-
Medical journals
- Career
Rosuvastatin-induced rhabdomyolysis due to medication errors
Authors: Martin Vodička 1; Ondřej Slanař 2; Michal Pisár 1; Tomáš Šálek 1
Authors‘ workplace: The Tomas Bata Hospital in Zlín, Czech Republic 1; Institute of Pharmacology, First Faculty of Medicine and General Teaching Hospital, Charles University in Prague, Prague, Czech Republic 2
Published in: Čes. slov. Farm., 2020; 69, 100-102
Category: Case report
Overview
Case (description): A 74 years old Caucasian suffering from chronic kidney disease presented with progressive asthenia and diffuse myalgia. It was revealed that the patient used three different rosuvastatin-containing preparations in a total daily dose of 120 mg for 76 days. Laboratory investigations revealed a marked elevation of serum urea, creatinine, myoglobin, creatine kinase (CK) and transaminases. Two serious medication errors have been identified as possible major factors that synergistically contributed to the development of rosuvastatin-induced rhabdomyolysis. First, 40 mg of rosuvastatin dose was prescribed to the patient, although the estimation of glomerular filtration rate (eGFR) declined below 40 ml/min/1.73 m2. Moreover, the patient used 3 different rosuvastatin formulations simultaneously in a total dose of 120 mg/day. The heterozygous CYP2C9*1/*3 genotype and warfarin co-administration could further contribute to the development of rhabdomyolysis. A number of preventive measures, notably in drug policy, are suggested to overcome unintended intoxications.
Conclusion: Rosuvastatin-induced myopathy is a rare, but serious adverse effect. This case report highlights the need for a proper treatment and dose adjustment during chronic medical therapy, the need for adequate patient education and application of adequate drug policy measures in the era of fragmented health care delivery and polypragmasia.
Keywords:
rosuvastatin – adverse effects – medication errors – rhabdomyolysis
Introduction
Rosuvastatin is a hydroxymethylglutaryl-CoA reductase inhibitor widely used in primary and secondary prevention of cardiovascular diseases. Rosuvastatin-induced myopathy is a well-known but fortunately rare adverse effect. The individual genetic susceptibilities in drug metabolism or transport and cytochrome P450 interactions are suspected to represent risk factors, although the exact mechanism of its development is unknown1, 2). The drug-induced reduction of isoprenoids causes a decreased production of isoprenylated proteins whose deficiency may cause disturbances in cell apoptosis regulation and skeletal muscle cell structure.
According to the New Drug Application (NDA) database, the incidence of rosuvastatin-related myopathy (symptomatic with CK elevations > 10 times upper limit of normal) is low, reported in up to 0.1% of patients taking rosuvastatin doses of up to 40 mg. It is a lower incidence than that observed with other statins3). However, myopathy appears to be dose-dependent. There were cases of rhabdomyolysis seen in higher than recommended doses in clinical studies (NDA).
Similar figures are shown in the meta-analysis of 18 clinical trials (301,374 person-years). It was found that myopathy, CK elevations and rhabdomyolysis occurred in 316, 81 and 9 cases, respectively, compared to the placebo group with 253, 64 and 1 respective cases4).
Despite the rosuvastatin is eliminated primarily by excretion in the feces, plasma concentrations increase in patients with severe renal impairment.
Impacts on practice
- Management of chronic medication should reflect a decrease in the elimination functions of organs as well as changes in medication habits, especially if the cognitive functions of the patient are likely to fluctuate or deteriorate.
- Frequent switching of generic formulations may be confusing for patients.
- Switching of medicinal products must be accompanied with adequate information for the patient to avoid overdose.
Case description
A 74-years Caucasian male patient visited primary care physician due to weakness, myalgia, progressive immobility and urinary incontinence lasting for the past few days. He shuffled slowly, unable to raise his head due to muscle pain. His medical history included ischemic stroke, atrial flutter, hypertension, type 2 diabetes, obesity (BMI = 31.6 kg/m2), diabetic retinopathy, chronic kidney disease (eGFR of 37.8 ml/min/1.73 m2), and benign prostatic hyperplasia. The physician discovered that the patient had been using three different rosuvastatin containing preparations in a total daily dose of 120 mg for 76 days. He advised to withdraw rosuvastatin and referred him to the hospital. Warfarin was stopped due to high international normalized ratio (INR) at the same time.
The patient came to the hospital 3 days later, he was afebrile, hemodynamically stable, eupneic, slightly dehydrated with a mild symmetric ankle edema. He complained of constipation since a month ago. Progressive fatigue left him bedridden for the last three days. He denied chills or fever, dyspnea, chest pain, cough, bleeding or weight loss. Laboratory investigations revealed a marked elevation of serum urea, creatinine, myoglobin, creatinine phosphokinase, INR (5.35) and transaminases. The patient was admitted to the intensive care unit (ICU). The time course of selected biochemistry parameters in relation to the rosuvastatin administration is summarized in Table 1.
1. Time course of laboratory parameters in relation to the administration of rosuvastatin dose of 120 mg/day 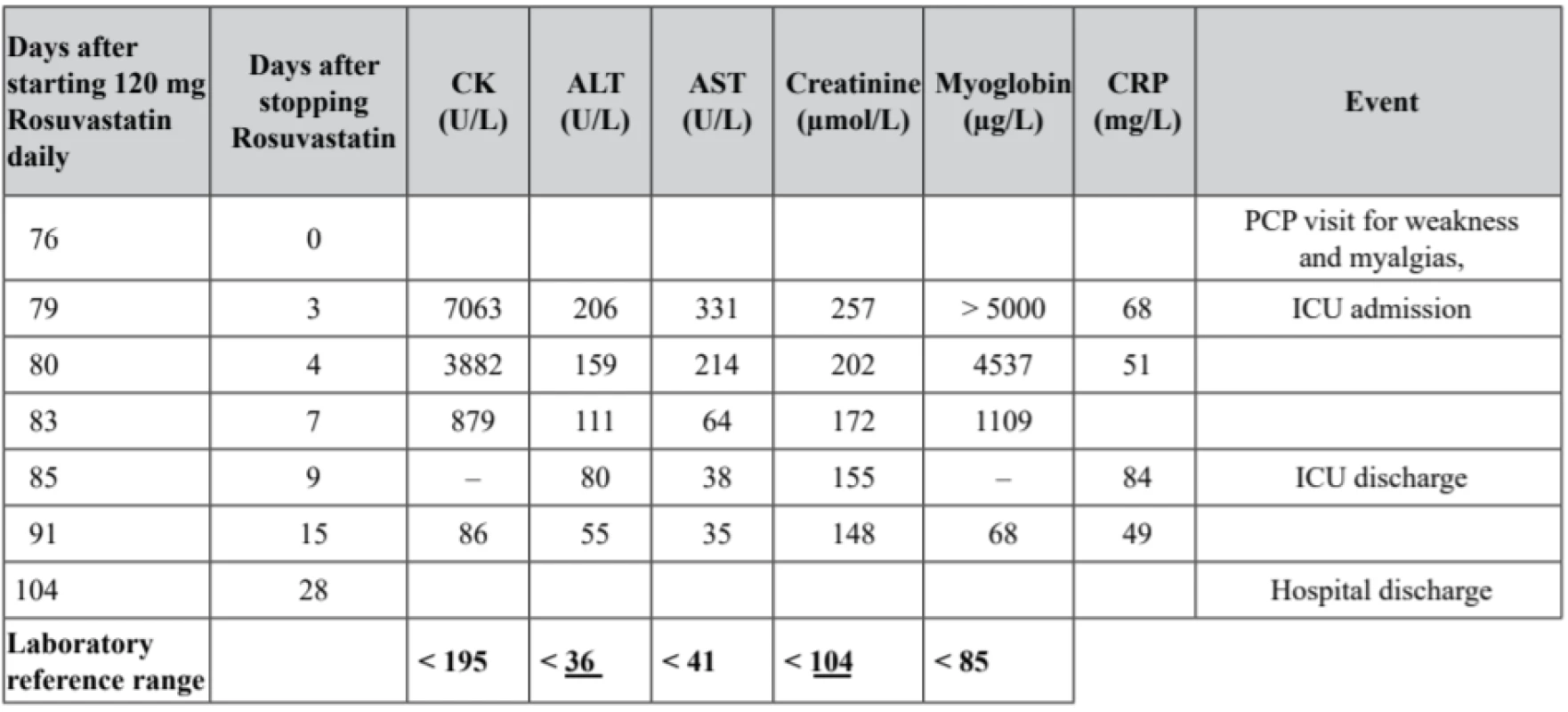
The medication prior to admission included: rosuva-statin 120 mg/day (withdrawn for 3 days), solifenacin 5–10 mg/day, finasteride 5 mg/day, tamsulosin 0.4 mg/day, rilmenidin 1 mg/day, warfarin 3 mg/day (withdrawn for 3 days), insulin lispro 42 IU/day, and insulin glargin 44 IU/day.
Intravenous rehydration and fluid balance monitoring was initiated in the ICU. A consistent rise in eGFR was observed in the next days. Transient polyuria was noted on day 5.
Attention was paid to the fluid balance and serum electrolyte levels. Despite the rapid improvement in renal functions, the patient required a considerable assistance in activities of daily living (ADLs of 30, Mini-Mental State Examination MMS of 7). He was therefore transferred to a long-term facility until the day 28 when his ADLs and MMS scores improved to 75 and 17, respectively. The warfarin administration has been continued under laboratory monitoring. The hydroxymethylglutaryl-CoA reductase inhibitor administration (atorvastatin 20 mg daily) was resumed later.
Discussion
The character of toxicity and time course in relation to drug use, chronic overdose, lack of other possible causes and substantial improvement after the drug discontinuation led to the diagnosis of rosuvastatin-induced rhabdomyolysis complicated with acute kidney injury in a patient with chronic kidney disease (CKD). While the biochemistry parameters improved within few days in the ICU, the cognitive function and mobility recovery required 4 more weeks.
When reviewing this case, we identified two serious medication errors. In the best intent, the patient and his caregivers mistakenly co-administered 3 rosuvastatin formulations with different brand names in a total dose of 120 mg/day. Next, in some countries including the Czech Republic, rosuvastatin 40 mg is contraindicated in patients with impaired renal function. However, this patient had not been given a reduced rosuvastatin dose although his eGFR declined below 40 ml/min/1.73 m2. These medication errors likely represent the major factors that synergistically contributed to the development of rosuvastatin-induced rhabdomyolysis.
Some other factors, possibly contributing to rosuvastatin toxicity should be mentioned. Competitive CYP2C9 saturation by warfarin could, in addition to renal function impairment, decrease the enzymatic clearance of rosuvastastin as hypothesized previously5).
Furthermore, our patient is heterozygous for CYP2C9*1/*3 predicting the enzyme CYP2C9 activity less than in wild-type homozygotes. The altered PK/PD of warfarin due to rosuvastatin co-administration has been reported previously in interaction studies6, 7), although the impact of CYP2C9 genotype on risk of enzyme saturation is not fully documented. The time course of the INR increase in our patient indicates prolonged time to reach steady state, i.e. decreased elimination of warfarin rather than increased sensitivity towards the drug.
However, the unintended triple dose was identified as the major factor contributing to rosuvastatin toxicity. First, the diabetologist switched from Rosumop® to Rosuvastatin Polpharma® in history. Second, the primary care physician prescribed Rosucard® (insisted on this brand-name).
The main reasons for the error are suggested. Confusing drug names: Up to date, there are 23 registered brand names of rosuvastatin in the Czech republic, 14 of them could be confused (Corvapro®, Crestor®, Delipid®, Mertenil®, Rosi®, Rosucard®, Rosuchen®, Rosumop®, Rosuvastatin®, Rovasyn®, Roxilip®, Sorvasta®, Tintaros® and Zahron®). The extraordinary creativity of Marketing Authorization Holders compromised safety in this case. There was insufficient sharing of information among the patient’s diabetologist and family physician. There was no clinical pharmacist care on discharge from the hospital.
To prevent the medication errors, we suggest:
- Implementation of a communication platform for sharing information among physicians and pharmacists about prescribed drugs. The electronic prescription holds the potential to solve this problem. In addition to the benefit of shared structured documentation, the software algorithms might allow a broad automatic screening for medication errors.
- Reduction of creativity in drug names and/or highlighting the information of active substance on the drug box.
- Implementation of clinical pharmacist discharge service.
Conclusions
Rosuvastatin-induced myopathy is a rare but serious adverse effect. This case report highlights the need for proper treatment and dose adjustment during chronic medical therapy, the need for adequate patient education and applying preventive measures.
Acknowledgements
This paper has been supported by the Charles University project PRVOUK P25/LF1/2.
Conflict of interest: none.
Mgr. Martin Vodička (∗) • M. Pisár • T. Šálek
The Tomas Bata Hospital in Zlín, Czech Republic
Krajská nemocnice T. Bati, a. s.
Havlíčkovo nábřeží 600, 762 75 Zlín
e-mail: martin_vodicka@yahoo.com
O. Slanař
Institute of Pharmacology, First Faculty of Medicine and General Teaching Hospital,
Charles University in Prague, Prague, Czech Republic
Sources
1. Abd T. T., Jacobson T. A. Statin-induced myopathy: a review and update. Expert Opin. Drug Saf. 2011; 10, 373–387.
2. Generaux G. T., Bonomo F. M., Johnson M., Doan K. M. Impact of SLCO1B1 (OATP1B1) and ABCG2 (BCRP) genetic polymorphisms and inhibition on LDL-C lowering and myopathy of statins. Xenobiotica 2011; 41, 639–651.
3. McKenney J. M. An assessment of statin safety. Am. J. Manag. Care 2006; 12, S310–S317.
4. Silva M. A., Swanson A. C., Gandhi P. J., Tataronis G. R. Statin-related adverse events: a meta-analysis. Clinical therapeutics 2006; 28, 26–35.
5. Gallelli L., Ferraro M., Spagnuolo V., Rende P., Mauro G. F., de Sarro G. Rosuvastatin induced rhabdomyolysis probably via CYP2C9 saturation. Drug Metabol. Drug Interact. 2009; 24, 83–87.
6. Jindal D., Tandon M., Sharma S., Pillai K. K. Pharmacodynamic evaluation of warfarin and rosuvastatin co-administration in healthy subjects. Eur. J. Clin. Pharmacol. 2005; 61, 621–625.
7. Simonson S. G., Martin P. D., Mitchell P. D., Lasseter K., Gibson G., Schneck D. W. Effect of rosuvastatin on warfarin pharmacodynamics and pharmacokinetics. J. Clin. Pharmacol. 2005; 45, 927–934.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 2-
All articles in this issue
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
- Nové knihy
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- The influence of «Saprogel» in the wound healing process on rats with a full-thickness wound model
- A cost minimization analysis of α2b-interferon supplementation in complex pharmacotherapy of rotavirus infection in newborns
- Formulation and technology development of vaginal pessaries with probiotic activity
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Formulation and technology development of vaginal pessaries with probiotic activity
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

