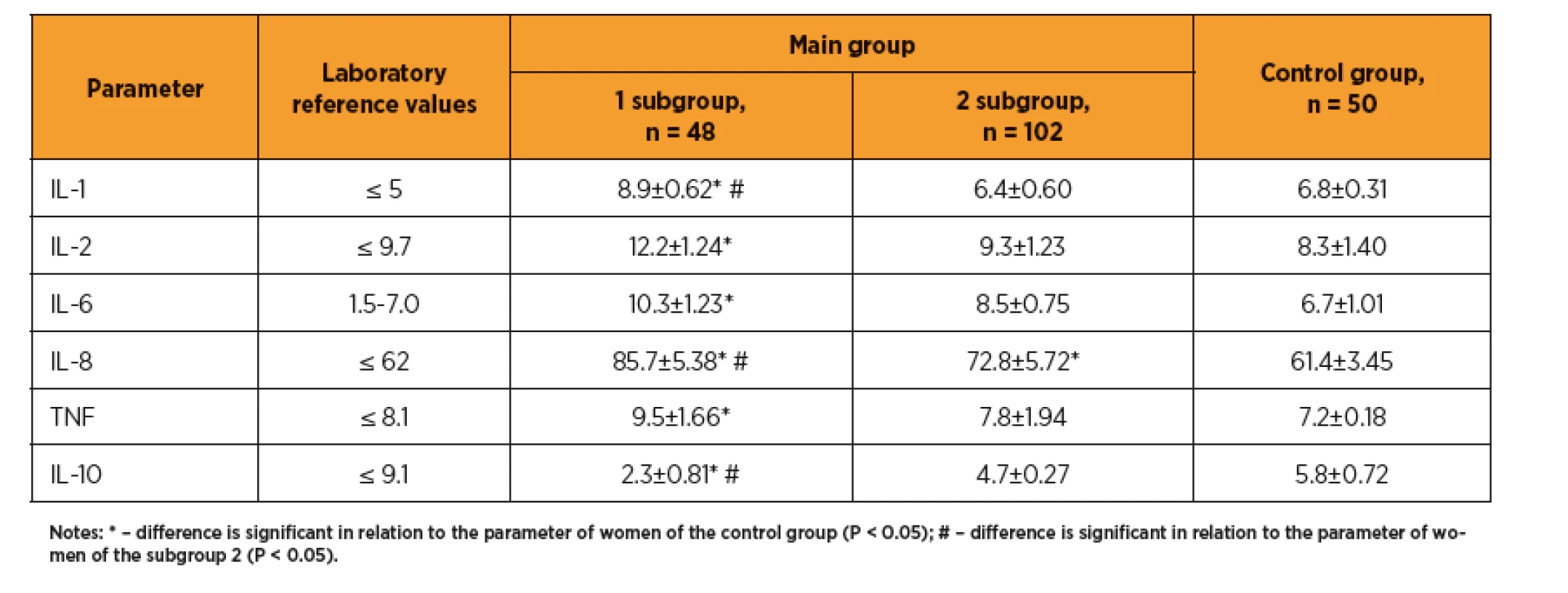
The immune system is closely related with the hemostasis system. Many clotting factors are produced in leukocytes, monocytes, and macrophages. Immunocompetent cells are able to switch the immune response to the activation of blood clotting for localization of the vascular clot formations. One of the cytokines produced at immune response activation, IL-1, is able to significantly reduce the antithrombogenic properties of the vascular endothelium, while releasing of fibrinolysis inhibitors triggers the disseminated intravascular coagulation.
Study of the hemostasis system (table 3) revealed in patients with preeclampsia an increase in platelet aggregation on the relative thrombocytopenia background, significantly higher levels of fibrinogen and soluble fibrin-monomer complexes (SFMC), significantly prolonged aPTT, indicating the procoagulative shifting of the homeostasis system in this group compared with the control pregnant women and patients without preeclampsia. Noteworthy is a significantly increased level of D-dimer (328.1±23.6 vs. 186.2±15.4 and 158.3±18.4 ng/mL, respectively, in subgroup 2 and control group, p <0. 05), which corresponds to the increase in procoagulative potential. The observed increase in von Willebrand factor (1.48±0.21 vs. 0.76±0.27 and 0.52±0.16 IU/mL, respectively, p <0.05) indicates more pronounced endothelial damage at the early pregnancy in those women who were later diagnosed with preeclampsia.
3. Indicators of hemostasis of pregnant woman after ART 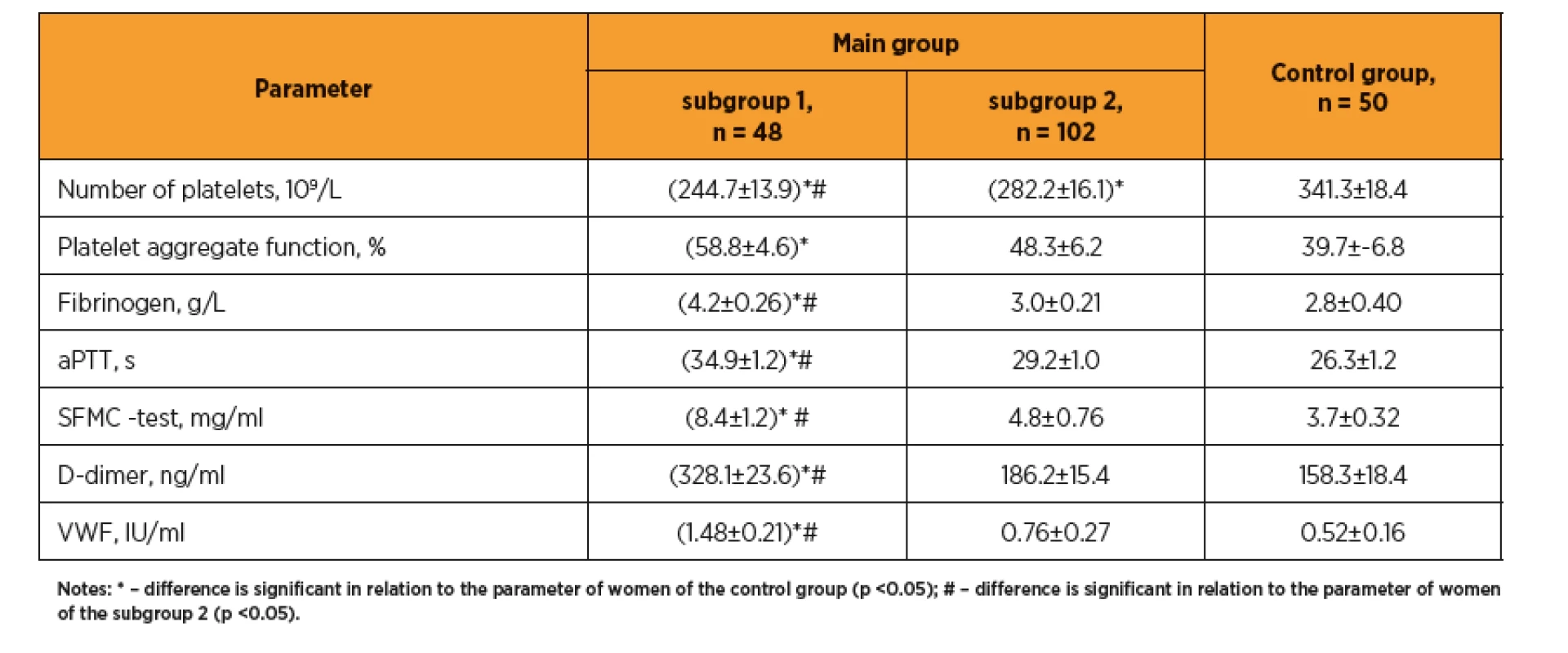
DISCUSSION
In women after ART with preeclampsia the impaired liver enzymatic activity was revealed, as evidenced by transaminase levels and lipid metabolism increasing, significant changes in lipidogram (TG, VLDL-C, LDL and AI increasing), which indicates a predominance of proatherogenic fractions of the cholesterol, and corresponds to a high rate of obesity and metabolic syndrome. Some researchers [29] even consider triglycerides as a potential easy-to-perform and not costly biomarker for preeclampsia prediction, although no changes in other cholesterol fractions have been found in this study. At the same time, in the recent USA study [32] the significant changes in lipid profile (involving all cholesterol fractions) in pregnant women after ART were contrast to spontaneous pregnancies, which was associated with obstetric and perinatal pathology (preeclampsia, intrauterine growth retardation, etc.).
The findings regarding lowering of the vitamin D at induced pregnancy are consistent with the data of recent studies, in which pregnant women and newborns are in group risk for vitamin D hypovitaminosis [27], and vitamin D deficiency related with the obstetric and perinatal risks [10]. There are evidences about role of vitamin D deficiency in the negative pregnancy outcomes after ART [36]. However there are conflicting data, in one clinical study winter vitamin D deficiency had not effect on fertility rates or pregnancy outcomes in married couples with in vitro fertilization [22].
Furthermore, some researchers [21, 28] indicate that preeclampsia is more commonly in pregnant women with vitamin D deficiency, which may be related to role of this vitamin as a universal modulator of the immune system [1, 8] with the effect on production and action of cytokines. Vitamin D deficiency has been linked to autoimmune diseases, including antiphospholipid syndrome (APS), which is considered as prothrombogenic condition [8].
As per our findings preeclampsia develops in women with aggravated inflammatory response in 1st trimester, as indicated by an increase of proinflammatory cytokines (IL-1, IL-2, IL-6, IL-8, TNF) and decrease of protective anti-inflammatory interleukins (IL-10), that causes damage of endothelial cells. Similar results have been reported by other researchers about correlation of the preeclampsia with the misbalance of pro - and anti-inflammatory cytokines ratio [2].
It is known that the treatment of infertility using ART per se significantly increases the risk of thromboembolism [19, 20]. Our results confirm the correlation of the preeclampsia with the prothrombogenic conditions of hemostasis system (fibrinogen and soluble fibrin monomer complexes increasing, considerable prolonged aPTT, significantly increased D-dimer), which according to review [13] is in line with the conclusions of other investigators about the role of haemostasis system activation in development of placenta-mediated pregnancy complications, including preeclampsia.
Noteworthy is the increase in von Willebrand factor, now considered as marker of endothelial damage. A pilot study also found a significant increase of von Willebrand factor in pregnant women with gestosis and US signs of placental insufficiency [34].
CONCLUSION
Preeclampsia in pregnancy after ART occurs in women with significant abnormalities of homeostasis in the first trimester. These are proatherogenic disorders of lipid metabolism, vitamin D deficiency, aggravated inflammatory response (shift of the cytokine profile towards proinflammatory cytokines), prothrombotic changes in the haemostasis system with increase of D-dimer and von Willebrand factor, resulting in damage of endothelial cells, vascular dismicrocirculation during fetoplacental complex formation, and this is the basis for preeclampsia development. These processes are a manifestation of the failure of systemic adaptation in the body. The identified changes in the studied parameters can be used as early markers of the possible development of preeclampsia in women after ART.
GRANTS
This research study was funded by the Ministry of Health of Ukraine within state budget (under the budget program of the CPCWC 2301020 “Scientific and research/technical activities in the field of health care”, “Reduction of the frequency of the large obstetric syndromes during pregnancy of high risk from the single genesis standpoint by introduction of pathogenetically-directed complex of prevention and treatment“, state registration number 118U001138).
Kolomiichenko Tetiana
Kyiv City Center for Reproductive and Perinatal Medicine,
16 Heroiv Stalinhrada Str.
Kyiv, Ukraine
e-mail: aagukrane@gmail.com,
Sources
1. Agarwal, S., Kovilam, O., Agrawal, DK. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit Rev Food Sci Nutr, 2018, 58(5), p. 755–769.
2. Aggarwal, R., Jain, AK., Mittal, P., et al. Association of pro - and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal, 33(4), e22834.
3. Ahmed, A., Rezai, H., Broadway-Stringer, S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv Exp Med Biol, 2017, 956, p. 355–374.
4. Ballesta-Castillejos, A., Gomez-Salgado, J., Rodriguez-Almagro, J., et al. Obstetric and perinatal complications associated with assisted reproductive treatment in Spain. J Assist Reprod Genet, 2019, 36(12), p. 2435–2445.
5. Boeldt, D., Bird, I. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia, J Endocrinol, 2017, 232(1), R27–R44.
6. Bokslag, A., van Weissenbruch, M., Mol, BW., de Groot, CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev, 2016, 102, p. 47–50.
7. Cavoretto, P., Candiani, M., Giorgione, V., et al. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol, 2018, 51(1), p. 43–53.
8. Cyprian, F., Lefkou, E., Varoudi, K., Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front Immunol, 2019, 10, p. 2739.
9. De Geyter, C., Calhaz-Jorge, C., Kupka, MS., et al. ART in Europe, 2014: results generated from European registries by ESHRE. Hum Reprod, 2018, 33(9), p. 1586–1601.
10. Eremkina, AK., Mokrysheva, NG., Pigarova, EA., Mirnaya, SS. Vitamin D: effects on pregnancy, maternal, fetal and postnatal outcomes. Ter Arkh, 2018, 90(10), p. 115–127.
11. Filipek, A., Jurewicz, E. Preeclampsia – a disease of pregnant women. Postepy Biochem, 2018, 64(4), p. 232–229.
12. Giorgione, V., Parazzini, F., Fesslova, V., et al. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol, 2018, 51(1), p. 33–42.
13. Gris, JC., Bouvier, S., Cochery-Nouvellon, É., et al. The role of haemostasis in placenta-mediated complications. Thromb Res, 2019, 181 (l), p. S10–S14.
14. Hoorsan, H., Mirmiran, P., Chaichian, S., et al. congenital malformations in infants of mothers undergoing assisted reproductive technologies: a systematic review and meta-analysis study. J Prev Med Public Health, 2017, 50(6), p. 347–360.
15. Kaminskyi, V., Ventskovskaia, I., Zhdanovych, O., et al. Peculiarities of psychoemotional status of pregnant women with perinatal losses in the history psychiatry psychotherapy and clinical psychology, 2020, 11 (1), p. 66–74.
16. Kaminskyi, VV., Zhdanovych, OI., Vorobey, LI., et al. Perinatal losses in anamnesis as a factor of fetus adaptation damage. Reprod Endocrinol (Ukraine), 2019, 47(3), p. 48–52.
17. Kawwass, JF, Badell, ML. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol, 2018, 132(3), p. 763–772.
18. Laššáková, S., Korabečná, M. New potential biomarkers for preeclampsia prediction. Čes Gynek, 2018, 83(6), p. 458–463.
19. Lattová, V., Dostál, J., Vodička, J., Procházka, M. The risk of thromboembolism in relation to in vitro fertilization. Čes Gynek. 2019, 84(3), p. 229–232.
20. Levente, T., Szilárd-Leó, K., Lujza-Katalin, B., et al. The prognostic role of thrombophilia in the treatment of infertility. Bulletin Med Sci, 2018, 91(1), p. 42–49.
21. Mirzakhani, H., Litonjua, AA., McElrath, TF., et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest, 2016, 126(12), p. 4702–4715.
22. Neville, G., Martyn, F., Kilbane, M., et al. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int J Gynaecol Obstet, 2016, 135(2), p. 172–176.
23. Niederberger, C., Pellicer, A., Cohen, J., et al. Forty years of IVF. Fertil Steril, 2018, 110(2), p. 185–324.
24. Okby, R., Harlev, A., Sacks, KN., et al. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch Gynecol Obstet, 2018, 297(3), p. 653–658.
25. Rana, S., Lemoine, E., Granger, JP., Karumanchi, SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res, 2019, 124(7), p. 1094–1112.
26. Roubalová, L., Vojtěch, J., Feyereisl, J., et al. First-trimester screening for preeclampsia. Čes Gynek, 2019, 84(5), p. 361–370.
27. Saraf, R., Morton, SM., Camargo, CAJr., Grant, CC. Global summary of maternal and newborn vitamin D status – a systematic review. Matern. Child Nutr, 2016, 12(4), p. 647–668.
28. Serrano-Díaz, NC., Gamboa-Delgado, EM., Domínguez-Urrego, CL., et al. Vitamin D and risk of preeclampsia: A systematic review and meta-analysis. Biomedica, 2018, 38(1), p. 43–53.
29. Siddiqui, IA. Maternal serum lipids in women with pre-eclampsia. Ann Med Health Sci Res, 2014, 4(4), p. 638–641.
30. Sites, CK., Wilson, D., Barsky, M., et al. Embryo cryopreservation and preeclampsia risk. Fertil Steri., 2017, 108(5), p. 784–790.
31. Sullivan-Pyke, CS., Senapati, S., Mainigi, MA., Barnhart, KT. In vitro fertilization and adverse obstetric and perinatal outcomes. Semin Perinatol, 2017, 41(6), p. 345–353.
32. Sun, T., Lee, B., Kinchen, J., et al. Differences in first-trimester maternal metabolomic profiles in pregnancies conceived from fertility treatments. J Clin Endocrinol Metab, 2019, 104(4), p. 1005–1019.
33. Sunderam, S., Kissin, DM., Crawfordë, SB., et al. Assisted reproductive technology surveillance – United States, 2015. MMWR Surveill Sum,. 2018, 67(3), p. 1–28.
34. Szpera-Gozdziewicz, A., Gozdziewicz, T., Boruczkowski, M., et al. Relationship between the von Willebrand factor plasma concentration and ultrasonographic doppler findings in pregnancies complicated by hypertensive disorders: a pilot study. Gynecol Obstet Invest, 2018, 83(3), p. 252–258.
35. Taylor, BD., Robert, B., Ness, RB., et al. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens, 2016, 6(4), p. 388–393.
36. Zhao, J., Huang, X., Xu, B., et al. Whether vitamin D was associated with clinical outcome after IVF/ICSI: a systematic review and meta-analysis. Reprod Biol Endocrinol, 2018, 16(1), p. 13.
37. Zhdanovych, OI., Vorobey, LI., Anoshina, TN., Kolomiichenko, TV. Perinatal consequences of adaptation disorder with burdened obstetric history. World Med Biol, 2020, 1(71), p. 044–049.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2020 Issue 6-
All articles in this issue
-
Importance of addition of HPV DNA testing to the cytology based cervical cancer screening and triage of findings with p16/Ki67 immunocytochemistry staining in 35 and 45 years old women
LIBUSE trial data analysis - A model for predicting unscheduled caesarean section in nulliparae
- Total laparoscopic hysterectomy – clinical comparison of the method using two types of uterine manipulators
- Preeclampsia-associated homeostasis changes in pregnant woman after ART
- Laparotomic myomectomy of a spontaneous perforated leiomyoma mimicking pseudomyxoma peritonei at 27 weeks of pregnancy
- Immunological principle of development of red blood cell alloimmunization in pregnancy, hemolytic disease of the fetus and prevention of RhD alloimmunization in RhD negative women
- Idiopathic polyhydramnios
- Prenatal care for woman after fertility-sparing surgery for cervical cancer
- Conservative treatment options for polycystic ovary syndrome: the importance of exercise
-
Mythology and rational explanation in the history of medicine
The case of molar pregnancy - Dienogest in the treatment of endometriosis
- Anti-Nairobi: A Statement against the Nairobi Statement
- Suplementace vitaminem D a kalciem – význam v gynekologii
- NEKROLOG
-
Importance of addition of HPV DNA testing to the cytology based cervical cancer screening and triage of findings with p16/Ki67 immunocytochemistry staining in 35 and 45 years old women
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Idiopathic polyhydramnios
- Total laparoscopic hysterectomy – clinical comparison of the method using two types of uterine manipulators
- Dienogest in the treatment of endometriosis
-
Importance of addition of HPV DNA testing to the cytology based cervical cancer screening and triage of findings with p16/Ki67 immunocytochemistry staining in 35 and 45 years old women
LIBUSE trial data analysis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

