-
Medical journals
- Career
Carotid body paraganglioma, a very rare pediatric tumor
Authors: M. Formánek 1; T. Hrbáč 2; V. Procházka 3; L. Čábalová 1; Pavel Komínek 1
Authors‘ workplace: Department of Otorhinolaryngology, and Head and Neck Surgery, University, Hospital Ostrava, Czech Republic 1; Department of Neurosurgery, University, Hospital Ostrava, Czech Republic 2; Department of Radiology, University, Hospital Ostrava, Czech Republic 3
Published in: Cesk Slov Neurol N 2020; 83/116(4): 436-437
Category: Letter to Editor
doi: https://doi.org/10.14735/amcsnn2020436Dear editorial office,
The carotid body tumor (CBT) is the most common paraganglioma (PG) that occurs in the neck; it accounts for almost 60% of head and neck PGs [1]. This neoplasm arises from neural crest-derived ectoderm in the third branchial arch. It is located in the adventitia of the carotid bifurcation. CBT is benign in 90-95% of cases, and it is typically identified accidentally as a unilateral, painless, slow-growing mass on the neck [2, 3]. Only about 10% of patients have bilateral tumors [4].
A neck mass is often observed in infants and children. The differential diagnosis must consider many different diseases, including lymphomas, branchial cleft cysts, metastatic lymph nodes, etc. Although tumors involving blood vessels are not uncommon in children, CBTs are extremely rare in pediatric patients. The world literature has reported less than twenty cases of CBTs in children under the age of 14 [1]. This case study describes one.
A 13-year-old girl with a painless mass in the right upper neck area was referred to the Department of Otorhinolaryngology at a tertiary referral hospital. She had a 7-year history of a slow-growing swelling that first appeared at the age of 6 years, which raised the suspicion of a lateral neck cyst or lymphadenopathy. The clinical examination showed a 3 - to 4-cm painless mass on the right side of the neck, just below the angle of the mandible, at the level of the hyoid bone (Fig. 1). The mass was mobile in the horizontal plane, but not in the vertical plane. There were no signs of cranial nerve lesions.
1. CBT located on the right side of the neck. 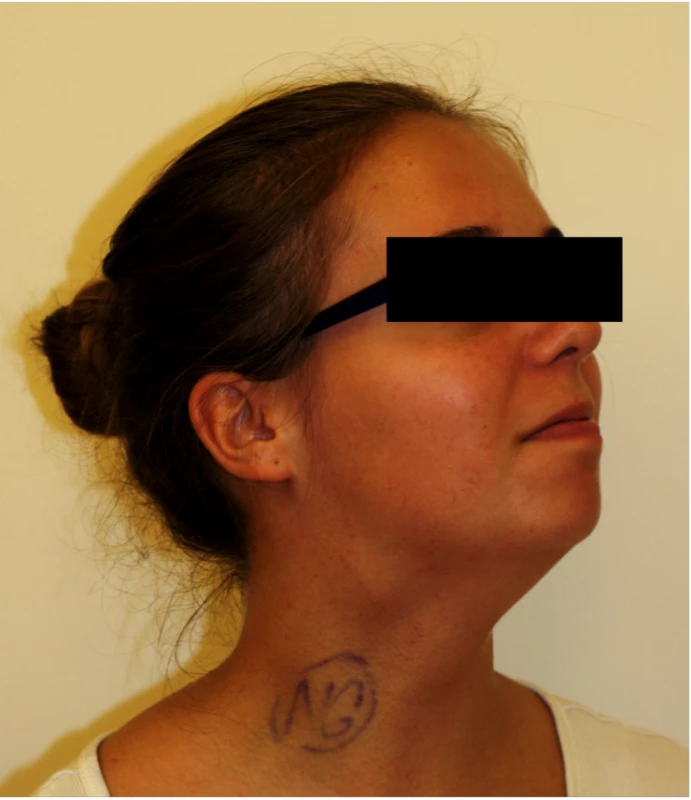
A Doppler ultrasound examination demonstrated a tumor 4×3×3.5 cm in size, with hypervascularization on the right side of the carotid bifurcation. Normal perfusion was observed in the internal and external carotid arteries. The tumor was adjacent to the submandibular gland. Magnetic resonance imaging confirmed that it was a tumor at the carotid bifurcation that had not infiltrated the surrounding tissues. We observed a hyperintense signal in the T2-weighted images and a distinct contrast enhancement in the T1-weighted images (Fig. 2). Carotid arteriography showed a highly vascularized tumor at the carotid bifurcation, with afferent vessels emerging from the external carotid artery and the ascending pharyngeal artery (Fig. 3). We did not perform a temporary balloon occlusion test. Angiography was performed under general anesthesia, prior to surgery, with Vortex coils 2/3 (2 pieces) 2/4 (2 pieces) and Deltaplush coils 2/3 and 2/4 for embolization. Then, with continued anesthesia, the patient was transferred to the operating theater for surgery.
2. T1-weighted MRI scan, after administration of gadolinium, demonstrates a mass within the right carotid space. Both the external carotid artery and the internal carotid artery are constricted.
Obr. 1. T1-vážený obraz MR po podání gadolinia. Viditelná masa karotické prostoru vpravo zaujímající a. carotis externa a interna.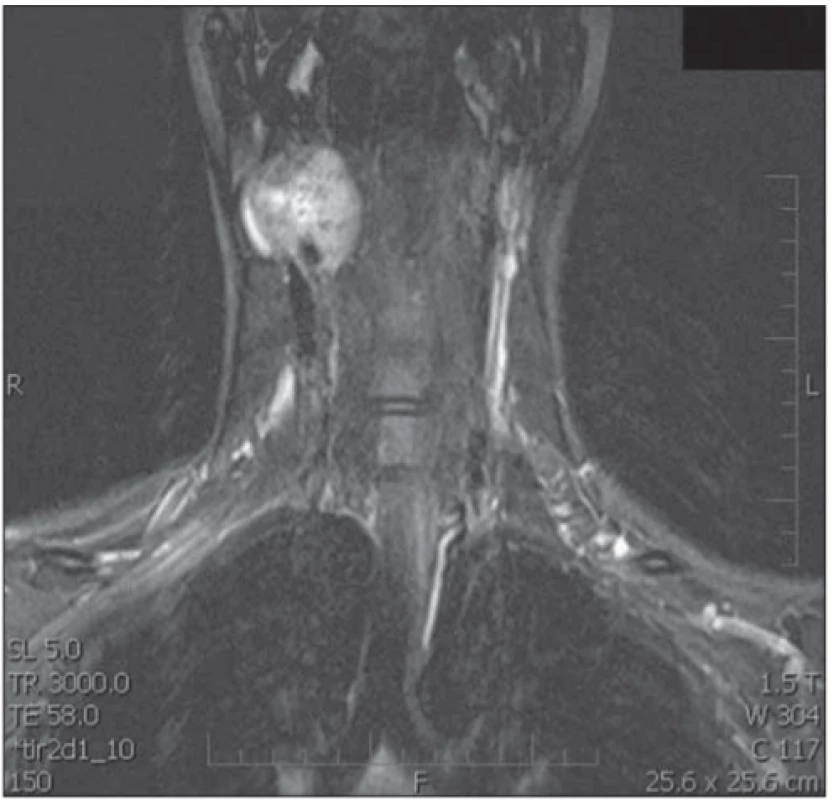
3. Carotid arteriography shows a strongly vascularized lesion in the right carotid bifurcation with a typical image for paraganglioma.
Obr. 2. Angiografi cký obraz ukazuje silně vaskularizované ložisko v pravé karotické bifurkaci s typickým obrazem pro paragangliom.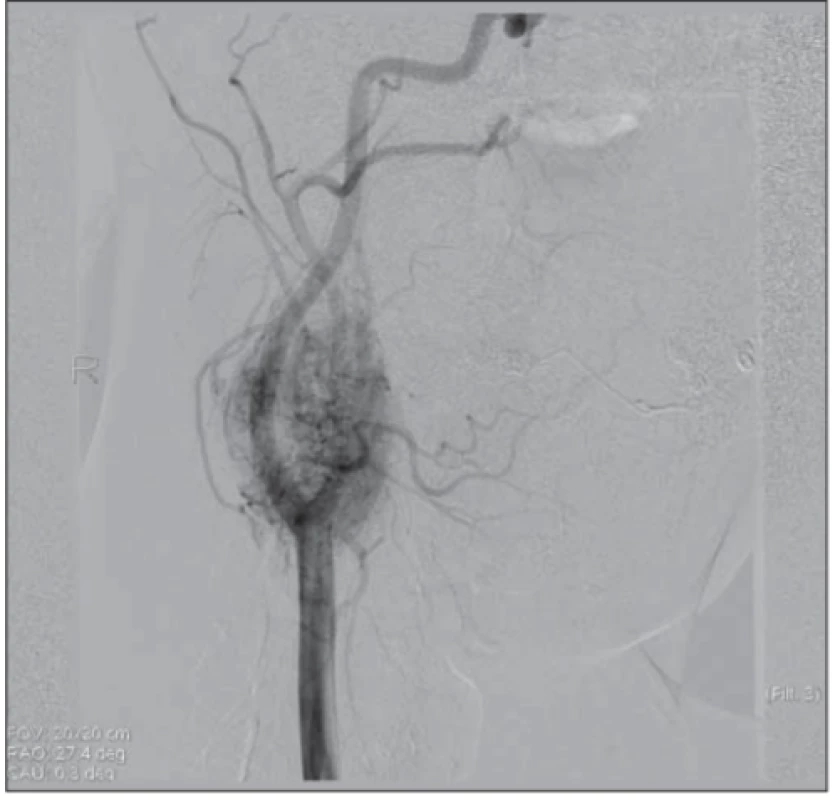
The surgery was performed by a head and neck surgeon and neurosurgeon. The jugular vein and the vagal nerve were exposed, and the carotid arteries were dissected and pulled aside. The median nerve was stimulated with somatosensory evoked potentials (SEPs) during the operation to monitor cerebral function, and after the surgery to prevent a potential neurologic deficit.
The tumor was separated from the external and internal carotid arteries. The superior thyroid artery and the ascending pharyngeal artery were ligated at the point where they emerged from the right side of the carotid bifurcation (a cross stitch was required). It was necessary to clamp the carotid artery for 7 min to perform this part of the surgery. SEP monitoring showed no changes in cerebral function, which suggested that collateral vessels adequately supplied the right side of the brain. The CBT was completely removed.
A histological examination of the tumor showed sharp margins that contained large polygonal cells with round nuclei, some of which were enlarged. Tumor proliferative activity was assessed with Ki67 biomarkers, and proliferation did not exceed 2%. The immunohistochemical study showed a weak positive signal for the S100 protein.
The patient exhibited no postoperative complications, and the wound healed per primam. The patient was discharged from the hospital 7 days after the surgery. No signs of tumor persistence or recurrence were observed (including sonography and magnetic resonance imaging performed one year and two years after the surgery, respectively), and no signs of neurological deficit were found during a 24‑month follow up. A genetic examination showed no genetic basis for the CBT in this patient.
These tumors typically occur sporadically; however, they can be associated with a genetic predisposition (familial or hereditary CBT). For example, they might be associated with Von Hippel Lindau syndrome, neurofibromatosis type 1, multiple endocrine neoplasia type 2A and 2B, or germline mutations in the succinate dehydrogenase gene [6]. They occur most frequently in individuals that reside at high altitudes, where atmospheric oxygen pressure is low; these conditions provide a chronic hypoxic stimulus [3].
Typically, CBT presents as a painless neck mass. Signs of cranial nerve deficits, such as dysphagia, dysphonia, or difficulties with mastication, only occur in 10–30% of patients [2]. In our patient, the CBT presented only as a neck mass with no other accompanying symptoms. A very rare symptom is excessive catecholamine release, which occurs in 1% to 3% of patients [7]. This rare occurrence and the absence of signs of catecholamine release (e.g. headache, palpitations, anxiety, sweating, pain in the chest, decreased thermal tolerance, tachycardia) in our patient was the reason why levels of catecholamines were not assessed preoperatively. However, it can be recommended as it could potentially seriously influence general anesthesia.The tumor growth is very slow (PGs grow <5 mm in size per year, and they double their size in 4.2 years). Usually, the diagnosis is not made until the CBT reaches a palpable volume. Thus, CBTs typically appear in the fourth to sixth decade of life, and they are extremely rare in children [8].
There is no consensus opinion on preoperative embolization, and sometimes, this is a controversial issue. Most authors prefer preoperative embolization, because it can significantly reduce intraoperative bleeding [9]. On the other hand, some authors avoid the use of preoperative embolization, due to the risk of stroke [10]. In our patient, the preoperative embolization applied had a significant effect on perioperative blood loss, which was minimal. In addition, intraoperative electroencephalographic monitoring is advantageous, because it can reduce the risk of brain injury caused by the restricted blood flow through the internal carotid artery. In our patient, even though the internal carotid artery was clamped for several minutes, no neurologic deficit occurred. A specific feature of our treatment was that the embolization was followed by surgery during a single general anesthesia administration. This approach reduced the anesthesia duration and the number of anesthesia administrations required.
CBT should be considered in the differential diagnosis of a neck mass in older children. We showed that the CBT could be successfully treated with preoperative embolization and surgical resection. Moreover, these procedures could be performed during a single anesthesia administration. When planning an early surgical resection, to avoid major surgery-related complications, the main factors to consider should be the slow growth of CBT, its low malignancy rate, and the high rate of postoperative morbidity in large tumors.
Acknowledgement
Supported by Ministry of Health, Czech Republic - conceptual development of research organization (FNOs/2019).
The Editorial Board declares that the manu script met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Accepted for review: 20. 2. 2020
Accepted for print: 14. 5. 2020
Martin Formánek, MD, PhD
Department of Otorhinolaryngology and Head and Neck Surgery University Hospital Ostrava
17. listopadu 1790
708 52 Ostrava
Czech Republic
e-mail address: martin.formanek@fno.cz
Sources
1. Georgiadis GS, Lazarides MK, Tsalkidis A et al. Carotid body tumor in a 13-year-old child: Case report and review of the literature. J Vasc Surg 2008; 47 (4): 874–880. doi: 10.1016/j.jvs.2007.10.040.
2. Naik SM, Shenoy AM, Nanjundappa et al. Paragangliomas of the carotid body: current management protocols and review of literature. Indian J Surg Oncol 2013; 4 (3): 305–312. doi: 10.1007/s13193-013-0249-4.
3. Čertík B, Třeška V. Why are carotid glomus tumours dangerous? Rozhl Chir 2014; 93 (10): 512–515.
4. Amato B, Bianco T, Compagna R et al. Surgical resection of carotid body paragangliomas: 10 years of experience. Am J Surg 2014; 207 (2): 293–298. doi: 10.1016/j.amjsurg.2013.06.002.
5. Gogoi G, Chelleng KK, Borgohain M et al. Carotid body paraganglioma in a child: a novel experience. CIBTech J Surg (Online) 2014; 3 : 37–41. doi: 10.1016/j.jvs.2007.10.040.
6. Sridhara SK, Yener M, Hann EY et al. Genetic testing in head and neck paraganglioma: who, what, and why? J Neurol Surg B Skull Base 2013; 74 (4): 236–240. doi: 10.1055/s-0033-1342924.
7. Offereld C, Brase C, Yaremchuk S et al. Head and neck paragangliomas: clinical and molecular classification. Clinics (Sao Paulo) 2012; 67 (Suppl 1): 19–28. doi: 10.6061/clinics/2012 (sup01) 05.
8. Boscarino G, Parente E, Minelli F et al. An evaluation on management of carotid body tumour (CBT). A twelve years experience. G Chir 2014; 35 (1–2): 47–51.
9. Litle VR, Reilly LM, Ramos TK. Preoperative embolization of carotid body tumors: when is it appropriate? Ann Vasc Surg 1996; 10 (5): 464–468. doi: 10.1007/BF02000594.
10. Hallet JW, Nora JD, Hollier JH et al. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a fifty-year experience with 153 tumors. J Vasc Surg 1988; 7 (2): 284–291.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2020 Issue 4-
All articles in this issue
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Radial nerve injury associated with humeral shaft fracture
- It is evident when to make a surgery for lumbar disc herniation?
- Current diagnostics of secondary progressive form of multiple sclerosis and its treatment with siponimod
- Airway clearance in patients with Parkinson‘s disease – overview and possibilities of physiotherapeutic intervention
- Clinical and social predictors of quality of life in children and young adults with autism spectrum disorder
- Safety of carotid endarterectomy in relation to the timing after ischemic stroke
- Glatirameracetate – the treatment of multiple sclerosis monitored in the ReMuS Registry
- Dropped head syndrome in patient with progressive bulbar palsy
- Transvenous embolization of a ruptured brain arteriovenous malformation
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Editorial
- Souběh dvou oportunních infekcí jako první projev HIV
- Využití kvantitativní MR venografie v indikaci stentingu stenózy žilního splavu
- Recenze knih
- 2020 AAN Highlights Dlouhodobá data o účinnosti deplece CD20+ B-buněk v léčbě RS
- 2020 AAN Highlights Jak mění malá molekula průběh spinální svalové atrofie?
- Multiple sclerosis – behind the immunity curtains
- Intensive computer-assisted cognitive rehabilitation in persons with multiple sclerosis – results of a 12-week randomized study
- Efficacy and safety of emergent microsurgical embolectomy in patients with acute ischemic stroke after the failure of intravenous thrombolysis and mechanical thrombectomy – a systematic review protocol
- Impact of the COVID-19 pandemic on sleep medicine in the Czech Republic and Slovakia
- The prevalence and characteristics of epilepsy in patients with relapsing-remitting multiple sclerosis treated with disease-modifying therapy
- Moyamoya syndrome associated with polycystic kidney disease – a rare case report and literature review
- Carotid body paraganglioma, a very rare pediatric tumor
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- It is evident when to make a surgery for lumbar disc herniation?
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Dropped head syndrome in patient with progressive bulbar palsy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

